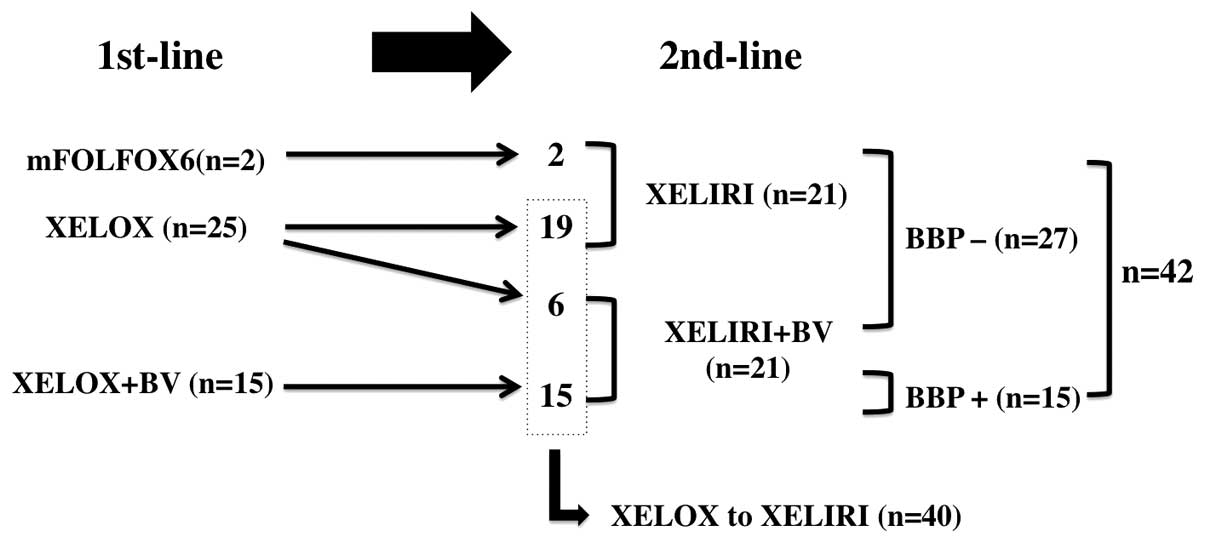
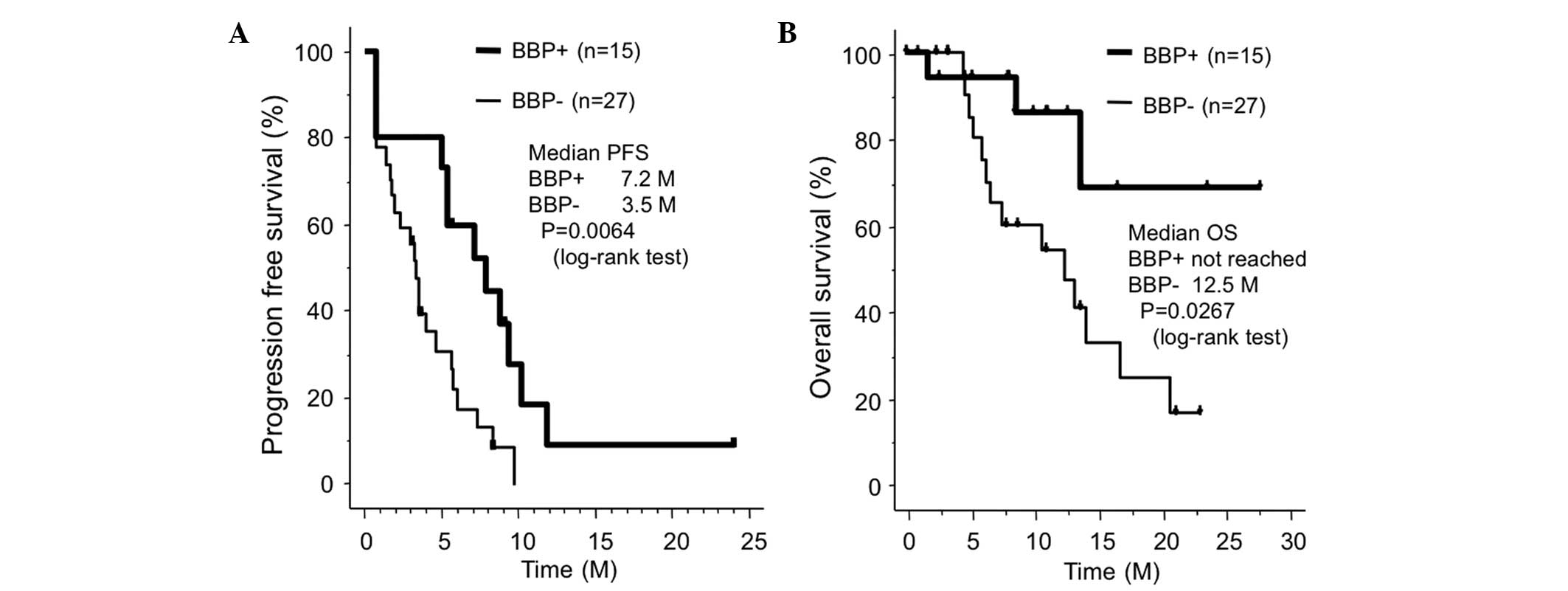
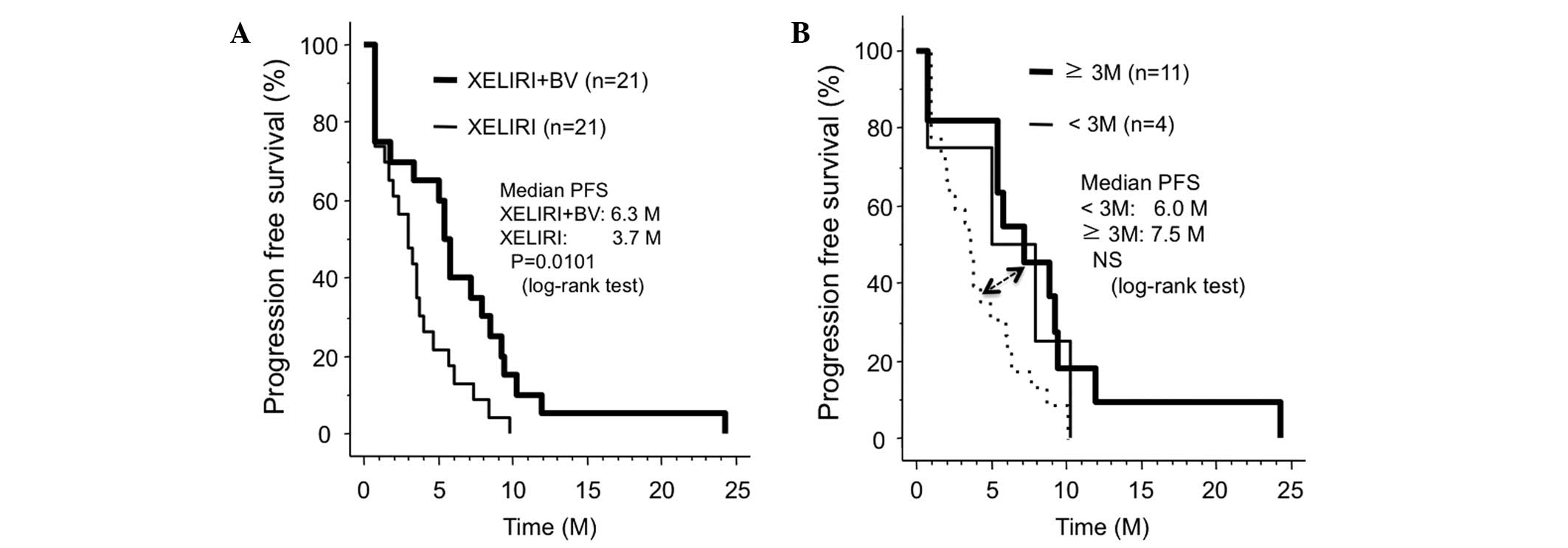
Spandidos Publications style
Suzuki K, Takaharu K, Muto Y, Ichida K, Fukui T, Takayama Y, Tsujinaka S, Sasaki J, Horie H, Kawamura YJ, Kawamura YJ, et al: XELIRI regimen plus continuous treatment with bevacizumab is well-tolerated and effective in metastatic colorectal cancer patients in a second‑line setting involving the sequential administration of XELOX and XELIRI. Mol Clin Oncol 2: 827-832, 2014.
APA
Suzuki, K., Takaharu, K., Muto, Y., Ichida, K., Fukui, T., Takayama, Y. ... Rikiyama, T. (2014). XELIRI regimen plus continuous treatment with bevacizumab is well-tolerated and effective in metastatic colorectal cancer patients in a second‑line setting involving the sequential administration of XELOX and XELIRI. Molecular and Clinical Oncology, 2, 827-832. https://doi.org/10.3892/mco.2014.306
MLA
Suzuki, K., Takaharu, K., Muto, Y., Ichida, K., Fukui, T., Takayama, Y., Tsujinaka, S., Sasaki, J., Horie, H., Kawamura, Y. J., Konishi, F., Rikiyama, T."XELIRI regimen plus continuous treatment with bevacizumab is well-tolerated and effective in metastatic colorectal cancer patients in a second‑line setting involving the sequential administration of XELOX and XELIRI". Molecular and Clinical Oncology 2.5 (2014): 827-832.
Chicago
Suzuki, K., Takaharu, K., Muto, Y., Ichida, K., Fukui, T., Takayama, Y., Tsujinaka, S., Sasaki, J., Horie, H., Kawamura, Y. J., Konishi, F., Rikiyama, T."XELIRI regimen plus continuous treatment with bevacizumab is well-tolerated and effective in metastatic colorectal cancer patients in a second‑line setting involving the sequential administration of XELOX and XELIRI". Molecular and Clinical Oncology 2, no. 5 (2014): 827-832. https://doi.org/10.3892/mco.2014.306