Introduction
Despite the recent advances in adjuvant therapy for
colorectal cancer (CRC), the peritoneal surface remains an
important failure site for patients with disease recurrence.
Peritoneal carcinomatosis (PC) is commonly associated with a poor
prognosis and a median survival of ∼6 months (1). However, the use of cytoreductive
surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) was
reported to be a novel approach to treating peritoneal surface
malignancies (2–6). Furthermore, as a positive peritoneal
lavage following macroscopic curative surgery has been associated
with decreased survival, increased recurrence and peritoneal
metastases (PM) (7–9), HIPEC is considered to be a viable
option for the treatment of patients at high risk of developing
PM.
Mitomycin C (MMC) is the most frequently used agent
for HIPEC and was shown to be efficient and safe for the treatment
of PM of colorectal origin (10).
In addition, 5-fluorouracil (5FU) was also shown to be effective as
intraperitoneal chemotherapy and was used as early postoperative
intraperitoneal chemotherapy (EPIC) following HIPEC in recent
trials (11,12). However, the efficacy of 5FU in the
HIPEC setting for patients with PM from CRC has not been
determined. We recently evaluated the clinical potential of HIPEC
with the combination of MMC and 5FU (MMC-5FU) in patients with PC
due to gastric cancer (unpublished data). In the present study, we
aimed to confirm the efficacy of HIPEC with MMC-5FU for CRC using
in vitro simulation. Furthermore, we conducted a clinical
study to investigate the feasibility and safety of HIPEC combining
these two agents in patients at high risk of developing colorectal
PM following cytoreductive surgery.
Materials and methods
In vitro simulation of HIPEC
HIPEC was simulated using a chemosensitivity test
for antitumor agents, the collagen gel droplet-embedded culture
drug sensitivity test, according to a modification of the
manufacturer’s instructions (Kurabo Industries Ltd., Osaka, Japan)
as previously described (13–15).
Briefly, a suspension of the HCT116 CRC cell line (ATCC, Manassas,
VA, USA) was added to a collagen solution to a final density of
1×105 cells/ml. Three drops (30 μl per drop) of
the collagen-cell mixture were placed in each well of a 6-well
plate on ice and allowed to gel at 37°C in a CO2
incubator. Each well was overlaid with PCM-2 medium (Kurabo
Industries Ltd.) after 1 h and incubated overnight. The plates were
then incubated with 5FU (final concentration, 200 μg/ml;
1,000 mg/5 l) and MMC (2 μg/ml; 10 mg/5 l) (Kyowa Hakko
Kogyo Co., Tokyo, Japan), as single agents or in combination, in 4
ml of saline solution for 30 min at 37 or 42°C. Following removal
of the saline solution containing the antitumor agents, each well
was rinsed twice with 3 ml of Hank’s balanced salt solution
(Nacalai Tesque, Inc., Kyoto, Japan), overlaid with 4 ml of PCM-2
medium and incubated for an additional 7 days. At the end of the
incubation period, neutral red was added to each well to a final
concentration of 50 μg/ml and the cancer cell colonies in
the collagen gel droplets were stained for 2 h. Each collagen
droplet was fixed with 10% neutral-buffered formalin, washed in
water, air-dried and quantified by image analysis. The cytotoxicity
(tumor reduction rate) was expressed as the T/C ratio percentage,
in which T was the image optical density of the treated group and C
was that of the control group.
Clinical evaluation
Subjects
This clinical study was performed at the Department
of Surgery, Shiga University of Medical Science (Shiga, Japan).
Patients who had received surgical intervention due to CRC between
August, 2009 and December, 2012 were evaluated and five patients
were found to be eligible and were enrolled in the study. The
toxicities were graded in accordance with the Common Terminology
Criteria for Adverse Events, version 4.0 (National Cancer
Institute; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf).
The toxicities observed during the first 30 days after surgery,
including hematological toxicity (anemia, neutropenia and
thrombocytopenia), organ dysfunction (renal and hepatic
dysfunction) and surgical complications, were recorded.
