Introduction
It was reported that in 2013, lung cancer was
expected to account for 26% of all female and 28% of all male
cancer-related deaths in the USA (1). Approximately 85% of lung cancers are
non-small-cell lung cancer (NSCLC) and the majority of patients are
diagnosed at an advanced stage. The standard first-line
platinum-based chemotherapy has achieved modest improvements in
overall survival (2). Pemetrexed
is an antifolate antineoplastic agent that exerts its effects by
disrupting folate-dependent metabolism. Continued chemotherapeutic
treatment prior to disease progression with single-agent pemetrexed
was found to improve progression-free and overall survival in
advanced lung cancer (3,4). Crizotinib was approved in 2011 by the
Food and Drug Administration for the treatment of anaplastic
lymphoma kinase (ALK)-rearranged NSCLC. Crizotinib is an orally
active small-molecule inhibitor of ALK and the c-Met receptor
tyrosine kinase and belongs to the family of
3-benzyloxy-2-aminopyridines. Recently, a phase 3 clinical trial
indicated that, compared to chemotherapy, crizotinib prolonged
progression-free survival, increased response rates and improved
the quality of life in patients with advanced, previously treated
ALK-positive NSCLC (5,6).
In this study, we present the case report of a
patient with NSCLC harboring echinoderm microtubule-associated
protein-like 4 (EML4)-ALK rearrangement. The patient, following
discontinuation of second-line therapy, experienced slowly
