Introduction
The existence of hypoxic areas is an important
parameter for solid tumors, which is due to the excessive
consumption of oxygen for tumor growth (1,2).
Previous studies have characterized critical resistant roles of
tumor hypoxia for radiation therapy and most anticancer drugs as
the shortage of blood vessels (3).
Moreover, extensive research has indicated that tumor hypoxia
promotes malignant progression and metastasis (4). Therefore, strategies or drugs
targeting hypoxia have become promising in the implementation of
tumor-specific treatments.
Attenuated Salmonella typhimurium (S.
typhimurium) strains accumulate and grow in tumor environments
(5). Therefore, the potential of
S. typhimurium in tumor-targeting therapy has been
evaluated. S. typhimurium was initially used as a
single-agent to treat cancer. Results from prostate cancer, breast
cancer and hepatocellular carcinoma demonstrated potential
anticancer activities of S. typhimurium(6). Moreover, S. typhimurium is
increasingly utilized as a vector to deliver antiangiogenic or
proapoptotic genes into the hypoxic area of a tumor (7). We recently showed that the
facultative anaerobe S. typhimurium strain VNP20009 is able
to replicate in hypoxic tumors and function as a potential vector
in an established mouse melanoma tumor model (8–11).
Cytokines have been shown to have significant
effects on tumor development. IL-21, one of newly identified
cytokines, belongs to a large family of cytokines identified by
their receptors which share a common γ chain (12). IL-21 is produced by activated T and
natural killer T (NKT) cells. The major function of IL-21 is to
increase the pool of cytotoxic CD8+ T, natural killer
(NK) and NKT cells (13).
Therefore, it is not unexpected that IL-21 has antitumor activity.
IL-21 has been proven to possess anticancer activities in various
tumors such as melanoma, colon and pancreatic carcinoma (13). Based on this, IL-21 has been used
in phase I clinical trials in advanced-stage melanoma patients
(13). Thus, IL-21 is becoming a
promising cytokine in antitumor research.
In this study, we applied a novel strategy to treat
melanoma with S. typhimurium VNP20009 in combination with
IL-21 gene therapy, which was delivered by hydrodynamic tail vein
(HTV) injection. The combination increased the inhibitory effects
on tumor growth compared with S. typhimurium or IL-21
treatment alone, and improved the survival time of animals. More
infiltrating NK and T cells were observed in the tumor area of the
mice that were administered VNP20009 in combination with IL-21
compared with S. typhimurium VNP20009 treatment alone. These
data suggest a novel strategy that can be employed to treat
melanoma in vivo.
Materials and methods
Plasmid construction
The full-length coding sequence of mouse IL-21 was
amplified from the mouse T cell cDNA library and inserted into a
vector pBLAST42-mcs (Invivogen, Inc., San Diego, CA, USA). The
coding sequence and open reading frame of positive clones were
confirmed to be correct by DNA sequencing (GenScript Corporation,
Nanjing, China).
Animals
Six- to seven-week-old female C57BL/6 mice were
purchased from the Model Animal Research Center of Nanjing
University (Nanjing, China) and housed in a specific pathogen-free
(SPF) animal facility (Gulou Hospital, Nanjing, China) which was
environmentally controlled (22°C; with a 12:12 h light/dark cycle;
light cycle, 08:00–20:00; dark cycle 20:00-08:00) with ad
libitum access to standard laboratory chow and water. The
protocols were approved by the local Institution Review Boards and
the animal experiments were performed in accordance with the
Ethical Guidelines for Animal Use and Care established by the
Nanjing University (Nanjing, China).
Cell culture and tumor model
B16F10 melanoma cells were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen,
Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Invitrogen).