The inclusion criteria were as follows: i) patients
at high risk of developing PM, defined as either the presence of PM
from CRC, positive intraoperative peritoneal cytology, or stage
cT4b CRC according to the 7th edition of the American Joint
Committee on Cancer TNM staging system for CRC, or both; and ii)
patients with PM considered to be resectable. The exclusion
criteria were as follows: i) age <18 or >75 years; ii) World
Health Organization performance status of >2; iii) cardiac
dysfunction (New York Heart Association classification class >II
or left ventricular ejection fraction <60%); iv) renal
dysfunction (serum creatinine levels >1.5 mg/dl); v) hepatic
dysfunction (total bilirubin levels >1.5 mg/dl); vi) leukopenia
(white blood cell count <4,000/μl); vii) thrombocytopenia
(platelet count <100,000/μl); viii) anemia (hemoglobin
concentration <9.5 mg/dl); ix) history of severe disease, such
as central nervous system disease, arrhythmia, myocardial
infarction within 6 months or uncontrolled diabetes); x)
unresectable distant metastases; and xi) pregnancy.
This study was conducted in accordance with the
Helsinki Declaration and was approved by the Ethics Committee of
the Shiga University of Medical Science. Informed consent was
obtained from all the patients or their family members prior to
HIPEC.
Surgical procedure
All the patients underwent resection of the primary
lesions, recurrent lesions and PM (as appropriate) with
open-abdomen HIPEC under general anesthesia. Following complete
adhesiolysis, the diagnosis of PM was confirmed by frozen section
biopsy when possible and the extent of PM was scored according to
the Sugarbaker peritoneal cancer index (16). Macroscopically detectable disease
was completely resected prior to HIPEC.
The HIPEC protocol was performed according to our
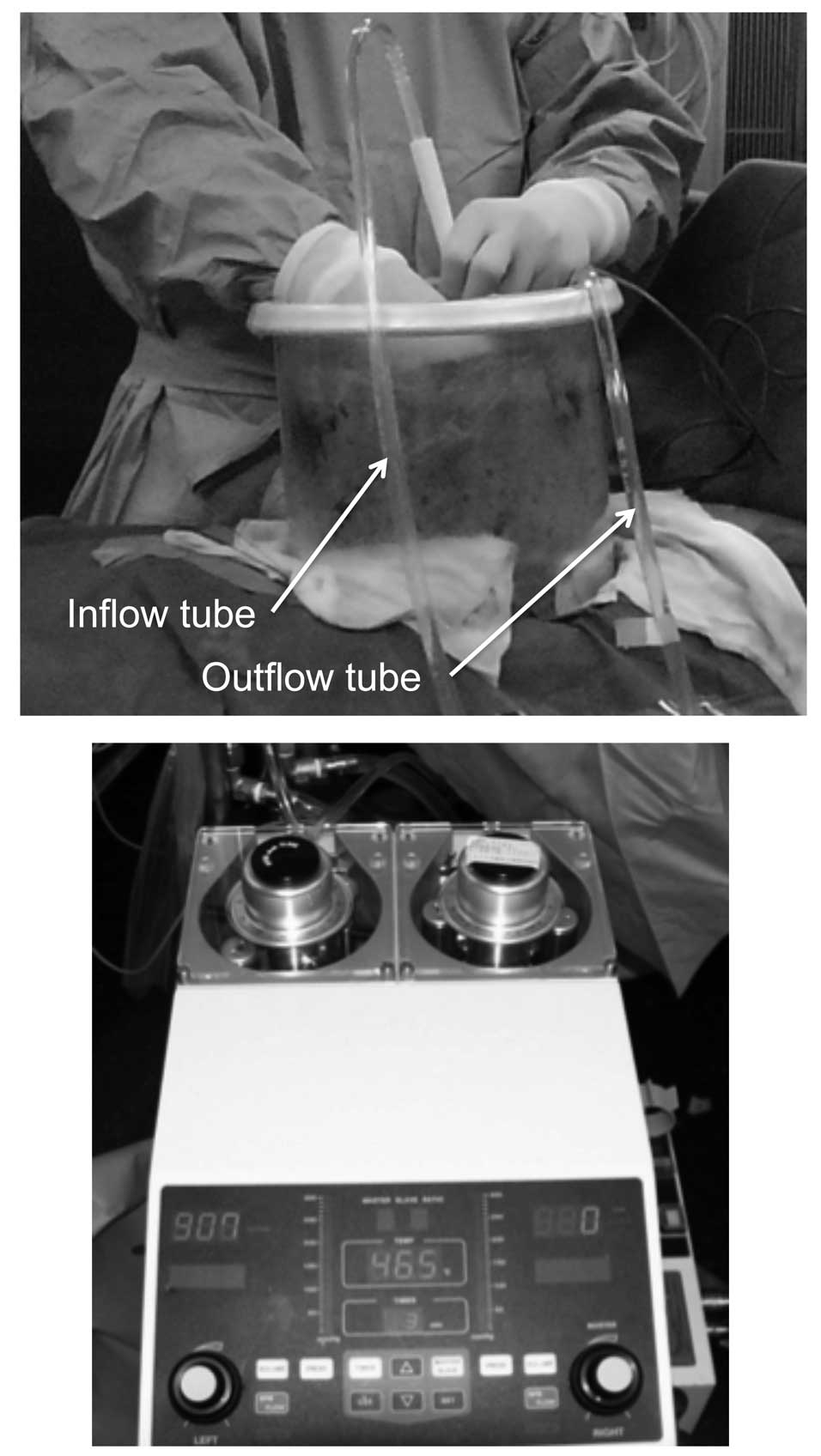
experience using an open sterile circuit. Briefly, the laparotomy
incision was closed, maintaining an 18-cm opening. An Arexis Wound
