Introduction
Lung cancer remains the leading cause of
cancer-related mortality worldwide (1). This disease is more common among the
elderly, with >2/3 of lung cancer cases occurring in individuals
aged ≥65 years and a median age at diagnosis of 70 years (2). The majority of lung cancer patients
present with unresectable disease and are candidates for thoracic
radiation therapy (TRT) and/or chemotherapy. Previously randomized
clinical trials revealed a survival benefit for concurrent vs.
sequential chemoradiotherapy (3,4) and
for sequential chemoradiotherapy vs. radiotherapy alone for
patients with stage III non-small-cell lung cancer (NSCLC)
(5,6).
Concurrent chemoradiotherapy with platinum-based
doublet chemotherapy is currently considered as the standard
treatment for patients with inoperable stage III NSCLC. However,
data from the Surveillance Epidemiology and End Results database
reveal that the majority of elderly patients do not receive
combined modality treatment (7).
This finding may reflect the uncertainty regarding concurrent
chemoradiotherapy as a treatment for elderly patients with locally
advanced NSCLC.
In elderly patients, the use of concurrent
chemoradiotherapy is often limited by poor functional status,
coexisting illnesses, limited life expectancy and the physicians’
concerns regarding toxicity and the effect of the treatment on the
quality of life (QOL) of the patients. In addition, the number of
available clinical trials designed to specifically evaluate the
treatment of elderly patients with stage III NSCLC is limited.
Therefore, it is crucial to establish an effective and feasible
chemoradiotherapy regimen for elderly patients with stage III
NSCLC.
The Japan Clinical Oncology Group 0301 trial
(JCOG0301) recently reported a significant survival advantage for
elderly patients who received chemoradiotherapy (daily low-dose
carboplatin plus radiotherapy) for locally advanced NSCLC (8). That trial provided reasonably strong
evidence that single-agent carboplatin-based chemoradiotherapy is
well-tolerated by elderly patients with locally advanced NSCLC and
may achieve improved survival rates compared to radiotherapy alone.
However, it has not been determined whether a carboplatin-based
doublet regimen with TRT is feasible for elderly patients with
locally advanced NSCLC.
The present study focused on the effectiveness of
weekly paclitaxel in combination with carboplatin and concurrent
TRT, since the efficacy and safety of this regimen in younger
patients with locally advanced NSCLC was confirmed by phase III
trials (9). The aim of our
retrospective analysis was to assess the anticancer effect and
toxicity of weekly paclitaxel and carboplatin with concurrent TRT
in patients aged ≥75 years with previously untreated locally
advanced NSCLC.
Patients and methods
Patients
This retrospective study was performed at the
Institute of Biomedical Research and Innovation and the Kobe City
Medical Center General Hospital, Hyogo, Japan. The data from 20
consecutive patients, aged ≥75 years, who were treated with weekly
paclitaxel and carboplatin for 6 weeks, plus TRT (60 Gy) for
locally advanced unresectable NSCLC (stage IIIA or IIIB) between
February, 2004 and July, 2013 were retrospectively evaluated. This
study was approved by the Institutional Review Board of the two
hospitals.
The diagnosis of locally advanced unresectable stage
III NSCLC was confirmed by a multidisciplinary council consisting
of radiologists, radiation oncologists and medical oncologists
prior to the initiation of the treatment. All the patients were
diagnosed with NSCLC and the diagnosis was histopathologically
confirmed. Tumour staging was performed by chest radiography,
computed tomography (CT) scan or magnetic resonance imaging of the
head, CT scan of the chest and the abdomen, CT scan or
ultrasonography of the abdomen and bone scintigraphy or
fluorodeoxyglucose positron emission tomography and CT scan. The
patients were staged according to the tumour-node-metastasis (TNM)
classification (10). An Eastern
Cooperative Oncology Group (ECOG) performance status (PS) of 0–2
was required for inclusion in this study (11).
Radiotherapy
Radiotherapy for all the patients consisted of 60 Gy
administered as 30 fractions over 6 weeks. A total of 40 Gy was
delivered with 6–10 MV photons using anterior-posterior opposed
fields that included the primary tumour, metastatic lymph nodes and
regional nodes. A booster dose of 20 Gy was delivered to the
primary tumour and the metastatic lymph nodes with off-cord fields,
for a total dose of 60 Gy. Three-dimensional CT simulation was used
for treatment planning. The clinical target volume included the
gross tumour volume, including the primary tumour and metastatic
nodes (>1 cm at the shortest dimension), plus a 0.5-cm margin.