C57BL/6 mice were inoculated subcutaneously (s.c.) on the mid-right
flank with 5×105 B16F10 cells in 0.1 ml
phosphate-buffered saline (PBS). S. typhimurium strain
VNP20009 (ATCC) was cultured and prepared as described and then
injected intraperitoneally (i.p.) with 0.2 ml PBS containing
1×106 colony-forming units (cfu) bacteria into the
tumor-bearing mice 6 days post-inoculation. IL-21 expression or
control plasmid was injected through the hydrodynamic tail vein
(HTV) twice at 6 and 13 days following inoculation. The tumor
volume was measured individually with a caliper and determined by
the formula: tumor volume = length × width × width × 0.52.
Transfection and western blot
analysis
293T cells were cultured in DMEM with 10% FBS. IL-21
expression or control plasmid was transfected by Lipofectamine 2000
(Invitrogen) following the manufacturer’s instructions. Following
transfection (24 h), the cells were collected and homogenized in
lysis buffer containing 50 mM Tris-HCl (pH 7.4), 250 mM NaCl, 0.5%
Triton X-100, 50 mM NaF, 2 mM EDTA and 1 mM
Na3VO4 on ice for 30 min. Anti-IL-21 antibody
was purchased from R&D Systems, Inc. (Minneapolis, MN,
USA).
Enzyme-linked immunosorbent assay
(ELISA)
Serum samples were prepared following standard
protocol. Samples were assayed using the an ELISA kit (eBioscience,
Inc., San Diego, CA, USA). The reagents used were supplied in the
