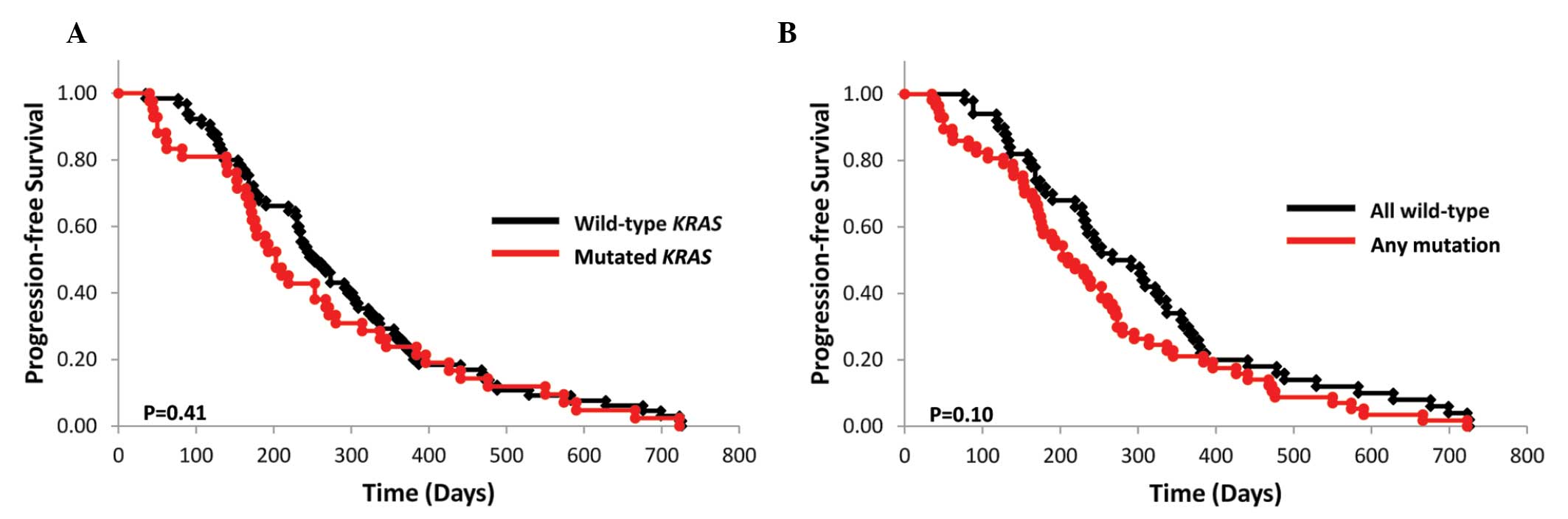
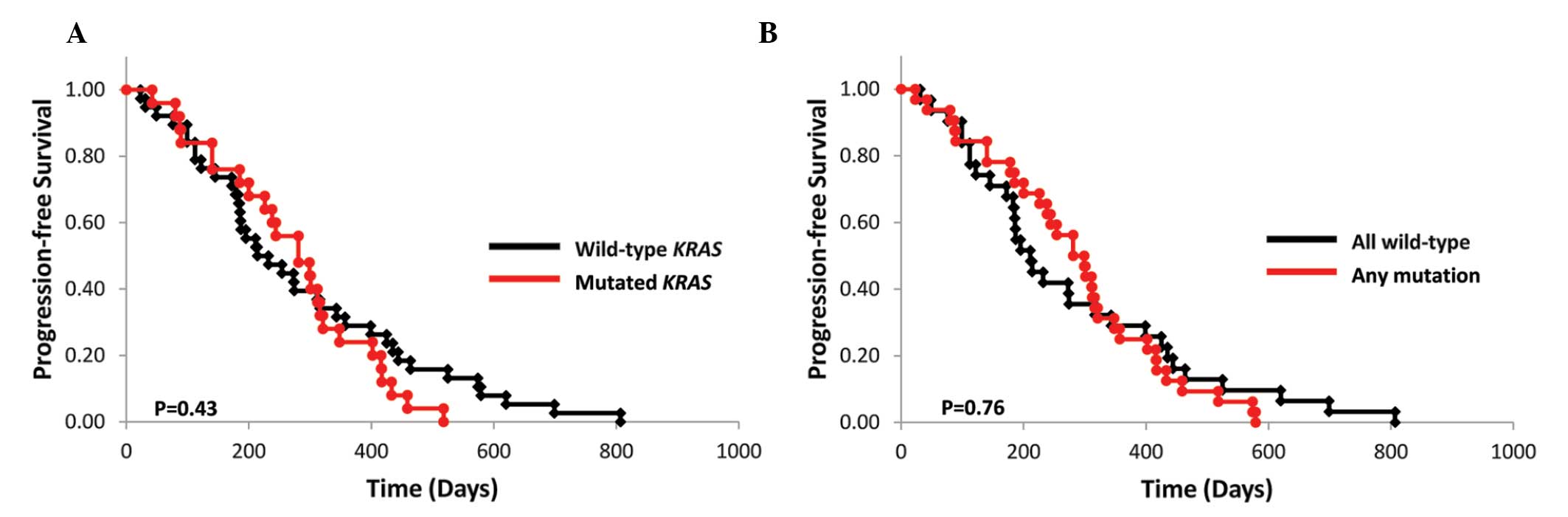
|
1.
|
Van Cutsem E, Kohne CH, Hitre E, et al:
Cetuximab and chemo-therapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009.PubMed/NCBI
|
|
2.
|
Bokemeyer C, Bondarenko I, Makhson A, et
al: Fluorouracil, leucovorin, and oxaliplatin with and without
cetuximab in the first-line treatment of metastatic colorectal
cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Cunningham D, Humblet Y, Siena S, et al:
Cetuximab mono-therapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bos JL, Fearon ER, Hamilton SR, et al:
Prevalence of ras gene mutations in human colorectal cancers.
