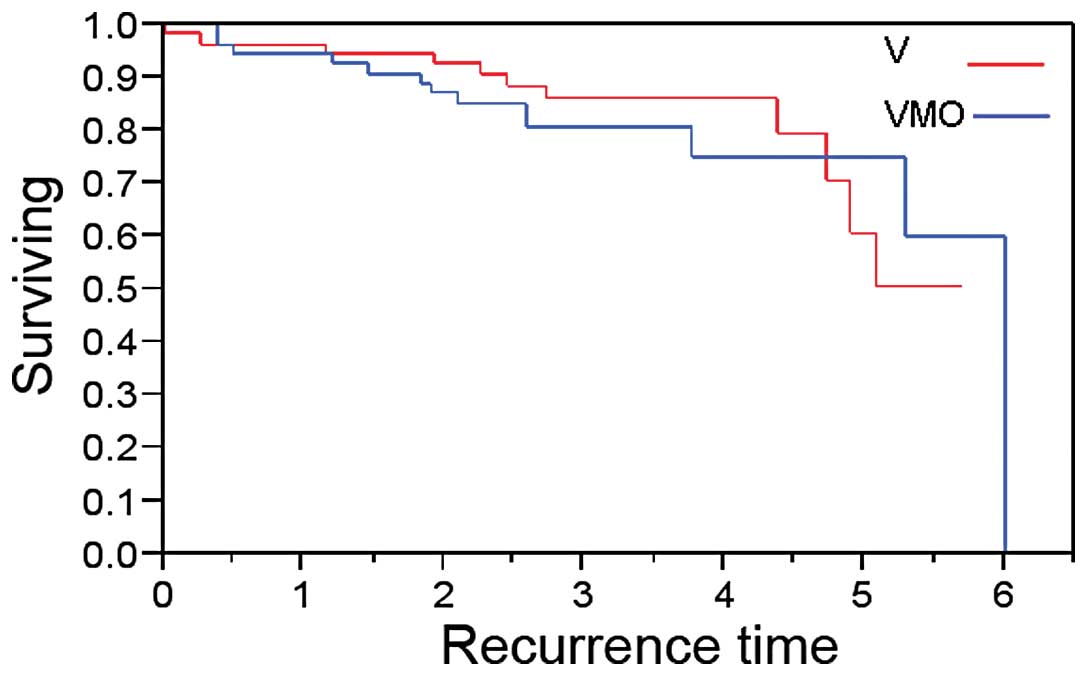
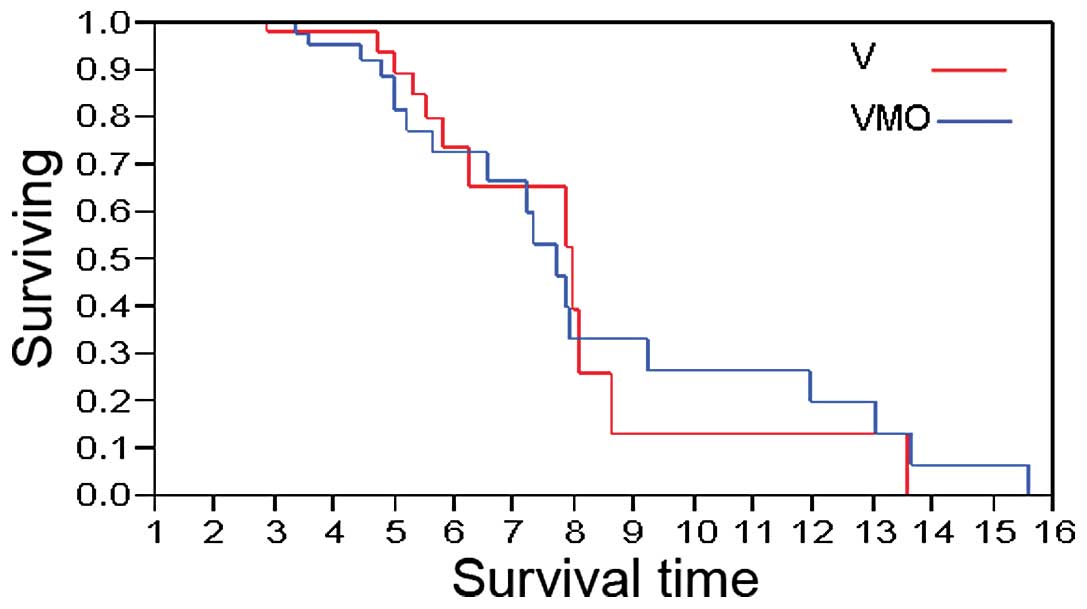
|
1.