The regional nodes, excluding the contra-lateral hilar nodes, were
also included in the clinical target volume. The planning target
volumes for the primary tumour, metastatic lymph nodes and regional
nodes were calculated as the clinical target volume plus adequate
margins (typically 0.5–1.0 cm laterally and 1.0–2.0 cm
craniocaudally).
The treatment plan was designed not to exceed the
maximum doses tolerated by intrathoracic structures, such as the
lung, spinal cord and heart. The spinal cord was excluded from the
boost field by the oblique opposing method. If the dose of the
spinal cord exceeded 40 Gy when planning the treatment, the minimum
dose of the planning target volumes was modified and the dose to
the spinal cord was restricted within 40 Gy. Patients were excluded
from this study if the initial radiation field exceeded half of the
ipsilateral lung. If ≥grade 3 oesophagitis occurred and the
physician decided that the RT could not be continued, the treatment
was suspended and reinitiated following recovery of the
oesophagitis to ≤grade 2.
Chemotherapy
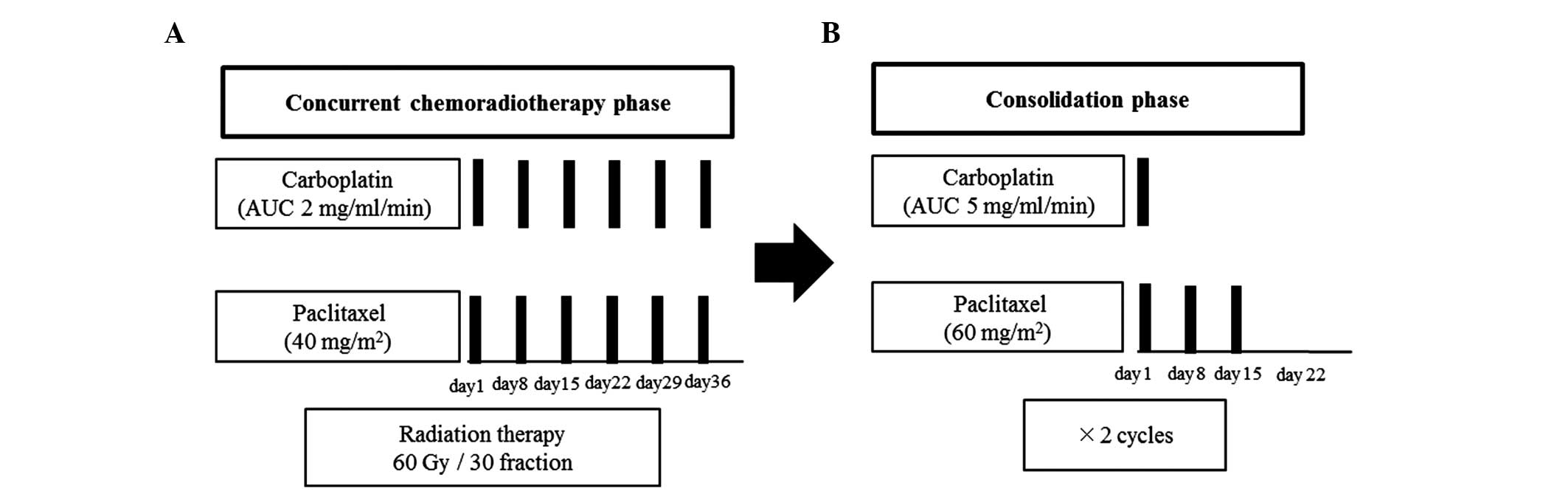
The treatment schedule is shown in Fig. 1. During radiotherapy, paclitaxel
(40 mg/m2) and carboplatin area under the curve (AUC) at
2 mg/ml/min were administered concomitantly on the first day of the
week. The consolidation phase chemotherapy, initiated 3–4 weeks
after the concurrent chemoradiotherapy, was administered in 2
cycles. The consolidation chemotherapy consisted of 3 weekly cycles
of paclitaxel (200 mg/m2 administered over 3 h) followed
by carboplatin (AUC, 5 mg/ml/min on day 1) or paclitaxel (70
mg/m2) weekly for 3 of 4 weeks with carboplatin (AUC, 5
mg/ml/min) on day 1 of each 4-week cycle. During radiation
treatment, paclitaxel and carboplatin administration was suspended
if grade 4 hematological toxicity occurred and chemotherapy was
reinitiated following recovery to ≤grade 3. A maximum of one dose
level reduction was permitted per patient in the consolidation
phase. The dose of carboplatin was reduced to achieve an AUC of 4
mg/ml/min and the dose of paclitaxel was reduced to 175
mg/m2, or the weekly paclitaxel dose was reduced to 60
mg/m2. Both paclitaxel and carboplatin were reduced by
one dose level if grade 4 hematological or ≥grade 3
non-hematological toxicity occurred and the physician decided that
chemotherapy should be discontinued.