Nature. 327:293–297. 1987. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Benvenuti S, Sartore-Bianchi A, Di
Nicolantonio F, et al: Oncogenic activation of the RAS/RAF
signaling pathway impairs the response of metastatic colorectal
cancers to anti-epidermal growth factor receptor antibody
therapies. Cancer Res. 67:2643–2648. 2007. View Article : Google Scholar
|
|
7.
|
Di Fiore F, Blanchard F, Charbonnier F, et
al: Clinical relevance of KRAS mutation detection in metastatic
colorectal cancer treated by cetuximab plus chemotherapy. Br J
Cancer. 96:1166–1169. 2007.PubMed/NCBI
|
|
8.
|
Amado RG, Wolf M, Peeters M, et al:
Wild-type KRAS is required for panitumumab efficacy in patients
with metastatic colorectal cancer. J Clin Oncol. 26:1626–1634.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hecht JR, Mitchell E, Chidiac T, et al: A
randomized phase IIIB trial of chemotherapy, bevacizumab, and
panitumumab compared with chemotherapy and bevacizumab alone for
metastatic colorectal cancer. J Clin Oncol. 27:672–680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Van Cutsem E, Lang I, D’haens G, et al:
KRAS status and efficacy in the first-line treatment of patients
with metastatic colorectal cancer (mCRC) treated with FOLFIRI with
or without cetuximab: the CRYSTAL experience. J Clin Oncol.
26:abstract 2. 2008.PubMed/NCBI
|
|
11.
|
Punt CJ, Tol J, Rodenburg CJ, et al:
Randomized phase III study of capecitabine, oxaliplatin, and
bevacizumab with or without cetuximab in advanced colorectal cancer
(ACC), the CAIRO2 study of the Dutch Colorectal Cancer Group
(DCCG). J Clin Oncol. 26:abstract LBA4011. 2008.PubMed/NCBI
|
|
12.
|
Bokemeyer C, Bondarenko I, Hartmann JT, et
al: KRAS status and efficacy of first-line treatment of patients
with metastatic colorectal cancer (mCRC) with FOLFOX with or
without cetuximab: the OPUS experience. J Clin Oncol. 26:abstract
4000. 2008.
|
|
13.
|
Karapetis CS, Khambata-Ford S, Jonker DJ,
et al: K-ras mutations and benefit from cetuximab in advanced
colorectal cancer. N Engl J Med. 359:1757–1765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Di Nicolantonio F, Martini M, Molinari F,
et al: Wild-type BRAF is required for response to panitumumab or
cetuximab in meta-static colorectal cancer. J Clin Oncol.
26:5705–5712. 2008.
|
|
15.
|
Tol J, Nagtegaal ID and Punt CJ: BRAF
mutation in metastatic colorectal cancer. N Engl J Med. 361:98–99.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
De Roock W, Claes B, Bernasconi D, et al:
Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy
of cetuximab plus chemotherapy in chemotherapy-refractory
meta-static colorectal cancer: a retrospective consortium analysis.
Lancet Oncol. 11:753–762. 2010.PubMed/NCBI
|
|
17.
|
Watanabe T, Yoshino T, Uetake H, et al:
KRAS mutational status in Japanese patients with colorectal cancer:
results from a nationwide, multicenter, cross-sectional study. Jpn
J Clin Oncol. 43(7): 706–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Andreyev HJ, Norman AR, Cunningham D, et
al: Kirsten ras mutations in patients with colorectal cancer: the
multicenter ‘RASCAL’ study. J Natl Cancer Inst. 90:675–684.
1998.
|
|
19.
|
Andreyev HJ, Norman AR, Cunningham D, et
al: Kirsten ras mutations in patients with colorectal cancer: the
‘RASCAL II’ study. Br J Cancer. 85:692–696. 2001.
|
|
20.
|
Barault L, Veyrie N, Jooste V, et al:
Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH
kinase) signaling network correlate with poor survival in a
population-based series of colon cancers. Int J Cancer.
122:2255–2259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ogino S, Nosho K, Kirkner GJ, et al:
PIK3CA mutation is associated with poor prognosis among patients
with curatively resected colon cancer. J Clin Oncol. 27:1477–1484.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Carpten JD, Faber AL, Horn C, et al: A
transforming mutation in the pleckstrin homology domain of AKT1 in
cancer. Nature. 448:439–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Sartore-Bianchi A, Martini M, Molinari F,
et al: PIK3CA mutations in colorectal cancer are associated with
clinical resistance to EGFR-targeted monoclonal antibodies. Cancer
Research. 69:1851–1857. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ince WL, Jubb AM, Holden SN, et al:
Association of k-ras, b-raf, and p53 status with the treatment
effect of bevacizumab. J Natl Cancer Inst. 97:981–989. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hurwitz HI, Yi J, Ince W, et al: The
clinical benefit of bevacizumab in metastatic colorectal cancer is
independent of K-ras mutation status: analysis of a phase III study
of bevacizumab with chemotherapy in previously untreated metastatic
colorectal cancer. Oncologist. 14:22–28. 2009. View Article : Google Scholar
|
|
26.
|
Richman SD, Seymour MT, Chambers P, et al:
KRAS and BRAF mutations in advanced colorectal cancer are
associated with poor prognosis but do not preclude benefit from
oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin
Oncol. 27:5931–5937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Souglakos J, Philips J, Wang R, et al:
Prognostic and predictive value of common mutations for treatment
response and survival in patients with metastatic colorectal
cancer. Br J Cancer. 101:465–472. 2009. View Article : Google Scholar : PubMed/NCBI
|