progressive disease within 4 years of maintenance pemetrexed and
later exhibited a favorable response to crizotinib. This case
indicates that selected patients may continue to benefit from
pemetrexed after disease progression.
Case report
In May 2007, a 63-year-old man presented with a
mildly productive cough. The patient had been initially treated for
pneumonia in a community hospital; however, as the symptoms did not
improve despite treatment, the patient underwent a chest computed
tomography (CT) scan in the Sichuan Provincial Hospital, which
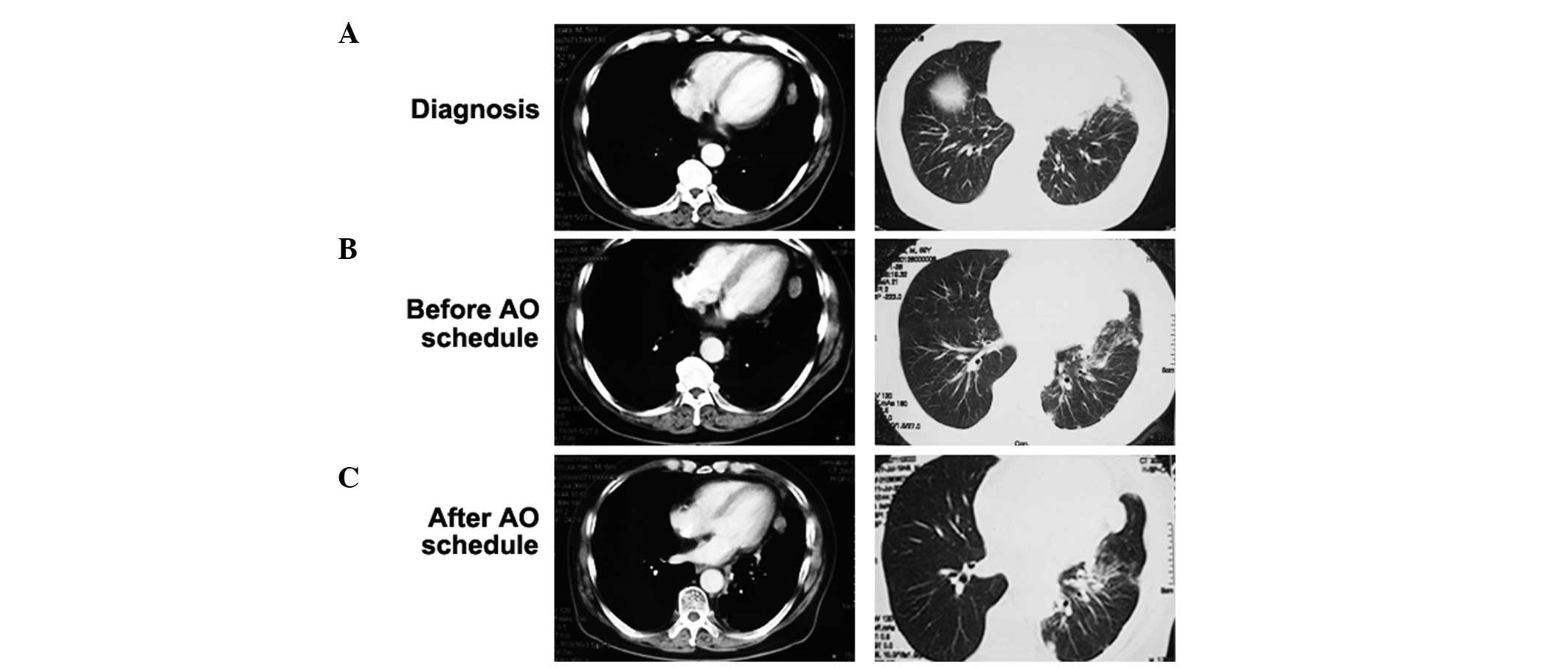
revealed multiple nodes in the left lower pulmonary lobe and left
pleura (Fig. 1A). Biopsy of the
pleural nodes was performed by thoracoscopy and the histological
examination confirmed the diagnosis of mucinous adenocarcinoma.
Immunohistochemical staining was positive for thyroid transcription
factor-1, epithelial membrane antigen and lymphocyte-specific
protein tyrosine kinase. The patient had an Eastern Cooperative
Oncology Group performance status of 1 and received a gemcitabine
and cisplatin regimen for 2 cycles. However, treatment was
discontinued due to persistent gastrointestinal upset. The patient
then received erlotinib orally for 6 months (150 mg daily) and the
tumor response according to the Response Evaluation Criteria in
Solid Tumors (RECIST) guidelines was stable disease. However, the
treatment was discontinued due to erlotinib-associated pulmonary
toxicity (interstitial pneumonia).
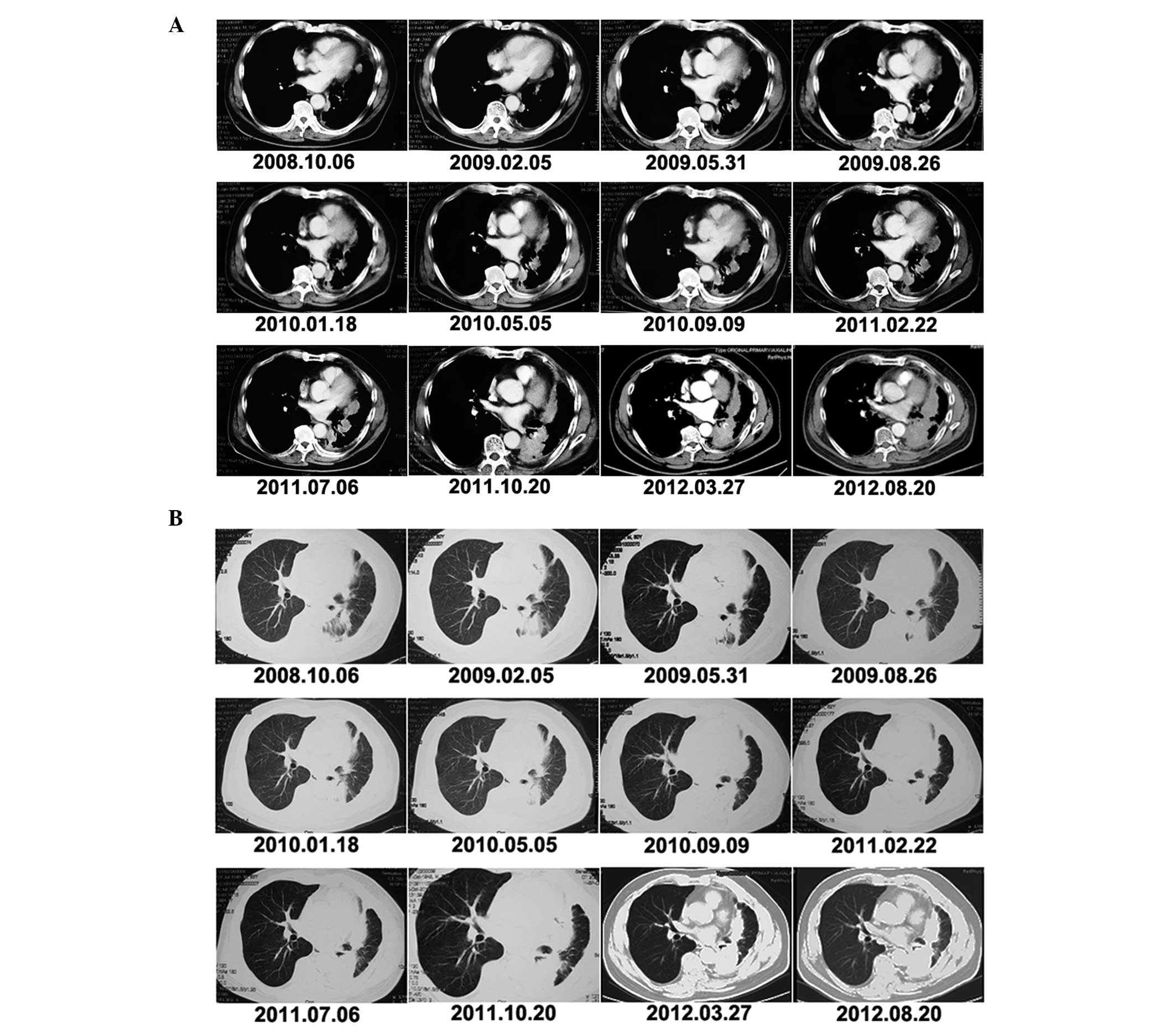