Treatment and toxicity evaluation
The treatment efficacy and toxicity were assessed in
all the treated patients. The patients were assessed for response
by CT scans within 8 weeks of completing treatment. Following
treatment completion, chest radiographs were obtained monthly and
thoracic CT scans every 6 months. The patients underwent follow-up
monthly for 1 year and at least every 3 months thereafter.
The treatment response evaluation was performed
according to the Response Evaluation Criteria in Solid Tumours,
version 1.09 (12), based only on
the longest diameter of all the lesions as follows: Complete
response (CR), disappearance of all the lesions; partial response
(PR), ≥30% reduction of the sum of the longest diameters of all the
lesions, referring to the sum of baseline longest diameters;
progressive disease (PD), ≥20% increase in the sum of the longest
diameters of the target lesions, referring to the smallest sum of
the longest diameters recorded since the initiation of the
treatment or the appearance of one or more new lesions; stable
disease (SD), neither sufficient lesion shrinkage to qualify for
PR, nor sufficient lesion growth to qualify for PD, referring to
the smallest sum of the longest diameters since the initiation of
the treatment. The toxicity was evaluated in accordance with the
National Cancer Institute Common Toxicity Criteria 4.0.3 (13).
Statistical analysis
Median overall survival (OS) was defined as the time
from the initiation of the treatment to death from any cause or the
last follow-up. The patients who remained alive were evaluated at
the date of the last follow-up. Progression-free survival (PFS) was
defined as the time from the initiation of the treatment to disease
progression (local recurrence and/or distant metastasis) or death.
The respective contribution of local and distant progression to PFS
and the rate of implementation of chemotherapy were estimated. The
median follow-up time was calculated with the reverse Kaplan-Meier
method and the Kaplan-Meier method was used for survival analysis.
JMP software, version 9.0.0 (SAS Institute, Cary, NC, USA), was
used for statistical analysis.
Results
Patient characteristics
Between February, 2004 and July, 2013, the data of
20 patients who were treated with weekly paclitaxel and carboplatin
for 6 weeks plus 60 Gy TRT, were collected at the two
abovementioned institutions. The median follow-up for censored
cases was 21.1 months (interquartile range, 16.0–21.6). The
pretreatment characteristics of the patients are summarized in
Table I. The median age of the
patients was 78 years, 85% of the patients were men, 90% had a
history of smoking and 85% had an ECOG PS of 0–1. A total of 45% of
the patients had stage IIIA and the remaining 55% had stage IIIB
disease. The reported comorbidities, listed by decreasing
frequency, were hypertension (25%), diabetes (10%), cerebrovascular
disease (10%), ischemic heart disease (10%) and arrhythmia
(5%).
 | Table I.Patient characteristics (n=20). |
Table I.
Patient characteristics (n=20).