kit. Each ELISA plate was read in a Bio-Tex EL×800™ plate reader
(Cole-Palmer, Vernon Hills, IL, USA). Reader software was used to
calculate the data.
Flow cytometric analysis
Single-cell suspension was prepared from the tumor
tissues. The suspension was blocked with 10% FBS for 30 min.
Immunofluorescent staining of the cell surface markers was
performed using PE-Cy™5 rat anti-mouse CD4 for the CD4 T cells,
FITC rat anti-mouse CD8α for the CD8 T cells, and APC mouse
anti-mouse NK-1.1 for the NK cells (Becton-Dickinson, Franklin
Lakes, NJ, USA). Samples were incubated in the dark by agitation
for 45 min. Following washing in PBS, the cells were resuspended
and then analyzed.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD) and data analysis was performed using SPSS software (SPSS
17.0, Chicago, IL, USA). Paired Student’s t-test analysis was
conducted to assess statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effective expression of IL-21 plasmid in
vivo
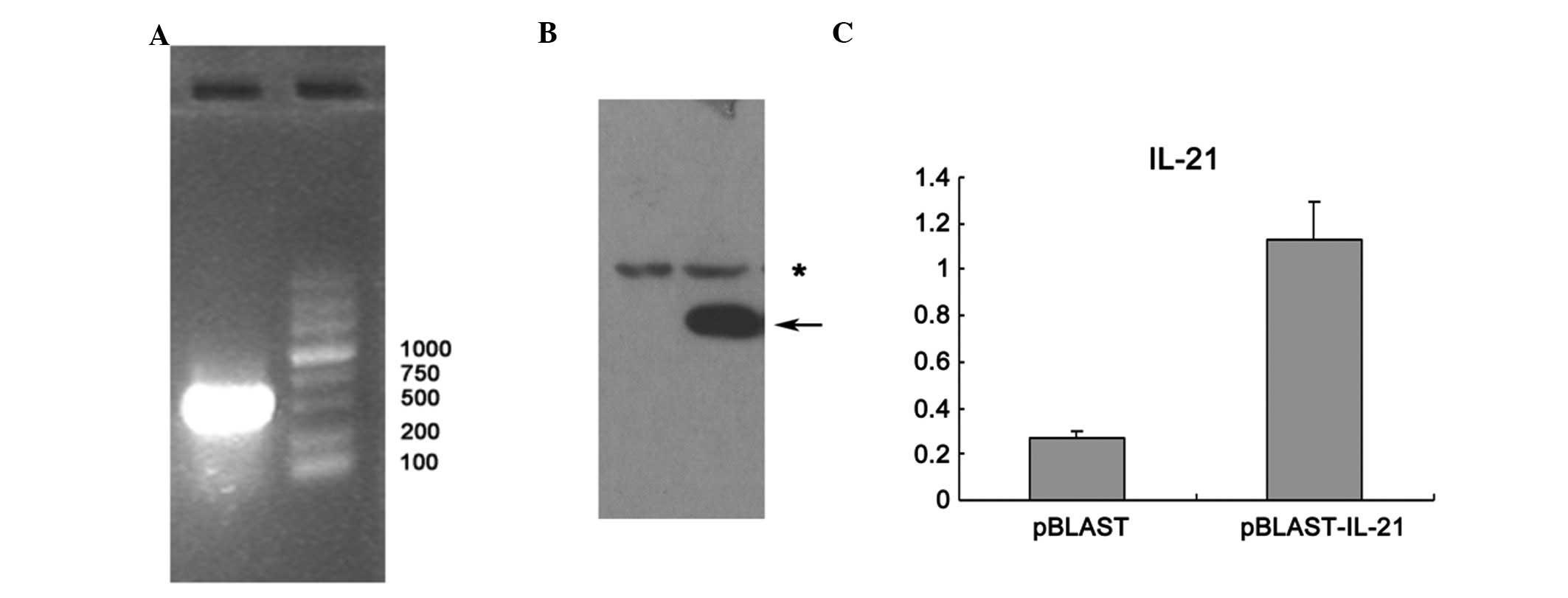
To perform the HTV injection of IL-21 plasmid, we
firstly constructed the IL-21 expressing plasmid. The full-length
cDNA of the IL-21 gene was amplified from a cDNA library of mouse T
cells with specific primers (Fig.
1A). The positive clones containing IL-21 gene were identified
by enzyme digestion and then confirmed by DNA sequencing. The
plasmid was then transfected into 293T cells in order to evaluate