Retractor (Applied Medical Resource, Rancho Santa Margarita, CA,
USA) and plastic cylinder were used to create a water bath space
for HIPEC (Fig. 1A). Outflow and
inflow drain tubes were inserted in the rectouterine or
rectovesical pouch and in the plastic cylinder bath space,
respectively. Temperature probes were inserted into the abdominal
cavity behind the mesentery. A perfusate of 5 l saline solution
(Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) was
circulated at a flow rate of 500–750 ml/min using a CP-3000 system
(Tonokura Ika Kogyo Co. Ltd., Tokyo, Japan) (Fig. 1B). Once the temperature of the
perfusate reached 42–43°C, 10 mg MMC and 1,000 mg 5FU were added.
HIPEC was performed for 30 min after the addition of the antitumor
agents. The intra-abdominal perfusate and blood samples were
obtained to measure the concentrations of MMC and 5FU during and
after HIPEC.
Measurement of agent concentrations in
the perfusate and plasma
The concentrations of MMC and 5FU in the plasma and
peritoneal perfusate were determined using high-performance liquid
chromatography on an LC-6A system (Shimadzu Corp., Kyoto, Japan) as
previously described (17).
Follow-up after surgery and HIPEC
Following surgery, all the patients were transferred
to a general surgical ward for postoperative management. The
patients underwent blood sampling (complete blood count, renal
function tests, electrolyte levels and liver function tests)
following surgery. Radiological imaging was performed on the basis
of clinical and biochemical parameters when postoperative
complications were suspected. All the toxicities and postoperative
complications that occurred during the first 30 days after surgery
and HIPEC were recorded. The patients were monitored every 3 months
in an outpatient clinic, where a physical examination was performed
and tumor markers were assessed. Chest and abdominopelvic computed
tomographic scans were performed every 6 months. The patients