In May, 2008, the patient was initiated on 4 cycles
of pemetrexed and oxaliplatin chemotherapy (Fig. 1B and C), followed by maintenance
pemetrexed (900 mg per cycle). Imaging for response and progression
was performed every 2 cycles, with the baseline scans performed
after the completion of induction treatment. In May, 2010, after 13
cycles of maintenance pemetrexed, the tumor response was disease
progression without any associated symptoms. After consulting with
the patient, we continued the administration of pemetrexed and
monitored the patient for the development of obvious clinical
symptoms or a rapid acceleration of disease progression over a
short period of time. The total number of maintenance cycles
delivered was 24 (Fig. 2). The
patient suffered from grade 1 fatigue, skin pigmentation and
periodic fever and requested an increase in the treatment interval.
In August, 2012, the patient presented with severe cough and
dyspnea following physical activity and the CT images revealed left
pleural thickening and an enhancing mass in left lower pulmonary
lobe. Rebiopsy was performed with a bronchofiberscope and the
histological examination confirmed the diagnosis of adenocarcinoma.
The molecular analysis revealed wild-type epidermal growth factor
receptor (EGFR) gene and absence of K-ras mutation. However,
fluorescence in situ hybridization revealed a translocation
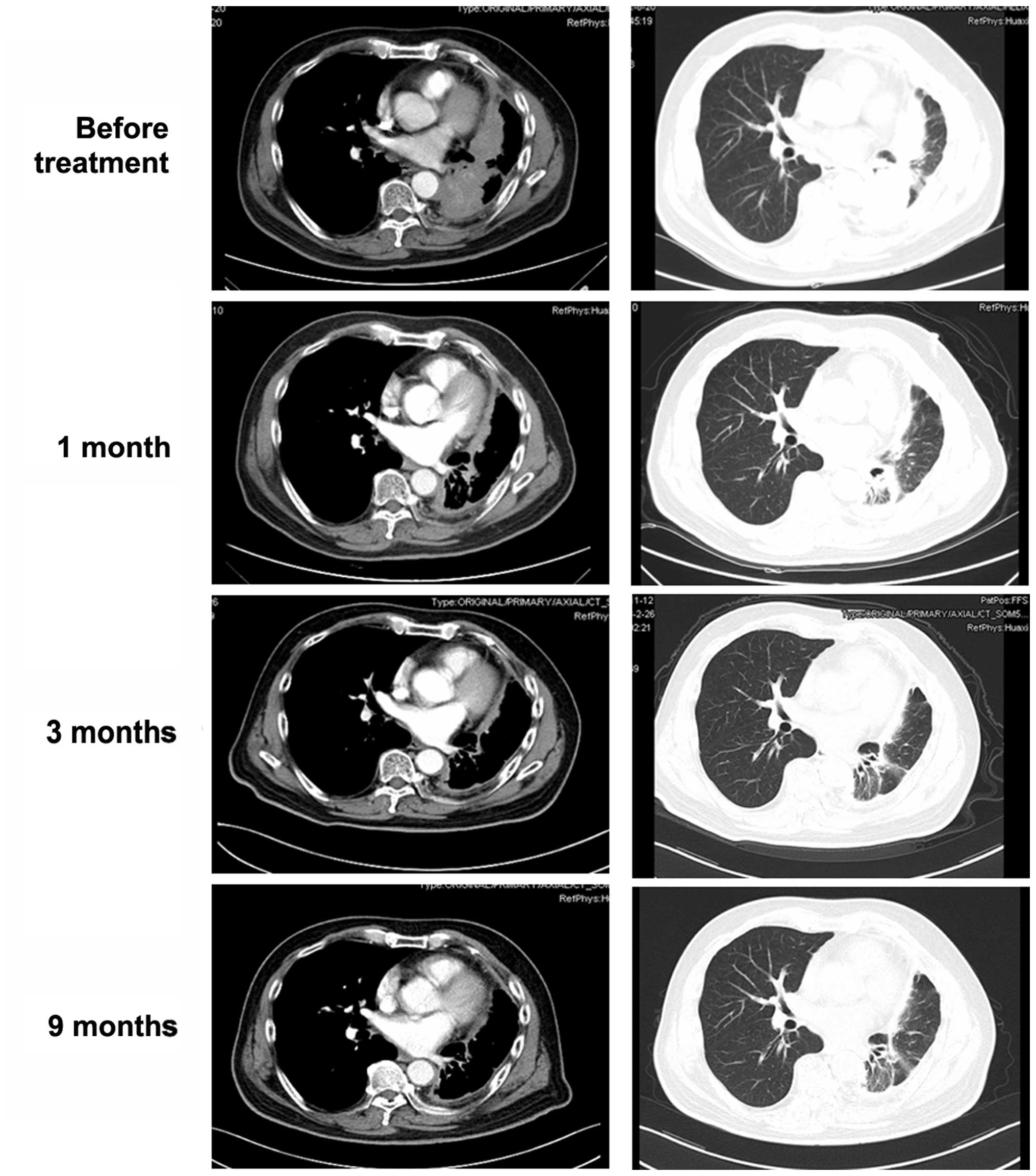
of ALK. In November, 2012, oral crizotinib (250 mg twice per day)
was initiated. Transient mild diarrhea and visual disturbances were