|
Haass NK, Smalley KS and Herlyn M: The
role of altered cell-cell communication in melanoma progression. J
Mol Histol. 35:309–318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Inamdar GS, Madhunapantula SV and
Robertson GP: Targeting the MAPK pathway in melanoma: why some
approaches succeed and other fail. Biochem Pharmacol. 80:624–637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Atkins MB, Hsu J, Lee S, Cohen GI,
Flaherty LE, Sosman JA, Sondak VK and Kirkwood JM; Eastern
Cooperative Oncology Group: Phase III trial comparing concurrent
biochemotherapy with cisplatin, vinblastine, dacarbazine,
interleukin-2, and inter-feron alfa-2b with cisplatin, vinblastine,
and dacarbazine alone in patients with metastatic malignant
melanoma (E3695): a trial coordinated by the Eastern Cooperative
Oncology Group. J Clin Oncol. 26:5748–5754. 2008.
|
|
5.
|
Bedikian AY, Millward M, Pehamberger H,
Conry R, Gore M, Trefzer U, et al: Bcl-2 antisense (oblimersen
sodium) plus dacarbazine in patients with advanced melanoma: the
Oblimersen Melanoma Study Group. J Clin Oncol. 24:4738–4745. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Weber J: Immunotherapy for melanoma. Curr
Opin Oncol. 23:163–169. 2011. View Article : Google Scholar
|
|
7.
|
Block MS, Suman VJ, Nevala WK, Kottschade
LA, Creagan ET, Kaur JS, et al: Pilot study of
granulocyte-macrophage colony-stimulating factor and interleukin-2
as immune adjuvants for a melanoma peptide vaccine. Melanoma Res.
21:438–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hodi FS, O’Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, et al: Improved survival with ipilimumab in
patients with metastatic melanoma. N Engl J Med. 363:711–723. 2010.
View Article : Google Scholar
|
|
9.
|
Flaherty KT, Yasothan U and Kirkpatrick P:
Vemurafenib. Nat Rev Drug Discov. 10:811–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
McCardle TW, Messina JL and Sondak VK:
Completely regressed cutaneous melanocytic lesion revisited. Semin
Oncol. 36:498–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Overwijk WW and Restifo NP: Autoimmunity
and the immunotherapy of cancer: targeting the ‘self’ to destroy
the ‘other’. Crit Rev Immunol. 20:433–450. 2000.
|
|
12.
|
Pittet MJ, Zippelius A, Valmori D, Speiser
DE, Cerottini JC and Romero P: Melan-A/MART-1-specific CD8 T cells:
from thymus to tumor. Trends Immunol. 23:325–328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gnjatic S, Nishikawa H, Jungbluth AA, Güre
AO, Ritter G, Jäger E, et al: NY-ESO-1: review of an immunogenic
tumor antigen. Adv Cancer Res. 95:1–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Boon T: Tumor antigens recognized by
cytolytic T lymphocytes: present perspectives for specific
immunotherapy. Int J Cancer. 54:177–180. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Rosenberg SA: A new era of cancer
immunotherapy: converting theory to performance. CA Cancer J Clin.
49:70–73. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Reynolds SR, Celis E, Sette A, Oratz R,
Shapiro RL, Johnston D, Fotino M and Bystryn JC: Identification of
HLA-A*03, A*11 and B*07-restricted
melanoma-associated peptides that are immunogenic in vivo by
vaccine-induced immune response (VIIR) analysis. J Immunol Methods.
244:59–67. 2000.
|
|
17.
|
Reynolds SR, Celis E, Sette A, Oratz R,
Shapiro RL, Johnston D, Fotino M and Bystryn JC: HLA-independent
heterogeneity of CD8+ T cell responses to MAGE-3, Melan-A/MART-1,
gp100, tyrosinase, MC1R, and TRP-2 in vaccine-treated melanoma
patients. J Immunol. 161:6970–6976. 1998.PubMed/NCBI
|
|
18.
|
Reynolds SR, Oratz R, Shapiro RL, Hao P,
Yun Z, Fotino M, Vukmanović S and Bystryn JC: Stimulation of CD8+ T
cell responses to MAGE-3 and Melan A/MART-1 by immunization to a
polyvalent melanoma vaccine. Int J Cancer. 72:972–976. 1997.
|
|
19.
|
Baurain J, Stas M, Hammouch F, Gillain A,
Feyens A, Van Baren N, et al: Association of primary melanoma
ulceration and clinical benefit of adjuvant vaccination with
tumor-specific antigenic peptides. J Clin Oncol. 27:abs. 3022.
2009.
|
|
20.
|
Kruit WH, van Ojik HH, Brichard VG,
Escudier B, Dorval T, Dréno B, et al: Phase 1/2 study of
subcutaneous and intradermal immunization with a recombinant MAGE-3
protein in patients with detectable metastatic melanoma. Int J
Cancer. 117:596–604. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Slingluff CL Jr, Petroni GR, Olson WC,
Smolkin ME, Ross MI, Haas NB, et al: Effect of
granulocyte/macrophage colony-stimulating factor on circulating
CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine:
outcome of a multicenter randomized trial. Clin Cancer Res.
15:7036–7044. 2009.PubMed/NCBI
|
|
22.
|
Filipazzi P, Pilla L, Patuzzo R, Castelli
C, Maurichi A, Tragni G, et al: Adjuvant multipeptide vaccination
in high-risk early melanoma patients. J Clin Oncol. 26:abs. 3014.