the expression efficiency of IL-21 protein. The plasmid containing
IL-21 cDNA successfully expressed IL-21 in 293T cells, compared
with the vehicle control (Fig.
1B). The plasmid was then delivered into mice by HTV injection.
Higher IL-21 expression in the serum of mice that received IL-21
expression plasmid was detected using ELISA compared with that of
the control (Fig. 1C).
Combination of S. typhimurium and IL-21
inhibits tumor growth and animal survival
Once the IL-21 expressing plasmid was constructed,
the antitumor efficiency of S. typhimurium VNP20009 combined
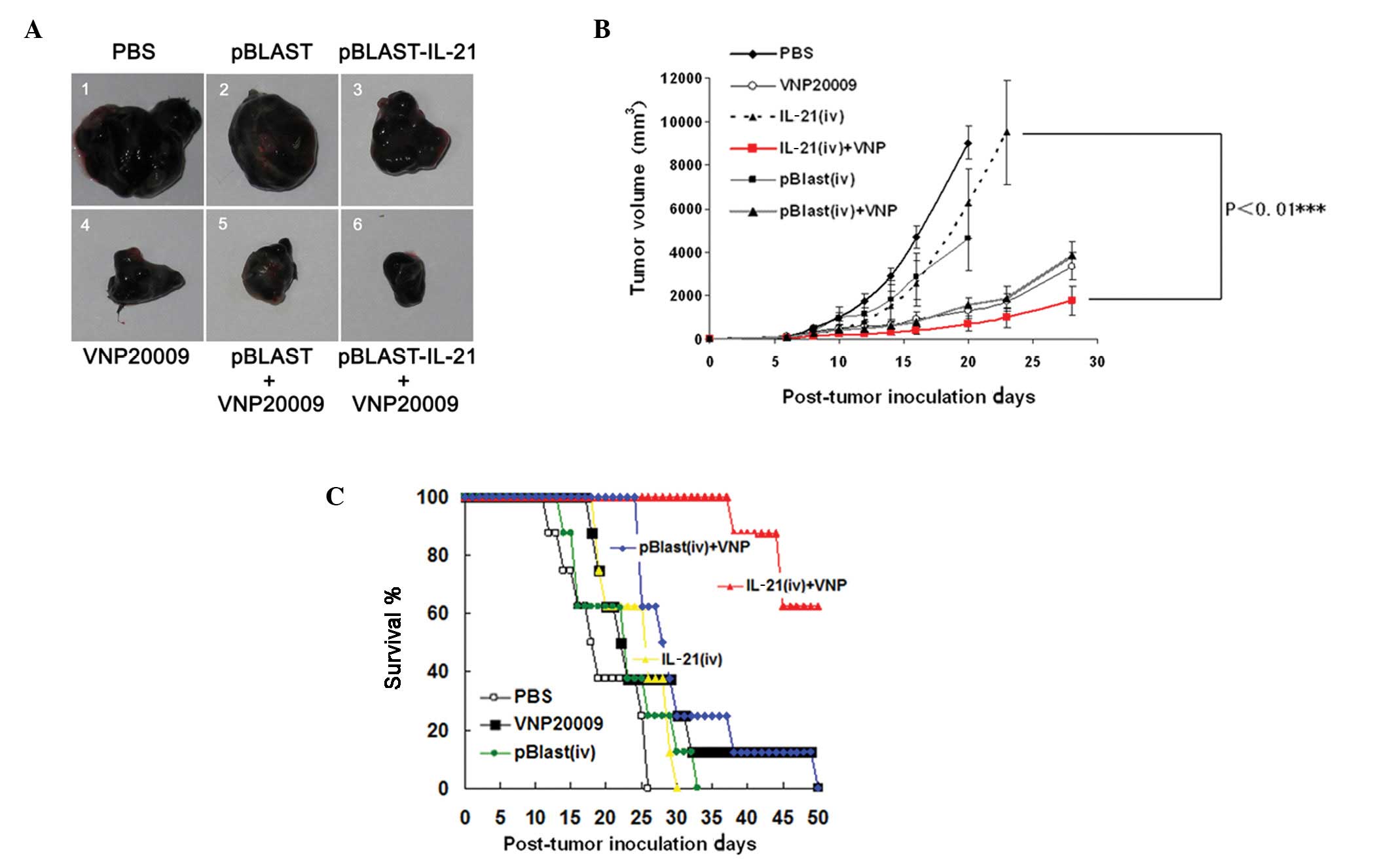
with IL-21 was examined in vivo. Six days following the
establishment of the B16F10 melanoma mouse model, VNP20009 and
IL-21 plasmids were delivered by i.p. and HTV injection,
respectively. The tumor volumes were monitored from 6 to 28 days.
The tumor that received combination treatment exhibited smaller
size compared with single treatment controls (Fig. 2A). We found that the combination
strategy significantly inhibited the tumor volumes of melanoma
compared with that of VNP20009 or IL-21 treatment alone (P<0.01,
Fig. 2B). Compared with vehicle
controls, the single treatment of VNP20009 and IL-21 prolonged the
survival of the mice bearing the melanoma. Notably, the combination
strategy significantly prolonged the survival time (Fig. 2C).