observed for the first 2 weeks, but were well controlled in the
subsequent course of treatment. After 1 month, a chest CT revealed
a significant decrease in the maximum aggregate tumor measurement
(Fig. 3). The tumor response
according to the RECIST guidelines was partial response. At 52
weeks after the initiation of crizotinib, the patient remained
alive and is in confirmed partial response.
Discussion
Continuation maintenance with pemetrexed is an
effective and well tolerated treatment choice for patients with
responsive or stable disease after first-line systematic therapy
for advanced non-squamous NSCLC (3,4). In
this case, first- and second-line therapy failed, mainly due to the
side effects; subsequently, the patient entered an induction phase
which consisted of 4 cycles of induction pemetrexed plus
oxaliplatin. The patient did not exhibit disease progression
following completion of the 4 cycles of induction and received
maintenance therapy with pemetrexed (900 mg per cycle). According
to the RECIST guidelines, the maintenance therapy should be
discontinued after 13 cycles of maintenance pemetrexed. As an
optimal strategy has not been established for patients receiving
third-line therapy and the patient had a good quality of life and
exhibited a relatively slow progression on CT imaging, we continued
the maintenance therapy, after consulting with the patient, until
the development of obvious symptoms. Surprisingly, the patient
completed 24 cycles of maintenance pemetrexed.