received systemic chemotherapy according to their recurrent
conditions.
Statistical analysis
The values were compared using the Wilcoxon and
Kruskal-Wallis rank order tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
In vitro simulation of HIPEC
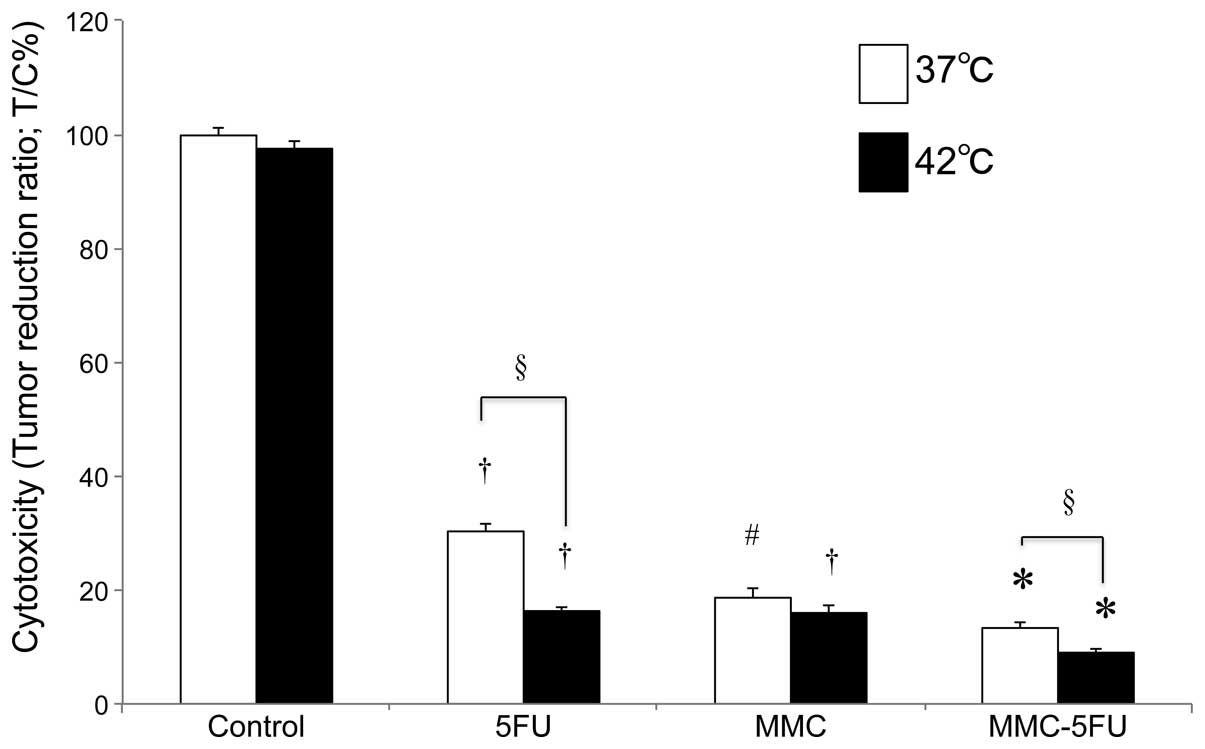
The combination treatment with 5FU and MMC at 37°C
exerted a significant suppressive effect on HCT116 cell
proliferation compared to the untreated control cells
(P<0.0001). Additionally, the combined use of MMC-5FU at 37 and
42°C suppressed tumor cell proliferation more effectively compared
to either agent used alone at 37 and 42°C. Compared to normothermia
(37°C), hyperthermia at 42°C significantly enhanced the sensitivity
of the tumor cells to these antitumor agents, regardless of whether
the drugs were used as single agents or in combination
(P<0.0001). Overall, MMC-5FU at 42°C exhibited the highest
cytotoxic activity (91.0% cytotoxity) against HCT116 cells in
vitro (Fig. 2).
Clinical evaluation
Patient characteristics
The characteristics of the five patients at a high
risk of developing colorectal PM who underwent surgical
intervention at our institution are shown in Table I. The study sample comprised three
men and two women (median age, 61 years; range, 47–72 years).
Patients 1 and 2 had developed peritoneal recurrence of CRC at 12
and 19 months following resection of their primary tumors,
respectively. Three patients had primary CRC. In patient 3, the
primary tumor had invaded the uterus and urinary bladder; PM was
suspected during surgery but could not be pathologically confirmed.
Patient 4 had positive intraoperative peritoneal cytology and in
the patient 5 PMs were detected in the Douglas pouch during surgery
(Table I).
 | Table I.Patient characteristics and
observations during and after surgery and hyperthermic
intraperitoneal chemotherapy (HIPEC). |
Table I.
Patient characteristics and
observations during and after surgery and hyperthermic
intraperitoneal chemotherapy (HIPEC).