| Characteristics | Patients (%) |
|---|
| Median age, years
(range) | 78 (75–86) |
| Gender | |
| Male | 17 (85) |
| Female | 3 (15) |
| Histology | |
| Adenocarcinoma | 8 (40) |
| Squamous cell
carcinoma | 12 (60) |
| ECOG performance
status | |
| 0 | 2 (10) |
| 1 | 15 (75) |
| 2 | 3 (15) |
| Disease stage | |
| IIIA | 9 (45) |
| IIIB | 11 (55) |
| Tumour stage | |
| 1 | 2 (10) |
| 2 | 7 (35) |
| 3 | 4 (20) |
| 4 | 7 (35) |
| Nodal stage | |
| 1 | 2 (10) |
| 2 | 12 (60) |
| 3 | 6 (30) |
| Comorbidity | |
| Hypertension | 5 (25) |
| Diabetes | 2 (10) |
| Cerebrovascular
disease | 2 (10) |
| Arrhythmia | 1 (5) |
| Ischemic heart
disease | 2 (10) |
| Smoking history | |
| Negative | 2 (10) |
| Positive | 18 (90) |
Treatment
The compliance to the protocol was considered as
acceptable. The status of chemotherapy implementation is shown in
Table II. During the concurrent
phase, 60% of the patients received 6 weekly cycles of
chemotherapy. In the consolidation phase, 60% of the patients
received the two scheduled courses of therapy. All the patients
completed TRT with a total dose of 60 Gy.
 | Table II.Chemotherapy administered. |
Table II.
Chemotherapy administered.
| Chemotherapy | Cycles | Patients (%) |
|---|
| Concurrent | 3 | 1 (5) |
| 4 | 1 (5) |
| 5 | 6 (30) |
| 6 | 12 (60) |
| Consolidation | 0 | 6 (30) |
| 1 | 2 (10) |
| 2 | 12 (60) |
Efficacy
In total, 1 patient achieved a CR, 17 achieved a PR,
1 had SD and 1 had PD (Table III).
The objective response rate (ORR) was 90% and the disease control
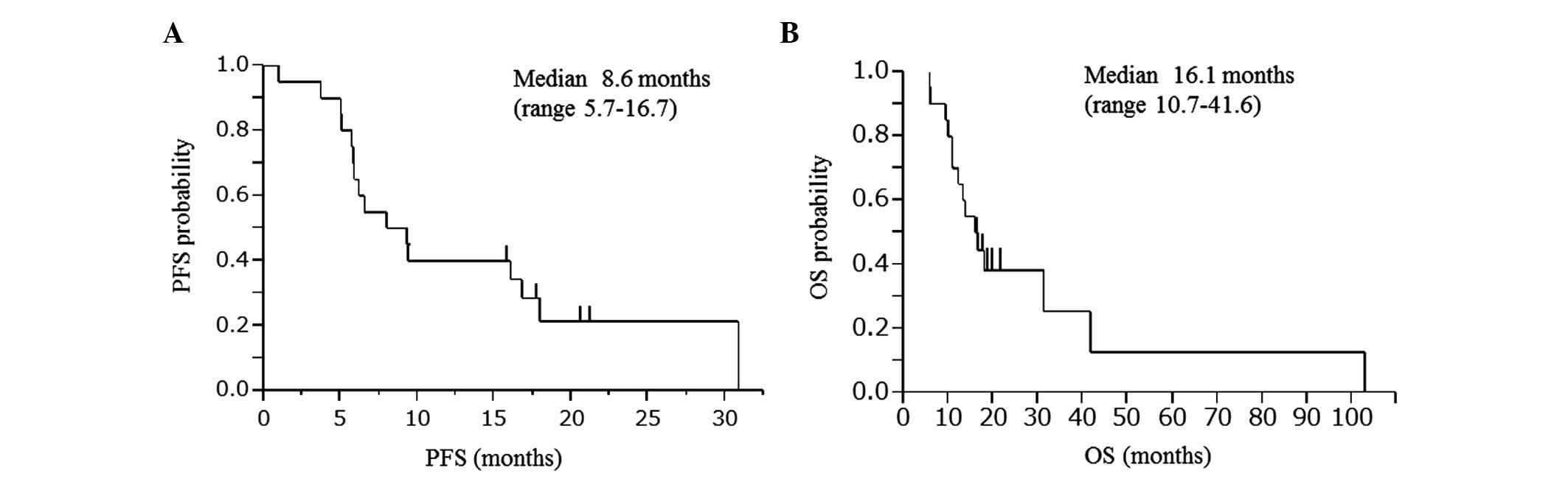
rate (DCR) was 95%. The PFS and OS of the patients who were
included in the trial are shown in Fig. 2. The median PFS was 8.6 months [95%
confidence interval (CI): 5.7–16.7] and the median OS was 16.1
months (95% CI: 10.7–41.6).
 | Table III.Objective response. |
Table III.