2008.
|
|
23.
|
Schwartzentruber DJ, Lawson DH, Richards
JM, Conry RM, Miller DM, Treisman J, et al: gp100 peptide vaccine
and interleukin-2 in patients with advanced melanoma. N Engl J Med.
364:2119–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wallack MK, Steplewski Z, Koprowski H,
Rosato E, George J, Hulihan B and Johnson J: A new approach in
specific, active immunotherapy. Cancer. 39:560–564. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wallack MK: Specific active immunotherapy
with vaccinia oncolysates. Tumor Progression. Crispen R:
Elsevier/North Holland Inc.; New York: pp. 277–287. 1980
|
|
26.
|
Berthier-Vergnes O, Portoukalian J,
Lefthériotis E and Doré JF: Induction of IgG antibodies directed to
a M(r) 31,000 melanoma antigen in patients immunized with vaccinia
virus melanoma oncolysates. Cancer Res. 54:2433–2439.
1994.PubMed/NCBI
|
|
27.
|
Shimizu Y, Hasumi K, Masubuchi K and
Okudaira Y: Immunotherapy of tumor-bearing mice utilizing virus
help. Cancer Immunol Immunother. 27:223–227. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wallack MK, Meyer M, Bourgoin A, Doré JF,
Leftheriotis E, Carcagne J and Koprowski H: A preliminary trial of
vaccinia oncolysates in the treatment of recurrent melanoma with
serologic responses to the treatment. J Biol Response Mod.
2:586–596. 1983.PubMed/NCBI
|
|
29.
|
Wallack MK, McNally KR, Leftheriotis E,
Seigler H, Balch C, Wanebo H, Bartolucci AA and Bash JA: A
Southeastern Cancer Study Group phase I/Il trial with vaccinia
melanoma oncolysates. Cancer. 57:649–655. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Wallack MK, Bash JA, Leftheriotis E,
Seigler H, Bland K, Wanebo H, Balch C and Bartolucci AA: Positive
relationship of clinical and serological responses to vaccinia
melanoma oncolysate. Arch Surg. 122:1460–1463. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wallack MK, Sivanandham M, Balch CM, Urist
MM, Bland KI, Murray D, et al: A phase III randomized, double-blind
multiinstitutional trial of vaccinia melanoma oncolysate-active
specific immunotherapy for patients with stage II melanoma. Cancer.
75:34–42. 1995. View Article : Google Scholar
|
|
32.
|
Wallack MK, Sivanandham M, Ditaranto K,
Shaw P, Balch CM, Urist MM, et al: Increased survival of patients
treated with a vaccinia melanoma oncolysate vaccine. Second interim
analysis of data from a phase III, multi-institutional trial. Ann
Surg. 226:198–206. 1997. View Article : Google Scholar
|
|
33.
|
Wallack MK, Sivanandham M, Balch CM, Urist
MM, Bland KI, Murray D, et al: Surgical adjuvant active specific
immunotherapy for patients with stage III melanoma: the final
analysis of data from a phase III, randomized, double-blind,
multicenter vaccinia melanoma oncolysate trial. J Am Coll Surg.
187:69–79. 1998. View Article : Google Scholar
|
|
34.
|
Roenigk HH Jr, Deodhar S, Jacques R and
Burdick K: Immunotherapy of malignant melanoma with vaccinia virus.
Arch Dermatol. 109:668–673. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Linge C, Gewert D, Rossmann C, Bishop JA
and Crowe JS: Interferon system defects in human malignant
melanoma. Cancer Res. 55:4099–4104. 1995.PubMed/NCBI
|
|
36.
|
Joosse A, de Vries E, Eckel R, Nijsten T,
Eggermont AM, Hölzel D, Coebergh JW and Engel J; Munich Melanoma
Group: Gender differences in melanoma survival: female patients
have a decreased risk of metastasis. J Invest Dermatol.
131:719–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Morton DL, Hsueh EC, Essner R, Foshag LJ,
O’Day SJ, Bilchik A, et al: Prolonged survival of patients
receiving active immunotherapy with Canvaxin therapeutic polyvalent
vaccine after complete resection of melanoma metastatic to regional
lymph nodes. Ann Surg. 236:438–449. 2002. View Article : Google Scholar
|
|
38.
|
Hersey P, Coates AS, McCarthy WH, Thompson
JF, Sillar RW, McLeod R, et al: Adjuvant immunotherapy of patients
with high-risk melanoma using vaccinia viral lysates of melanoma:
results of a randomized trial. J Clin Oncol. 20:4181–4190. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Aydin N, Jack A, Montenegro G, Boyes C,
Alam K and Wallack MW: Expression of melanoma-associated antigens
in human dendritic cells pulsed with an interleukin-2 gene encoded
vaccinia melanoma oncolysate (rIL-2VMO). Cancer Biol Ther.
5:1654–1657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Jack AM, Aydin N, Montenegro G, Alam K and
Wallack MK: A novel dendritic cell-based cancer vaccine produces
promising results in a syngenic CC-36 murine colon adenocarcinoma
model. J Surg Res. 139:164–169. 2007. View Article : Google Scholar : PubMed/NCBI
|