| Variables | Patients |
|---|
|
|---|
| 1 | 2 | 3 | 4 | 5 |
|---|
| Age (years) | 69 | 60 | 73 | 60 | 47 |
| Gender | Male | Male | Female | Male | Female |
| Performance
statusa | 0 | 1 | 0 | 0 | 0 |
| Clinical
stageb | Peritoneal recurrence
(12 months after 1st surgery) | Peritoneal recurrence
(19 months after 1st surgery) | T4aN1aM0
Stage IIIB | T4aN0M0
Stage IIB | T3N2bM1b
Stage IVB |
| Histology
(adenocarcinoma) | Moderately
differentiated | Moderately
differentiated | Moderately
differentiated |
Well-differentiated | Moderately
differentiated |
| Invasion of other
organs | Rectum | Duodenum, right
kidney, small intestine | Uterus, bladder | None | None |
| PM | Present | Present | Suspected | Absent | Present |
| Peritoneal cancer
index | 2 | 2 | 0 | 0 | 1 |
| Intraoperative
cytology | Positive | Negative | Negative | Positive | Positive |
| Complications | Ileus (grade 3),
parotitis | Superficial surgical
site infection (grade 3) | None | None | None |
| Toxicity | Increased alanine
aminotransferase (grade 1) | Increased total
bilirubin and creatinine, decreased hemoglobin (grade 1) | Decreased hemoglobin
(grade 1) | None | None |
| Hospital stay
(days) | 22 | 11 | 15 | 17 | 9 |
| Observation
(months) | 44 | 40 | 38 | 35 | 16 |
| Recurrence after
HIPEC | Lung, liver (13
months) | Lung (7 months) | None | Liver (14
months) | Liver (3 months)
Ovary (12 months) |
| Treatment after
HIPEC | Chemotherapy | Chemotherapy | None | Hepatectomy | Chemotherapy; surgery
for ovarian metastasis |
| Second observation
after HIPEC | None | None | None | No evidence of PM;
cytology (−) | No evidence of PM;
cytology (−) |
Toxicity following HIPEC
One patient developed grade 3 post-surgical ileus
(Table I) and received
conservative treatment with long-tube drainage for 3 days; this
patient also developed parotitis. A single case of superficial
grade 3 surgical site infection was recorded in patient 2. Two
patients experienced a grade 1 decrease in the levels of hemoglobin
and three patients experienced a grade 1 increase in alanine
aminotransferase, total bilirubin and creatinine levels (Table I).
MMC and 5FU concentrations in the
perfusate and plasma
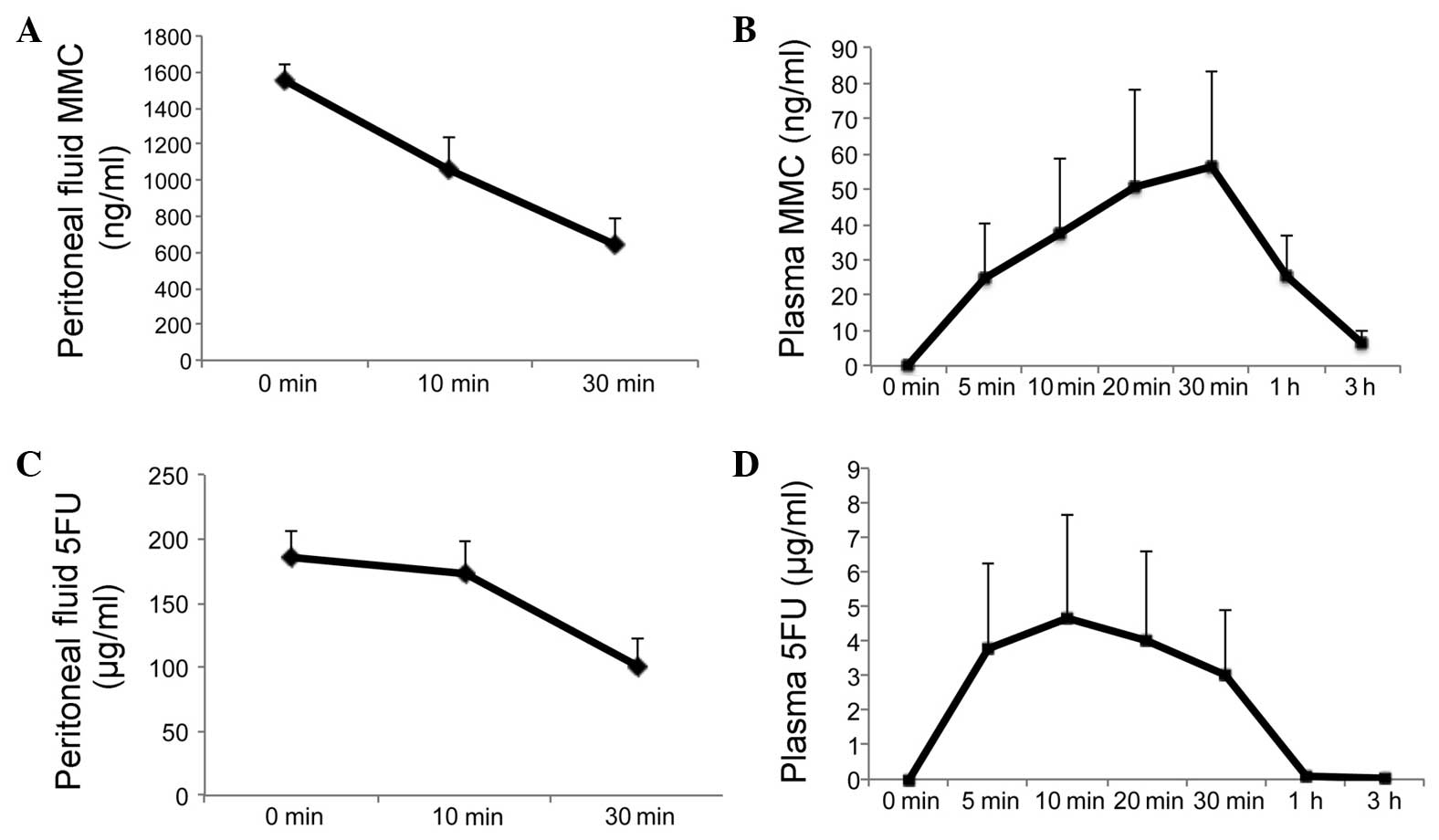
The area under the concentration-time curve (AUC) of
MMC was 563±78.2 ng•h/ml in the perfusate (Fig. 3A) and 71.9±35.2 ng•h/ml in the
plasma (Fig. 3B). The peak
concentration of plasma MMC was 56.2±27.4 ng/ml 30 min after the
initiation of HIPEC.
The AUC of 5FU was 82.4±9.9 μg•h/ml in the
perfusate (Fig. 3C) and 2.8±1.6
μg•h/ml in the plasma (Fig.
3D). The peak concentration of plasma 5FU was 4.7±2.9
μg/ml 10 min after the initiation of HIPEC.
Regarding the pharmacokinetics of intraperitoneal
MMC-5FU, the peritoneal exposure to MMC was 7.8-fold higher and to