Objective response.
| Type of
response | Patients (%) |
|---|
| Complete | 1 (5) |
| Partial | 17 (85) |
| Stable disease | 1 (5) |
| Progressive
disease | 1 (5) |
| Objective response
rate | 18 (90) |
| Disease control
rate | 19 (95) |
Toxicity
The treatment-related adverse events are shown in
Table IV. There were no reported
grade 4 hematological and non-hematological toxicities. Grade 3
leukopenia occurred in 8 (40%) and grade 3 neutropenia in 4
patients (20%). Grade 2 pneumonitis occurred in 3 patients (15%) on
day 17 and at 3 and 7 months following treatment. All the cases
required steroid therapy (prednisolone 20–40 mg/day). Grade 2
oesophagitis occurred in 5 patients (25%). There were no reported
treatment-related deaths.
 | Table IV.Adverse events reported during the
entire course of the treatment. |
Table IV.
Adverse events reported during the
entire course of the treatment.
| Adverse event | Toxicity (%)
|
|---|
| Grade 2 | Grade 3 | Grade 4 |
|---|
| Leukopenia | 8 | 8 | 0 |
| Neutropenia | 7 | 4 | 0 |
| Anaemia | 1 | 0 | 0 |
|
Thrombocytopenia | 0 | 0 | 0 |
| Pneumonitis | 3 | 0 | 0 |
| Esophagitis | 5 | 0 | 0 |
| Pleural effusion
(non-malignant) | 0 | 0 | 0 |
| Sensory
neuropathy | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 |
| Rash | 1 | 0 | 0 |
First site of disease progression
A total of 3 patients (15%) exhibited local relapse,
9 (45%) had distant metastasis and 2 (10%) had both. Overall, 16
patients (80%) exhibited disease progression and 17 (85%) succumbed
to the disease during the analysis, with 14 deaths due to the
primary disease and 1 due to acute myocardial infarction at 379
days after treatment.
Post-treatment
A total of 8 patients (40%) received second-line
therapy (pemetrexed, 2; docetaxel, 1; gemcitabine, 1; vinorelbine,
1; S1, 1; gefitinib, 1; and erlotinib, 1). All the patients
received palliative care and palliative radiation therapy as
required.
Discussion
The present study retrospectively assessed the
anticancer effect and toxicity of weekly paclitaxel in combination
with carboplatin and concurrent TRT in previously untreated elderly
patients (aged ≥75 years) with locally advanced NSCLC. According to
our results, the ORR was 90% and the DCR was 95%, which were
improved compared to those reported by previous studies.
Furthermore, the median PFS was 8.6 months and the median OS was
16.1 months.
The PFS was similar to that reported by previous
studies (8,9). The OS was different, although it was
similar to that reported for radiation alone (16.9 months) in the
JCOG0301 trial (8).
Concurrent chemoradiotherapy is considered to be the
standard treatment for locally advanced NSCLC in selected patients
with a good PS (14). Despite the
high frequency of NSCLC in the elderly population, elderly patients
are frequently underrepresented in clinical trials evaluating
chemoradiotherapy (15,16). This is due to elderly patients
generally being incapable of tolerating the treatment-related
toxicity. In addition, the expectations for long-term benefits may
be limited on the part of physicians, as well as on the part of the
patients or their families. Therefore, the number of clinical
trials designed to specifically study the treatment of elderly
patients with stage III NSCLC is limited (17,18).
The results of age-based retrospective subgroup
analyses of randomized phase III trials that evaluated concurrent
chemoradiotherapy were previously reported by 5 studies (18–22).
Those studies reported that healthy older adults with locally
advanced NSCLC benefitted from concurrent chemoradiotherapy similar
to younger patients, but experienced higher rates of
hospitalization and toxicity. A previous meta-analysis reported
that the benefit of concomitant chemotherapy appeared to be greater
in elderly compared to that in younger patients (23).
In the JCOG0301 study, Atagi et al (8), reported that the median OS for the
chemoradiotherapy (TRT with daily low-dose carboplatin) and
radiation therapy alone groups were 22.4 and 16.9 months,
respectively. Chemoradiotherapy was associated with a significantly
longer survival compared to radiation therapy alone. That study was
the first to demonstrate that combined chemoradiotherapy may
improve the outcome of stage III NSCLC in elderly patients. In the
present study, weekly paclitaxel and carboplatin with concurrent
TRT did not provide a survival benefit when compared to the results
of the JCOG0310 study.