IL-21 promotes T and NK cell
infiltration
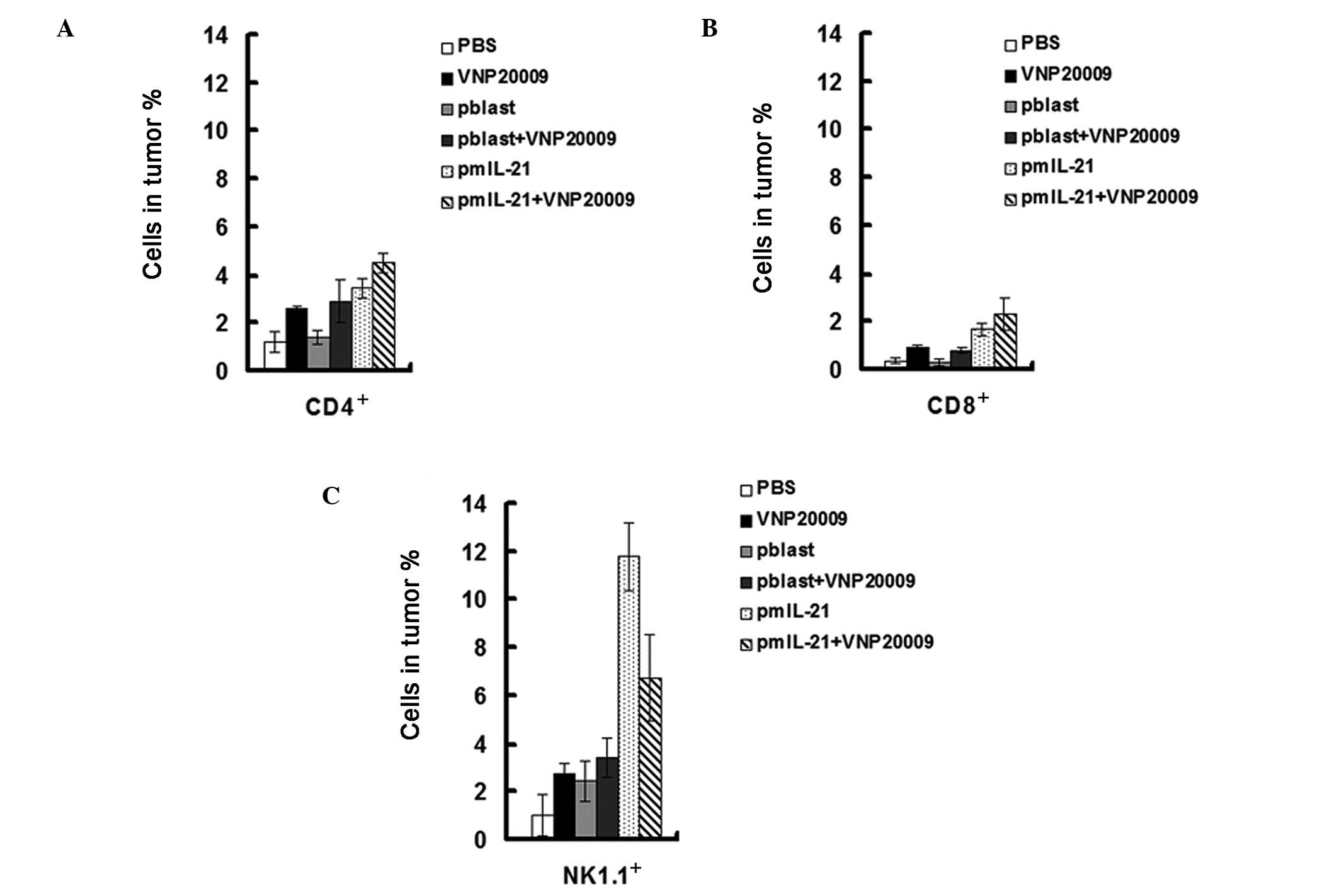
IL-21 has been shown to effectively induce the
infiltration of T and NK cells. Therefore, we investigated whether
or not it improved the treatment efficiency through promoting T and
NK cell infiltration in the tumor microenvironment. To investigate
this, we examined the T-helper cell distribution in tumors by their
specific marker CD4. As expected, IL-21 induced CD4+ T
cell infiltration in tumor ∼2 times compared with controls
(Fig. 3A). Similarly, more
cytotoxic T cells marked by CD8 were also observed in tumors
treated with IL-21 (Fig. 3B).
Furthermore, IL-21 induced more NK1.1 cell distribution in tumors
(Fig. 3C). These data suggest that
IL-21 facilitates the antitumor activities of VNP20009 by inducing
the infiltrated T and NK cells.