5FU 29.4-fold higher compared to that measured in the plasma. The
plasma MMC and 5FU levels were ∼0 at 3 and 1 h after the initiation
of HIPEC, respectively (Fig.
3).
Patient outcomes
No patients were lost during the median
observational period of 38 months (range, 16–44 months). Three of
the five patients developed pulmonary and/or hepatic metastases.
Two patients (patients 1 and 2) received systemic chemotherapy
alone. Patient 4 underwent hepatectomy for hepatic metastasis 14
months after HIPEC and patient 5 received systemic chemotherapy for
hepatic metastasis and underwent surgical resection for ovarian
metastasis 12 months after HIPEC. The two patients who underwent a
second surgery exhibited no evidence of positive cytology or
recurrent masses suggestive of PM. In addition, none of the five
patients exhibited clinical signs of PM during the follow-up period
(Table I).
Discussion
In our in vitro simulation of HIPEC, MMC-5FU
exhibited at least 91.0% cytotoxicity against HCT116 cells. The
combination of MMC-5FU was found to be superior to treatment with
either agent alone regarding antitumor efficacy. In addition,
although the follow-up period was brief, our results suggested that
HIPEC with MMC-5FU is a safe and feasible therapeutic option for
patients at high risk of PM from CRC.
MMC is the most frequent component of
chemotherapeutic regimens administered during HIPEC for patients
with PC of colorectal origin in single-center phase II studies,
where MMC was administered as a single agent or in combination with
other drugs, at doses ranging from 2.5 to 120 mg/m2
(12). In this study, we set the
maximal dose of MMC at 10 mg/5 l in the perfusate, according to the
maximal intraperitoneal dose (10 mg overall) permitted by the
National Health Insurance guidelines in Japan.
5FU has been extensively used in perioperative
cancer chemotherapy for colorectal PC. Its marked activity in the
prevention of adhesion formation following major surgery (18) merits further investigation. In an
initial study by Sugarbaker and Jablonski (19), 5FU was administered as
intraperitoneal chemotherapy during surgery; however,
chemotherapeutic agents that are enhanced by heat and are non-cell
cycle-specific are pharmacologically preferable for HIPEC (20). 5FU, a thymidylate synthase
inhibitor, binds covalently to this enzyme and prevents the
formation of the DNA nucleoside precursor thymidine monophosphate
(21). Therefore, 5FU is currently
used in EPIC. Furthermore, 5-fluorouridine diphosphate and
5-fluorouridine triphosphate, which are metabolites of 5FU, exert
cytotoxic effects via their incorporation into the RNA. In the
present study, a tumor-suppressive effect was observed after a
brief incubation of a large dose of 5FU with the HCT116 cells,
which may reflect its cytotoxic effects due to incorporation into
the RNA.