It was previously reported that ALK-positive NSCLC
patients achieved a prolongation of progression-free survival on
pemetrexed (7). A previous study
reported that an ALK-positive patient received 4 cycles of
chemotherapy with pemetrexed and carboplatin followed by
maintenance pemetrexed therapy for 19.3 months until disease
progression (8). The underlying
mechanism has not been clearly determined, but it was recently
demonstrated that certain enzymes, which catalyze purine
biosynthesis, are increased by ALK-mediated phosphorylation.
Therefore, ALK-positive tumors may be more susceptible to
antifolate agents (9).
Approximately 5% of the malignant transformation in
NSCLC is caused by EML4-ALK rearrangement. Tumor cells with
EML4-ALK rearrangement are dependent on its function, similar to
tumor cells harboring EGFR mutations. Previous studies reported
that crizotinib prolonged progression-free survival, increased
response rates and improved the quality of life in patients with
advanced ALK-positive NSCLC (10,11).
In this case, following a rapid acceleration of disease
progression, oral crizotinib (250 mg twice per day) was initiated.
The tumor response was remarkable and rapid and the chest CT
revealed a significant decrease in the maximum aggregate tumor
measurement within 1 month. The patient experienced transient mild
diarrhea and visual disturbances, which were well controlled in the
subsequent course of treatment.
Resistance to crizotinib has also been reported. An
alteration at C1156 of ALK may allosterically interfere with the
binding of tyrosine kinase inhibitors (12). The patient’s disease status will be
closely followed and further investigations should focus on
different treatment strategies using second-generation ALK
inhibitors.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Govindan R, Page N, Morgensztern D, et al:
Changing epidemiology of small-cell lung cancer in the United
States over the last 30 years: analysis of the surveillance,
epidemiologic, and end results database. J Clin Oncol.
24:4539–4544. 2006.PubMed/NCBI
|
|
3
|
Ciuleanu T, Brodowicz T, Zielinski C, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small-cell lung cancer: a
randomised, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paz-Ares L, de Marinis F, Dediu M, et al:
Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255. 2012.
|
|
5
|
Shaw AT, Kim DW, Nakagawa K, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SJ, Kim DW, Kim TM, et al: Remarkable
tumor response to crizotinib in a 14-year-old girl with
ALK-positive non-small-cell lung cancer. J Clin Oncol. 3:e147–e150.
2012.PubMed/NCBI
|
|
7
|
Camidge DR, Kono SA, Lu X, et al:
Anaplastic lymphoma kinase gene rearrangements in non-small cell
lung cancer are associated with prolonged progression-free survival
on pemetrexed. J Thorac Oncol. 6:774–780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeda M, Okamoto I, Sakai K, et al:
Clinical outcome for EML4-ALK-positive patients with advanced
non-small-cell lung cancer treated with first-line platinum-based
chemotherapy. Ann Oncol. 23:2931–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boccalatte FE, Voena C, Riganti C, et al:
The enzymatic activity of 5-aminoimidazole-4-carboxamide
ribonucleotide formyltransferase/IMP cyclohydrolase is enhanced by
NPM-ALK: new insights in ALK-mediated pathogenesis and the
treatment of ALCL. Blood. 113:2776–2790. 2009. View Article : Google Scholar
|
|
10
|
Kijima T, Takeuchi K, Tetsumoto S, et al:
Favorable response to crizotinib in three patients with echinoderm
microtubule-associated protein-like 4-anaplastic lymphoma kinase
fusion-type oncogene-positive non-small cell lung cancer. Cancer
Sci. 102:1602–1604. 2011. View Article : Google Scholar
|
|
11
|
Shaw AT, Yeap BY, Solomon BJ, et al:
Effect of crizotinib on overall survival in patients with advanced
non-small-cell lung cancer harbouring ALK gene rearrangement: a
retrospective analysis. Lancet Oncol. 12:1004–1012. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi YL, Soda M, Yamashita Y, et al:
EML4-ALK mutations in lung cancer that confer resistance to ALK
inhibitors. N Engl J Med. 363:1734–1739. 2010. View Article : Google Scholar : PubMed/NCBI
|