|
1
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Govindan R, Page N, Morgensztern D, et al:
Changing epidemiology of small-cell lung cancer in the United
States over the last 30 years: analysis of the surveillance,
epidemiologic, and end results database. J Clin Oncol.
24:4539–4544. 2006.PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
4
|
Patel AM, Dunn WF and Trastek VF: Staging
systems of lung cancer. Mayo Clin Proc. 68:475–482. 1993.
View Article : Google Scholar
|
|
5
|
Lu HY, Wang XJ and Mao WM: Targeted
therapies in small cell lung cancer (Review). Oncol Lett. 5:3–11.
2013.
|
|
6
|
Hanna NH and Einhorn LH: Small-cell lung
cancer: state of the art. Clin Lung Cancer. 4:87–94. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warde P and Payne D: Does thoracic
irradiation improve survival and local control in limited-stage
small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol.
10:890–895. 1992.PubMed/NCBI
|
|
8
|
Kubota K, Hida T, Ishikura S, et al:
Etoposide and cisplatin versus irinotecan and cisplatin in patients
with limited-stage small-cell lung cancer treated with etoposide
and cisplatin plus concurrent accelerated hyperfractionated
thoracic radiotherapy (JCOG0202): a randomised phase 3 study.
Lancet Oncol. 15:106–113. 2014. View Article : Google Scholar
|
|
9
|
Sundstrom S, Bremnes RM, Kaasa S, et al:
Cisplatin and etoposide regimen is superior to cyclophosphamide,
epirubicin, and vincristine regimen in small-cell lung cancer:
results from a randomized phase III trial with 5 years’ follow-up.
J Clin Oncol. 20:4665–4672. 2002.PubMed/NCBI
|
|
10
|
Johnson DH, Bass D, Einhorn LH, et al:
Combination chemotherapy with or without thoracic radiotherapy in
limited-stage small-cell lung cancer: a randomized trial of the
Southeastern Cancer Study Group. J Clin Oncol. 11:1223–1229.
1993.
|
|
11
|
Osterlind K, Hansen HH, Hansen HS, et al:
Chemotherapy versus chemotherapy plus irradiation in limited small
cell lung cancer. Results of a controlled trial with 5 years
follow-up. Br J Cancer. 54:7–17. 1986.PubMed/NCBI
|
|
12
|
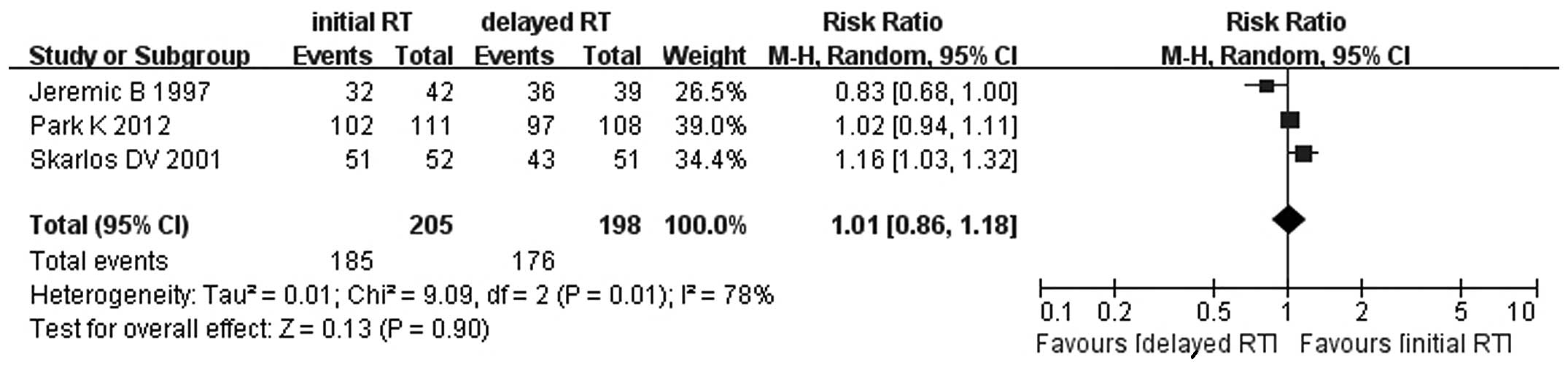
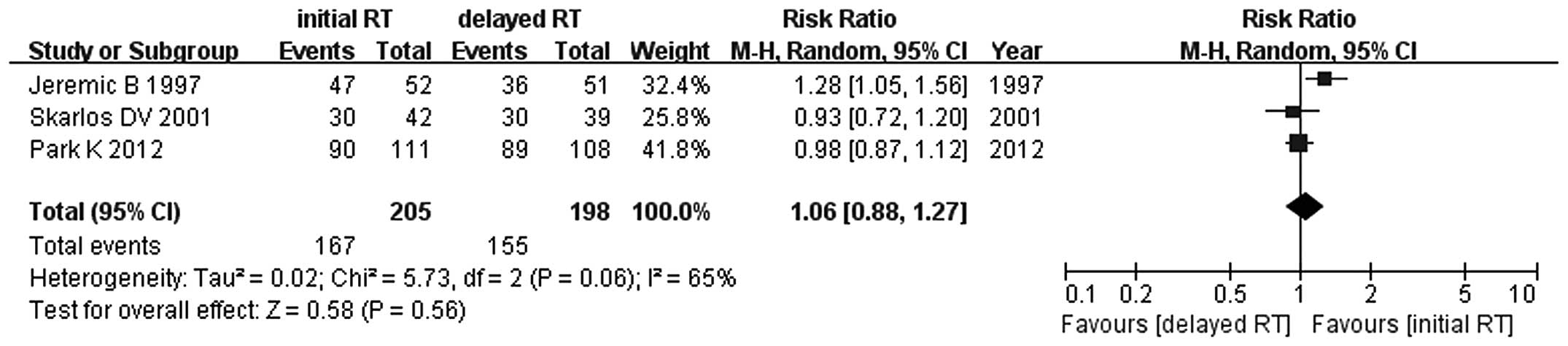
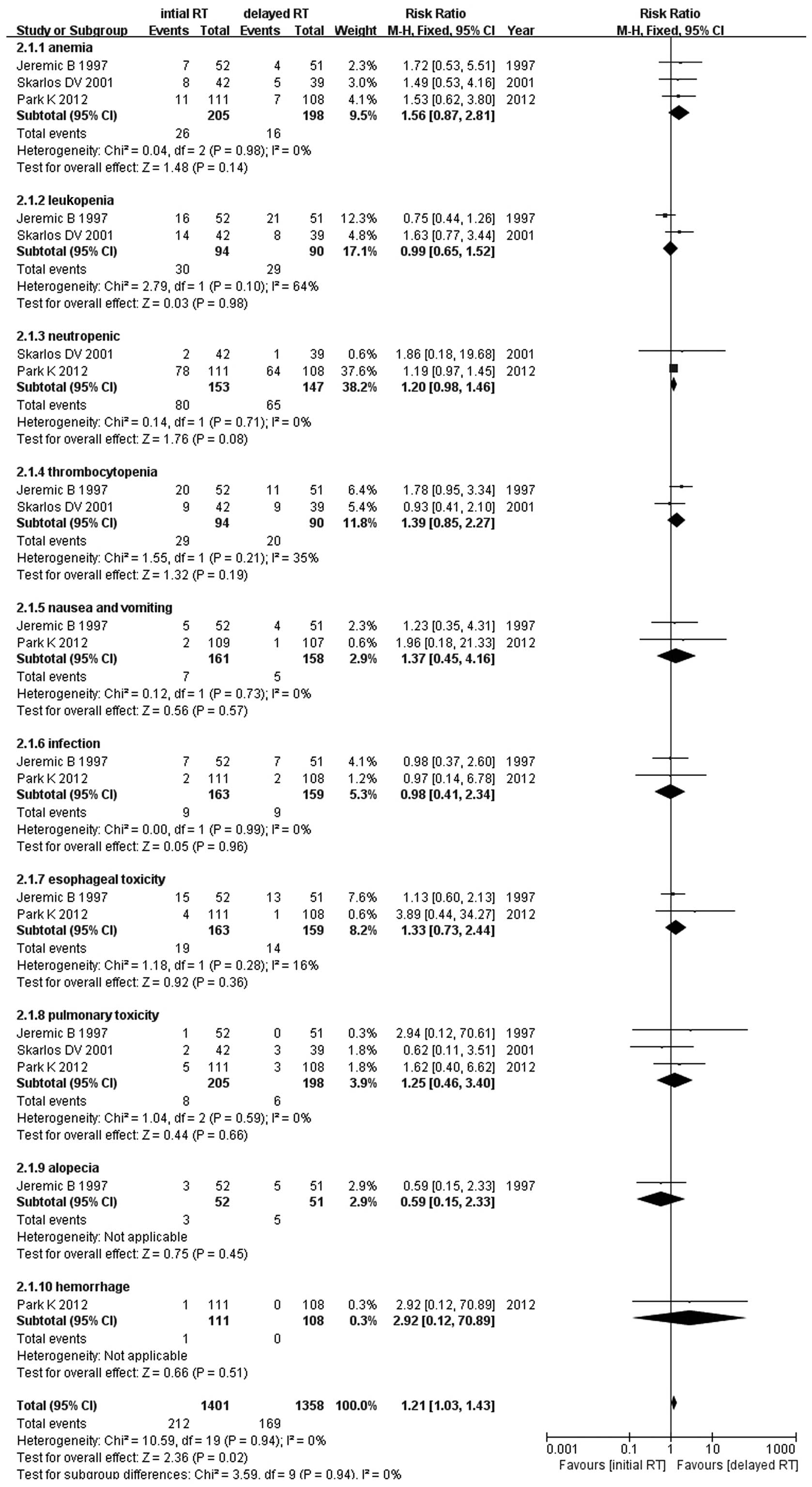
Skarlos DV, Samantas E, Kosmidis P, et al:
Randomized comparison of etoposide-cisplatin vs.
etoposide-carboplatin and irradiation in small-cell lung cancer. A
Hellenic Co-operative Oncology Group study. Ann Oncol. 5:601–607.
1994.PubMed/NCBI
|
|
13
|
Rossi A, Di Maio M, Chiodini P, et al:
Carboplatin- or cisplatin-based chemotherapy in first-line
treatment of small-cell lung cancer: the COCIS meta-analysis of
individual patient data. J Clin Oncol. 30:1692–1698. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takada M, Fukuoka M, Kawahara M, et al:
Phase III study of concurrent versus sequential thoracic
radiotherapy in combination with cisplatin and etoposide for
limited-stage small-cell lung cancer: results of the Japan Clinical
Oncology Group Study 9104. J Clin Oncol. 20:3054–3060. 2002.
View Article : Google Scholar
|
|
15
|
Skarlos DV, Samantas E, Briassoulis E, et
al: Randomized comparison of early versus late hyperfractionated
thoracic irradiation concurrently with chemotherapy in limited
disease small-cell lung cancer: a randomized phase II study of the
Hellenic Cooperative Oncology Group (HeCOG). Ann Oncol.
12:1231–1238. 2001. View Article : Google Scholar
|
|
16
|
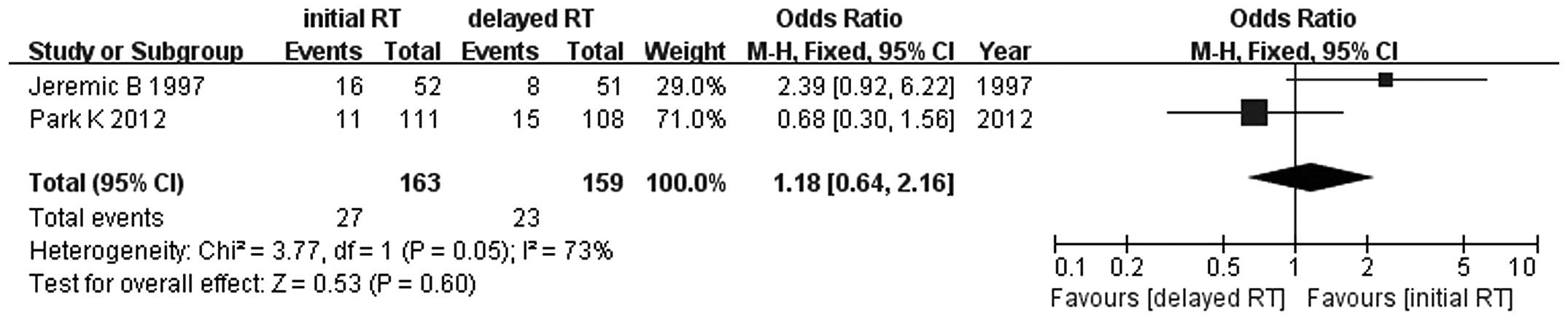
Park K, Sun JM, Kim SW, et al: Phase III
trial of concurrent thoracic radiotherapy (TRT) with either the
first cycle or the third cycle of cisplatin and etoposide
chemotherapy to determine the optimal timing of TRT for
limited-disease small cell lung cancer. J Clin Oncol. 30(Suppl):
abstract 7004. 2012.