5FU has been considered to be chemically
incompatible with other drugs in solutions for infusion or
instillation. Our study demonstrated that the combination of MMC
and 5FU exerted a 91.0% cytotoxic effect on HCT116 cells at 42°C in
an in vitro simulation of HIPEC, and this effect was more
prominent compared to that of 5FU as a single agent at 42°C.
Therefore, 5FU also retains its antitumor activity in the MMC-5FU
mixture. We set the maximal dose of 5FU to 1,000 mg/5 l in the
perfusate, according to the maximal intravenous bolus dose (10–20
mg/kg) permitted by the National Health Insurance guidelines in
Japan.
Various researchers reported the efficacy of
adjuvant HIPEC in the clinical setting in patients at high risk of
developing PC. Although the size of the patient sample was limited,
several randomized studies suggested that adjuvant HIPEC may
significantly improve survival and reduce the incidence rate of PC
in patients with gastric cancer and CRC (22–25).
A positive peritoneal lavage following macroscopic curative surgery
in patients with CRC appears to be associated with decreased
survival and increased recurrence of PM (7–9) and
HIPEC appears to be a suitable treatment for patients at high risk
of PM. Thus, in our preliminary clinical study, we enrolled
patients in whom complete resection of macroscopic disease was
achieved prior to HIPEC.
We acknowledge that there were several limitations
to the present study. Our results demonstrated that the combination
treatment with MMC-5FU was effective in an in vitro
simulation of HIPEC; however, the suppressive effects of this
combination in vivo have yet to be fully determined. In
addition, the dosage of antitumor agents, the perfusion technique
and the duration of the perfusion differ among different centers
that perform HIPEC. Additional studies are required to establish
the efficacy of HIPEC with MMC-5FU.
In conclusion, this study demonstrated that HIPEC
with MMC-5FU is feasible, safe and may prevent PM from CRC in
patients at high risk of this complication. These findings suggest
that HIPEC with MMC-5FU following cytoreductive surgery may be a
promising novel therapeutic option for such patients, which merits
further verification of its safety and efficacy in large-scale
clinical trials.
Acknowledgements
This study was supported by a
Grant-in-Aid for Scientific Research from the Japan Society for the
Promotion of Science (grant number 24591973).
References
|
1.
|
Stewart JH IV, Shen P and Levine EA:
Intraperitoneal hyperthermic chemotherapy for peritoneal surface
malignancy: current status and future directions. Ann Surg Oncol.
12:765–777. 2005. View Article : Google Scholar
|
|
2.
|
Chua TC, Esquivel J, Pelz JO and Morris
DL: Summary of current therapeutic options for peritoneal
metastases from colorectal cancer. J Surg Oncol. 107:566–573. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mohr Z, Hirche C, Liebeskind U, Rau B and
Hunerbein M: Feasibility of delayed hyperthermic intraperitoneal
chemotherapy in case of unforeseen complications. Eur Surg Res.
47:19–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Yonemura Y, Elnemr A, Endou Y, et al:
Multidisciplinary therapy for treatment of patients with peritoneal
carcinomatosis from gastric cancer. World J Gastrointest Oncol.
2:85–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Imano M, Imamoto H, Itoh T, et al: Impact
of intraperitoneal chemotherapy after gastrectomy with positive
cytological findings in peritoneal washings. Eur Surg Res.
47:254–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fujishima Y, Goi T, Kimura Y, Hirono Y,
Katayama K and Yamaguchi A: MUC2 protein expression status is
useful in assessing the effects of hyperthermic intraperitoneal
chemotherapy for peritoneal dissemination of colon cancer. Int J
Oncol. 40:960–964. 2012.
|
|
7.
|
Bosanquet DC, Harris DA, Evans MD and
Beynon J: Systematic review and meta-analysis of intraoperative
peritoneal lavage for colorectal cancer staging. Br J Surg.
100:853–862. 2013. View
Article : Google Scholar
|
|
8.
|
Mohan HM, O’Connor DB, O’Riordan JM and
Winter DC: Prognostic significance of detection of microscopic
peritoneal disease in colorectal cancer: a systematic review. Surg
Oncol. 22:e1–e6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Glockzin G, Rochon J, Arnold D, et al: A
prospective multicenter phase II study evaluating multimodality
treatment of patients with peritoneal carcinomatosis arising from
appendiceal and colorectal cancer: the COMBATAC trial. BMC Cancer.