Discussion
Conventional anticancer therapies such as
chemotherapy and radiotherapy have successfully been used in
treating different types of cancer. However, approximately half of
the patients exhibited resistance to these therapies (14). This encourages the development of
novel therapies with higher specificity and efficiency. One of the
most prevalent differences of tumors from normal tissues is the
existence of the hypoxia region. This characteristic is becoming a
candidate for novel tumor-targeted therapies. Among many emerging
novel anticancer experimental strategies targeting the hypoxic
area, bacterial treatment possesses certain advantages that render
it a promising candidate for novel cancer therapies (15). For example, some anaerobic bacteria
such as Salmonella, which preferentially replicate in hypoxic and
necrotic areas, have been extensively used in the development of
novel cancer treatment strategies. To reduce the side-effects of
Salmonella as a bacterium, attenuated S. typhimurium have
been developed and approved for clinical trials in cancer treatment
(16). It has been shown that
distinct strategies was developed for Salmonella typhimurium to
treat cancer (17). The primary
application of S. typhimurium in cancer treatment is to
directly inhibit tumor growth. Current research combines S.
typhimurium with other antitumor agents to develop novel
strategies. In the present study, we have developed a novel
strategy to treat melanoma by combining IL-21 with S.
typhimurium VNP20009. IL-21 significantly improved the
inhibitory effect on the tumor growth of S. typhimurium.
More CD4+ and CD8+ lymphocytes and NK were
observed in the tumor after IL-21 administration, indicating that
IL-21 is able to effectively promote immune cell infiltration in
melanoma tumors. As a newly identified interleukin, the regulatory
roles of IL-21 in cancer inhibition remain controversial (18). It has been proven to exhibit
inhibitory effects on tumors such as pancreatic carcinoma,
fibrosarcoma and renal cell carcinoma. However, it has also been
shown to possess accelerative roles in colitis-associated colon
cancer (19, 20). Therefore, IL-21 has been suggested
to have a cell context-dependent activity on cancer. Thus, IL-21
exhibits anticancer effects on melanoma in vivo.
HTV delivery of naked DNA or RNA is a simple and
effective in vivo gene delivery method (21). This non-viral strategy has been
successfully used to express exogenous genes in tissues such as
skeletal muscle and liver and has been successfully used in several
clinical trials (22,23). Whether this systematic delivery
strategy is useful to cancer treatment has not been fully
elucidated yet. In the present study, we showed that HTV injection
is an effective strategy to deliver an anticancer gene in
vivo, suggesting a novel combination strategy of bacteria and
non-viral delivery method to treat cancer. Additional studies are
required to evaluate the safety and efficiency of this novel
strategy.
Acknowledgements
The authors are grateful to grants
from the National Key Basic Research Program from the Ministry of
Science and Technology (2012CB967004), the Jiangsu Provincial
Nature Science Foundation (BK2011228, BZ2011048, BZ2010074,
BZ2012050), the Chinese National Nature Sciences Foundation
(81121062, 50973046, 31071196, 31070706) and the Bureau of Science
and Technology of Changzhou (CN20100016, CZ20100008, CJ20115006,
CE20115034, CZ20110028).
References
|
1.
|
Dachs GU, Patterson AV, Firth JD, et al:
Targeting gene expression to hypoxic tumor cells. Nat Med.
3:515–520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pouyssegur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Brown JM and Giaccia AJ: The unique
physiology of solid tumors: opportunities (and problems) for cancer
therapy. Cancer Res. 58:1408–1416. 1998.PubMed/NCBI
|
|
4.
|
Graham CH, Forsdike J, Fitzgerald CJ and
MacDonald-Goodfellow S: Hypoxia-mediated stimulation of carcinoma
cell invasiveness via upregulation of urokinase receptor
expression. Int J Cancer. 80:617–623. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Leschner S and Weiss S: Salmonella-allies
in the fight against cancer. J Mol Med (Berl). 88:763–773. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Luo X, Li Z, Lin S, et al: Antitumor
effect of VNP20009, an attenuated Salmonella, in murine tumor
models. Oncol Res. 12:501–508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pawelek JM, Low KB and Bermudes D:
Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res.