|
|
17
|
De Ruysscher D, Pijls-Johannesma M,
Vansteenkiste J, et al: Systematic review and meta-analysis of
randomised, controlled trials of the timing of chest radiotherapy
in patients with limited-stage, small-cell lung cancer. Ann Oncol.
17:543–552. 2006.
|
|
18
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0 [updated
March 2011]. The Cochrane Collaboration; 2011
|
|
19
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeremic B, Shibamoto Y, Acimovic L, et al:
Initial versus delayed accelerated hyperfractionated radiation
therapy and concurrent chemotherapy in limited small-cell lung
cancer: a randomized study. J Clin Oncol. 15:893–900. 1997.
|
|
21
|
Work E, Nielsen OS, Bentzen SM, et al:
Randomized study of initial versus late chest irradiation combined
with chemotherapy in limited-stage small-cell lung cancer. Aarhus
Lung Cancer Group. J Clin Oncol. 15:3030–3037. 1997.PubMed/NCBI
|
|
22
|
Spiro SG, James LE, Rudd RM, et al; London
Lung Cancer Group. Early compared with late radiotherapy in
combined modality treatment for limited disease small-cell lung
cancer: a London Lung Cancer Group multicenter randomized clinical
trial and meta-analysis. J Clin Oncol. 24:3823–3830. 2006.
View Article : Google Scholar
|
|
23
|
Murray N, Coy P, Pater JL, et al:
Importance of timing for thoracic irradiation in the combined
modality treatment of limited-stage small-cell lung cancer. The
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 11:336–344. 1993.PubMed/NCBI
|
|
24
|
Perry MC, Herndon JE III, Eaton WL and
Green MR: Thoracic radiation therapy added to chemotherapy for
small-cell lung cancer: an update of Cancer and Leukemia Group B
Study 8083. J Clin Oncol. 16:2466–2467. 1998.PubMed/NCBI
|
|
25
|
Jiang J, Liang X, Zhou X, et al: A
meta-analysis of randomized controlled trials comparing
irinotecan/platinum with etoposide/platinum in patients with
previously untreated extensive-stage small cell lung cancer. J
Thorac Oncol. 5:867–873. 2010. View Article : Google Scholar
|
|
26
|
Hu X, Bao Y, Zhang L, et al: Omitting
elective nodal irradiation and irradiating postinduction versus
preinduction chemotherapy tumor extent for limited-stage small cell
lung cancer: interim analysis of a prospective randomized
noninferiority trial. Cancer. 118:278–287. 2012. View Article : Google Scholar
|
|
27
|
Gridelli C, Perrone F and Monfardini S:
Lung cancer in the elderly. Eur J Cancer. 33:2313–2314. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Ruysscher D, Pijls-Johannesma M,
Bentzen SM, et al: Time between the first day of chemotherapy and
the last day of chest radiation is the most important predictor of
survival in limited-disease small-cell lung cancer. J Clin Oncol.
24:1057–1063. 2006.PubMed/NCBI
|
|
29
|
Pijls-Johannesma M, De Ruysscher D,
Vansteenkiste J, et al: Timing of chest radiotherapy in patients
with limited stage small cell lung cancer: a systematic review and
meta-analysis of randomised controlled trials. Cancer Treat Rev.
33:461–473. 2007. View Article : Google Scholar
|
|
30
|
Huncharek M and McGarry R: A meta-analysis
of the timing of chest irradiation in the combined modality
treatment of limited-stage small cell lung cancer. Oncologist.
9:665–672. 2004. View Article : Google Scholar : PubMed/NCBI
|