13:672013. View Article : Google Scholar
|
|
10.
|
Koppe MJ, Boerman OC, Oyen WJ and
Bleichrodt RP: Peritoneal carcinomatosis of colorectal origin:
incidence and current treatment strategies. Ann Surg. 243:212–222.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Klaver YL, Hendriks T, Lomme RM, Rutten
HJ, Bleichrodt RP and de Hingh IH: Intraoperative versus early
postoperative intraperitoneal chemotherapy after cytoreduction for
colorectal peritoneal carcinomatosis: an experimental study. Ann
Surg Oncol. 19(Suppl 3): S475–S482. 2012. View Article : Google Scholar
|
|
12.
|
Weber T, Roitman M and Link KH: Current
status of cytoreductive surgery with hyperthermic intraperitoneal
chemotherapy in patients with peritoneal carcinomatosis from
colorectal cancer. Clin Colorectal Cancer. 11:167–176. 2012.
View Article : Google Scholar
|
|
13.
|
Kobayashi H, Tanisaka K, Doi O, et al: An
in vitro chemosensitivity test for solid human tumors using
collagen gel droplet embedded cultures. Int J Oncol. 11:449–455.
1997.
|
|
14.
|
Okumura K, Shiomi H, Mekata E, et al:
Correlation between chemosensitivity and mRNA expression level of
5-fluorouracil-related metabolic enzymes during liver metastasis of
colorectal cancer. Oncol Rep. 15:875–882. 2006.
|
|
15.
|
Muller M, Cherel M, Dupre PF, Gouard S,
Collet M and Classe JM: The cytotoxic effect of combined
hyperthermia and taxane chemotherapy on ovarian cancer cells:
results of an in vitro study. Eur Surg Res. 48:55–63. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sugarbaker PH: Intraperitoneal
chemotherapy and cytoreductive surgery for the prevention and
treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg
Oncol. 14:254–261. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kuzuya T, Yamauchi M, Ito A, Hasegawa M,
Hasegawa T and Nabeshima T: Pharmacokinetic characteristics of
5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J
Pharm Pharmacol. 46:685–689. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Pestieau SR, Marchettini P, Stuart OA,
Chang D and Sugarbaker PH: Prevention of intraperitoneal adhesions
by intraperitoneal lavage and intraperitoneal 5-fluorouracil:
experimental studies. Int Surg. 87:195–200. 2002.PubMed/NCBI
|
|
19.
|
Sugarbaker PH and Jablonski KA: Prognostic
features of 51 colorectal and 130 appendiceal cancer patients with
peritoneal carcinomatosis treated by cytoreductive surgery and
intraperitoneal chemotherapy. Ann Surg. 221:124–132. 1995.
View Article : Google Scholar
|
|
20.
|
Sugarbaker PH: Cytoreductive surgery plus
hyperthermic perioperative chemotherapy for selected patients with
peritoneal metastases from colorectal cancer: a new standard of
care or an experimental approach? Gastroenterol Res Pract.
2012:3094172012. View Article : Google Scholar
|
|
21.
|
Wyatt MD and Wilson DM III: Participation
of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci.
66:788–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
De Roover A, Detroz B, Detry O, et al:
Adjuvant hyperthermic intraperitoneal peroperative chemotherapy
(HIPEC) associated with curative surgery for locally advanced
gastric carcinoma. An initial experience. Acta Chir Belg.
106:297–301. 2006.
|
|
23.
|
Tentes AA, Spiliotis ID, Korakianitis OS,
Vaxevanidou A and Kyziridis D: Adjuvant perioperative
intraperitoneal chemotherapy in locally advanced colorectal
carcinoma: preliminary results. ISRN Surg. 2011:5298762011.
View Article : Google Scholar
|
|
24.
|
Raue W, Tsilimparis N, Bloch A, Menenakos
C and Hartmann J: Volume therapy and cardiocircular function during
hyperthermic intraperitoneal chemotherapy. Eur Surg Res.
43:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Mizumoto A, Canbay E, Hirano M, et al:
Morbidity and mortality outcomes of cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy at a single institution
in Japan. Gastroenterol Res Pract. 2012:8364252012. View Article : Google Scholar : PubMed/NCBI
|