The West Japan Thoracic Oncology Group (WJTOG0105),
in a phase III trial, evaluated concurrent chemoradiotherapy using
second-generation regimens at full doses or third-generation
regimens at reduced doses in patients with locally advanced NSCLC
aged <75 years. The results of that trial demonstrated that
treatment with weekly paclitaxel and carboplatin with TRT was
equally efficacious and exhibited a more favorable toxicity profile
compared to the second-generation regimens (9). The ORR was 63.3%, the median PFS was
9.5 months and the median survival time and 5-year survival rate
were 22.0 months and 19.8%, respectively, with the weekly
paclitaxel and carboplatin treatment. In the WJTOG0105 study, the
status of chemotherapy implementation was reported as 58.5% of
patients who received 6 weekly cycles of chemotherapy during the
concurrent phase and 49.7% of patients who received the two
scheduled courses of therapy during the consolidation phase. The
incidence of grade 3 or worse hematological toxicity was leukopenia
in 66%, neutropenia in 61% and febrile neutropenia in 10.2% of the
patients. Grade 3 or worse pneumonitis occurred in 4.1% and
esophagitis in 8.2% of the patients in the weekly paclitaxel and
carboplatin group.
The present study revealed a lower incidence of
grade 3 or worse hematological toxicity, pneumonitis and
esophagitis compared to those reported by the WJTOG0105 study for
the weekly paclitaxel and carboplatin group. Although our study
exhibited similar chemotherapy implementation, ORR, PFS and milder
toxicity, it failed to demonstrate a survival benefit compared to
other studies (Table V),
indicating that our retrospective and small cohort group was not
sufficient to provide statistically valid results. Concurrent TRT
and chemotherapy with weekly paclitaxel and carboplatin is
potentially an effective and feasible treatment for elderly
patients; however, our retrospective cohort was insufficient to
demonstrate efficacy. Future larger and well-designed prospective
clinical trials are required to verify survival benefits.
 | Table V.Results of previous studies and the
present study. |
Table V.
Results of previous studies and the
present study.
| Studies | Patient age, years
median (range) | ORR (%) | PFS (months) | OS (months) | Grade 3 or worse
AEs (%) (neutropenia/pneumonitis/esophagitis) | Refs. |
|---|
| WJTOG0105 weekly
CBDCA+PAC arm | 63 (38–74) | 63.3 | 9.5 | 22.0 | 61.6/4.1/8.2 | (9) |
| JCOG0301
chemoradiotherapy arm | 77 (71–89) | 51.5 | 8.9 | 22.4 | 57.2/1.0/1.0 | (8) |
| Present study | 78 (75–86) | 90.0 | 8.6 | 16.1 | 20/0/0 | - |
The limitations of our study lie with its
retrospective nature, the small cohort and the lack of a QOL
assessment. The intervals between the evaluations of the lesions in
this study were not as accurate as those in a prospective study. In
addition, the severity of the adverse events may have been
underestimated in the present study due to its retrospective
nature. The patients were hospitalized during most of the treatment
period and the toxicity data were recorded in detail in the
patients’ medical records. The sample size in the present study was
limited; therefore, it was difficult to reach a definitive
conclusion. However, the collection of data on a large number of
patients with locally advanced NSCLC, aged ≥75 years, treated with
chemoradiotherapy, is difficult. This retrospective study may
therefore be useful for physicians to determine the optimal
treatment strategy for patients aged ≥75 years with locally
advanced NSCLC.
Previous studies demonstrated that QOL is an
important prognostic factor in patients with lung cancer (24–31).
One randomized phase III study reported QOL as a prognostic factor
for long-term survival among patients with locally advanced NSCLC
treated with chemoradiotherapy (32). However, in elderly NSCLC patients,
the adverse effects of chemoradiotherapy and their negative effect
on the QOL have not been determined. QOL assessment is required for
future clinical trials of chemoradiotherapy in elderly
patients.
In conclusion, weekly paclitaxel and carboplatin
with concurrent TRT failed to demonstrate a clinically significant
survival benefit in elderly patients with locally advanced NSCLC.