57:4537–4544. 1997.PubMed/NCBI
|
|
8.
|
Chen J, Wei D, Zhuang H, et al: Proteomic
screening of anaerobically regulated promoters from Salmonella and
its antitumor applications. Mol Cell Proteomics.
10:M111.0093992011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jia LJ, Wei DP, Sun QM, Jin GH, et al:
Tumor-targeting Salmonella typhimuriumimproves
cyclophosphamide chemotherapy at maximum tolerated dose and
low-dose metronomic regimens in a murine melanoma model. Int J
Cancer. 121:666–674. 2007.PubMed/NCBI
|
|
10.
|
Chen G, Tang B, Yang BY, Chen JX, et al:
Tumor-targeting Salmonella typhimurium, a natural tool for
activation of prodrug 6MePdR and their combination therapy in
murine melanoma model. Appl Microbiol Biotechnol. Aug 7–2012, (Epub
ahead of print).
|
|
11.
|
Chen J, Yang B, Cheng X, et al:
Salmonella-mediated tumor-targeting TRAIL gene therapy
significantly suppresses melanoma growth in mouse model. Cancer
Sci. 103:325–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Spolski R and Leonard WJ: Interleukin-21:
basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sondergaard H and Skak K: IL-21: roles in
immunopathology and cancer therapy. Tissue Antigens. 74:467–479.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Goluboff ET, Hirano D, Thrasher JB, Stark
G, Miller GJ and Glode LM: New approaches to the treatment of
advanced prostate cancer. Rev Urol. 3:S69–S78. 2001.PubMed/NCBI
|
|
15.
|
Patyar S, Joshi R, Byrav DS, Prakash A,
Medhi B and Das BK: Bacteria in cancer therapy: a novel
experimental strategy. J Biomed Sci. 17:212010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Low KB, Ittensohn M, Luo X, et al:
Construction of VNP20009: a novel, genetically stable
antibiotic-sensitive strain of tumor-targeting Salmonella for
parenteral administration in humans. Methods Mol Med. 90:47–60.
2004.
|
|
17.
|
Dang LH, Bettegowda C, Huso DL, Kinzler KW
and Vogelstein B: Combination bacteriolytic therapy for the
treatment of experimental tumors. Proc Natl Acad Sci USA.
98:15155–15160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Stolfi C, Pallone F, Macdonald TT and
Monteleone G: Interleukin-21 in cancer immunotherapy: friend or
foe? Oncoimmunology. 1:351–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Stolfi C, Rizzo A, Franze E, et al:
Involvement of interleukin-21 in the regulation of
colitis-associated colon cancer. J Exp Med. 208:2279–2290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Jauch D, Martin M, Schiechl G, et al:
Interleukin 21 controls tumour growth and tumour immunosurveillance
in colitis-associated tumorigenesis in mice. Gut. 60:1678–1686.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hodges BL and Scheule RK: Hydrodynamic
delivery of DNA. Expert Opin Biol Ther. 3:911–918. 2003. View Article : Google Scholar
|
|
22.
|
Romero NB, Benveniste O, Payan C, et al:
Current protocol of a research phase I clinical trial of
full-length dystrophin plasmid DNA in Duchenne/Becker muscular
dystrophies. Part II: clinical protocol. Neuromuscul Disord.
12:S45–S48. 2002. View Article : Google Scholar
|
|
23.
|
Budker V, Zhang G, Knechtle S and Wolff
JA: Naked DNA delivered intraportally expresses efficiently in
hepatocytes. Gene Ther. 3:593–598. 1996.PubMed/NCBI
|