However, this regimen had a tolerable safety profile and an
improved objective response. Therefore, it is suggested that this
regimen may be suitable for elderly patients; however, further
prospective clinical trials are required to evaluate the true
efficacy and safety of weekly paclitaxel and carboplatin with
concurrent TRT for the treatment of elderly patients with
NSCLC.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2.
|
National Cancer Institute: SEER Stat fact
sheets: Lung and bronchus cancer. http://seer.cancer.gov/statfacts/html/lungb.html.
Accessed August 12, 2013.
|
|
3.
|
Furuse K, Fukuoka M, Kawahara M, et al:
Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with mitomycin, vindesine, and
cisplatin in unresectable stage III non-small-cell lung cancer. J
Clin Oncol. 17:2692–2699. 1999.PubMed/NCBI
|
|
4.
|
Curran WJ Jr, Paulus R, Langer CJ, et al:
Sequential vs. concurrent chemoradiation for stage III non-small
cell lung cancer: randomized phase III trial RTOG 9410. J Natl
Cancer Inst. 103:1452–1460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Dillman RO, Herndon J, Seagren SL, Eaton
WL Jr and Green MR: Improved survival in stage III non-small-cell
lung cancer: seven-year follow-up of cancer and leukemia group B
(CALGB) 8433 trial. J Natl Cancer Inst. 88:1210–1215.
1996.PubMed/NCBI
|
|
6.
|
Sause W, Kolesar P, Taylor S IV, et al:
Final results of phase III trial in regionally advanced
unresectable non-small cell lung cancer: Radiation Therapy Oncology
Group, Eastern Cooperative Oncology Group, and Southwest Oncology
Group. Chest. 117:358–364. 2000. View Article : Google Scholar
|
|
7.
|
Edelman MJ, Tang M, Gardner JF, Mullins
CD, Seal B and Davidoff AJ: Therapy (Tx) of locally advanced (LA)
NSCLC in the elderly: Analysis of 6,325 patients from surveillance,
epidemiology and end results (SEER)-Medicare. J Clin Oncol.
26(Suppl 15): 75492008.
|
|
8.
|
Atagi S, Kawahara M, Yokoyama A, et al
Japan Clinical Oncology Group Lung Cancer Study Group: Thoracic
radiotherapy with or without daily low-dose carboplatin in elderly
patients with non-small-cell lung cancer: a randomised, controlled,
phase 3 trial by the Japan Clinical Oncology Group (JCOG0301).
Lancet Oncol. 13:671–678. 2012. View Article : Google Scholar
|
|
9.
|
Yamamoto N, Nakagawa K, Nishimura Y, et
al: Phase III study comparing second- and third-generation regimens
with concurrent thoracic radiotherapy in patients with unresectable
stage III non-small-cell lung cancer: West Japan Thoracic Oncology
Group WJTOG0105. J Clin Oncol. 28:3739–3745. 2010. View Article : Google Scholar
|
|
10.
|
Goldstraw P, Crowley J, Chansky K, et al
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar
|
|
11.
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
13.
|
National Cancer Institute: Common Toxicity
Criteria (NCI-CTC) version 4.0.3. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Accessed August 13, 2013.
|
|
14.
|
National Comprehensive Cancer Network:
Clinical practice guidelines in oncology: non-small cell lung
cancer, NCCN guidelines version 2 (2013). http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
Accessed August 13, 2013.
|
|
15.
|
Hutchins LF, Unger JM, Crowley JJ, Coltman
CA Jr and Albain KS: Underrepresentation of patients 65 years of
age or older in cancer-treatment trials. N Engl J Med.
341:2061–2067. 1999.PubMed/NCBI
|
|
16.
|
De Ruysscher D, Botterweck A, Dirx M, et
al: Eligibility for concurrent chemotherapy and radiotherapy of
locally advanced lung cancer patients: a prospective,
population-based study. Ann Oncol. 20:98–102. 2009.PubMed/NCBI
|
|
17.
|
Socinski MA, Zhang C, Herndon JE II, et
al: Combined modality trials of the Cancer and Leukemia Group B in
stage III non-small-cell lung cancer: analysis of factors
influencing survival and toxicity. Ann Oncol. 15:1033–1041. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rocha Lima CM, Herndon JE II, Kosty M,
Clamon G and Green MR: Therapy choices among older patients with
lung carcinoma: an evaluation of two trials of the Cancer and
Leukemia Group B. Cancer. 94:181–187. 2002.PubMed/NCBI
|
|
19.
|
Langer CJ, Hsu C, Curran WJ, et al:
Elderly patients (pts) with locally advanced non-small cell lung
cancer (LA-NSCLC) benefit from combined modality therapy: Secondary
analysis of radiation therapy oncology group (RTOG) 94-10. In:
Proceedings of the Annual Meeting of the American Society for
Clinical Oncology 21 (abstract 1193); pp. 299a2002
|
|
20.
|
Schild SE, Stella PJ, Geyer SM, et al: The
outcome of combined-modality therapy for stage III non-small-cell
lung cancer in the elderly. J Clin Oncol. 21:3201–3206. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Jalal SI, Riggs HD, Melnyk A, et al:
Updated survival and outcomes for older adults with inoperable
stage III non-small-cell lung cancer treated with cisplatin,
etoposide, and concurrent chest radiation with or without
consolidation docetaxel: analysis of a phase III trial from the
Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol.
23:1730–1738. 2012.
|
|
22.
|
Sgroi MM, Neubauer M, Ansari R, et al: An
analysis of elderly patients (pts) treated on a phase III trial of
cisplatin (P) plus etoposide (E) with concurrent radiotherapy (CRT)
followed by docetaxel (D) vs observation (O) in pts with stage III
non small cell lung cancer (NSCLC). J Clin Oncol. 25(Suppl 18):
90372007.
|
|
23.
|
Aupérin A, Le Péchoux C, Pignon JP, et al:
Meta-Analysis of Cisplatin/carboplatin based Concomitant
Chemotherapy in non-small cell Lung Cancer (MAC3-LC) Group:
Concomitant radio-chemotherapy based on platin compounds in
patients with locally advanced non-small cell lung cancer (NSCLC):
a meta-analysis of individual data from 1764 patients. Ann Oncol.
17:473–483. 2006.
|
|
24.
|
Montazeri A, Milroy R, Hole D, McEwen J
and Gillis CR: Quality of life in lung cancer patients: as an
important prognostic factor. Lung Cancer. 31:233–240. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Langendijk H, Aaronson NK, de Jong JM, ten
Velde GP, Muller MJ and Wouters M: The prognostic impact of quality
of life assessed with the EORTC QLQ-C30 in inoperable non-small
cell lung carcinoma treated with radiotherapy. Radiother Oncol.
55:19–25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Efficace F, Bottomley A, Smit EF, et al:
Is a patient’s self-reported health-related quality of life a
prognostic factor for survival in non-small-cell lung cancer
patients? A multivariate analysis of prognostic factors of EORTC
study 08975. Ann Oncol. 17:1698–1704. 2006.
|
|
27.
|
Maione P, Perrone F, Gallo C, et al:
Pretreatment quality of life and functional status assessment
significantly predict survival of elderly patients with advanced
non-small-cell lung cancer receiving chemotherapy: a prognostic
analysis of the multi-center Italian lung cancer in the elderly
study. J Clin Oncol. 23:6865–6872. 2005.
|
|
28.
|
Herndon JE II, Fleishman S, Kornblith AB,
Kosty M, Green MR and Holland J: Is quality of life predictive of
the survival of patients with advanced nonsmall cell lung
carcinoma? Cancer. 85:333–340. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Brown J, Thorpe H, Napp V, et al:
Assessment of quality of life in the supportive care setting of the
big lung trial in non-small-cell lung cancer. J Clin Oncol.
23:7417–7427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ganz PA, Lee JJ and Siau J: Quality of
life assessment. An independent prognostic variable for survival in
lung cancer. Cancer. 67:3131–3135. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Eton DT, Fairclough DL, Cella D, Yount SE,
Bonomi P and Johnson DH; Eastern Cooperative Oncology Group: Early
change in patient-reported health during lung cancer chemotherapy
predicts clinical outcomes beyond those predicted by baseline
report: results from Eastern Cooperative Oncology Group Study 5592.
J Clin Oncol. 21:1536–1543. 2003. View Article : Google Scholar
|
|
32.
|
Movsas B, Scott C, Langer C, et al:
Randomized trial of amifostine in locally advanced non-small-cell
lung cancer patients receiving chemotherapy and hyperfractionated
radiation: Radiation Therapy Oncology Group Trial 98-01. J Clin
Oncol. 23:2145–2154. 2005. View Article : Google Scholar
|