Introduction
Metadherin (MTDH), also known as astrocyte-elevated
gene (AEG)-1 and lysine-rich CEACAM-1-associated protein (LYRIC)
(1–2), was first identified in human fetal
astrocytes induced by human immunodeficiency virus-1 in 2002
(3). MTDH has been cloned for
nearly ten years (4–6) and is considered to be an important
oncogene in carcinogenesis, tumor progression and metastasis, via a
series of signaling pathways (7–9).
However, as the exact functions of MTDH in cancer remain unclear,
particularly in malignant tumors, future investigations are
required (10).
Recent studies have shown that high expressions of
MTDH are present in various tumors, involving the nervous,
urogenital, respiratory and digestive systems (9,11–19).
Evidence indicates that MTDH knockdown significantly inhibited the
growth of gastric cancer cells (9). MTDH is an anti-apoptotic gene in
glioma cells and could be used as a target of microRNA-136 in
anticancer therapeutic strategy (11). A study revealed that MTDH promotes
invasion and metastasis through the activation of nuclear
factor-κB (NF-κB), interleukin-8 and matrix
metalloproteinase-9 in breast cancer (12), which is consistent with studies in
non-small cell lung cancer and glioma (13,14).
MTDH has been shown to be associated with a poor prognosis in
malignancies, including non-small cell lung cancer, invasive breast
cancer, laryngeal squamous cell carcinoma, gallbladder
adenocarcinoma, tongue carcinoma and bladder cancer (15–19).
MTDH is also associated with the prognosis of carcinoma, possibly
due to chemoresistance and radiosensitivity in cancer therapy
(20,21).
Increasing evidence indicates that MTDH is an
important mediator in signal transduction pathways in malignancies,
a potential biomarker to predict cancer survival and provides
information to develop target chemotherapies. The present
systematic review aimed to investigate the expression, pathogenesis
and therapeutic strategies of MTDH in prostate, bladder and kidney
cancers.
Materials and methods
Literature selection
The inclusion criteria for the search are any
studies regarding MTDH and prostate, bladder and kidney cancer. The
exclusion criteria are as followings: i) Duplicate or series
reporting, in which the most detailed results were included; ii)
conference report, review or letter; and iii) study language not in
English or Chinese.
Literature search
A comprehensive literature search of the English and
Chinese databases was performed from the time when establishing the
databases to 25 March, 2014, using Pubmed, Highwire, Springerlink,
EBSCO, ProQuest, Wiley, Google Scholar, China National Knowledge
Infrastructure and Wanfang Data Knowledge Service Platform (Wanfang
data). The studies regarding MTDH and prostate, bladder and kidney
cancer respectively were searched for. The following example is the
search strategy of MTDH and prostate cancer in Pubmed: (metadherin
or MTDH or astrocyte elevated gene-1 or AEG-1 or lysine-rich
CEACAM1 or LYRIC) and (prostate or prostate neoplasm or prostate
cancer). The references of the associated studies were checked
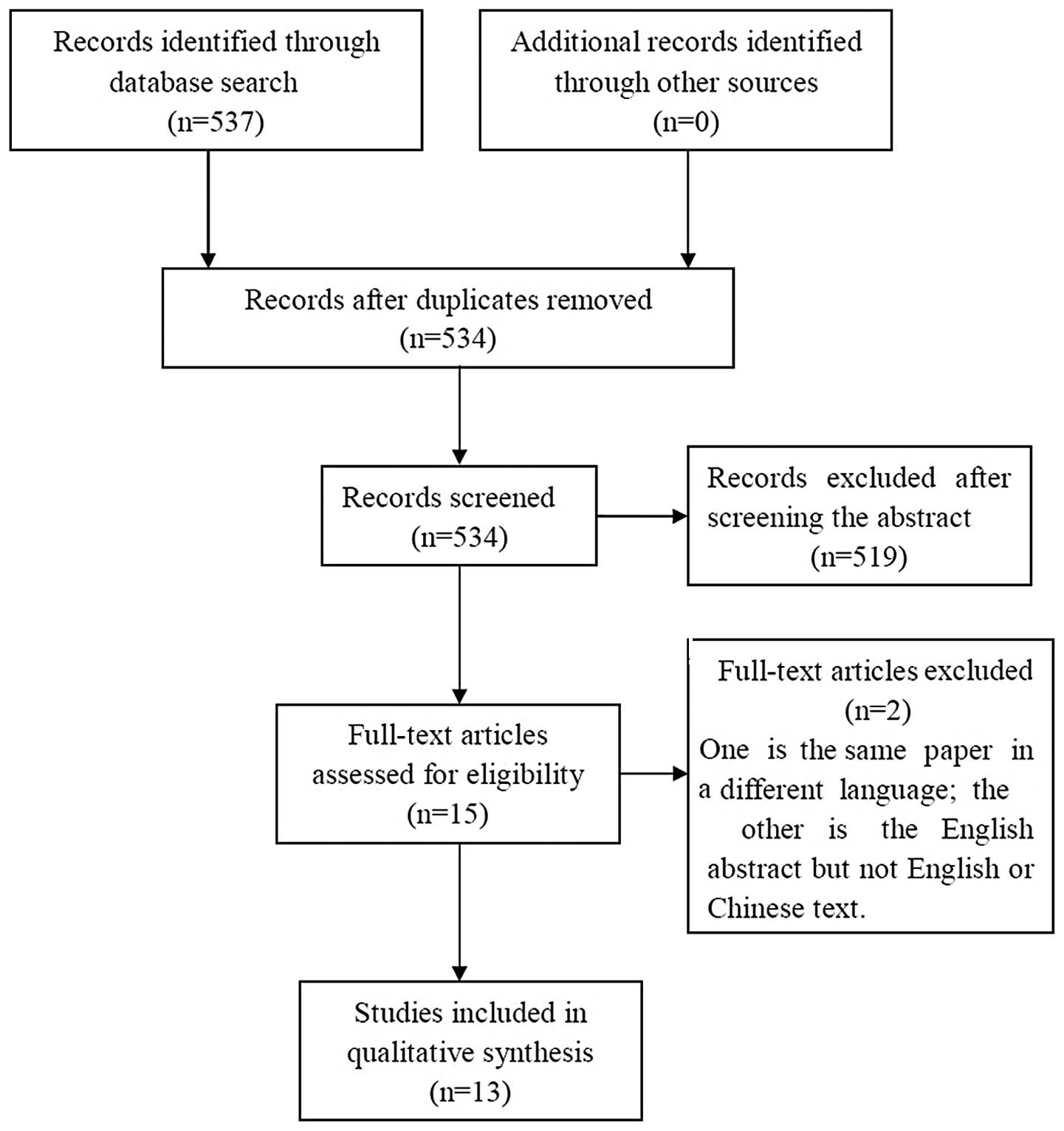
manually to avoid the loss of relevant studies. Approximately 537
studies were consistent with the search strategies in these
databases, and 13 were eligible for the search. Fig. 1 shows a flowchart of the search
process. The detailed search results of MTDH and prostate, bladder
and kidney cancers are listed in Table
I.
 | Table ISummary of the 13 eligible studies
regarding MTDH and prostate, bladder and kidney cancer. |
Table I
Summary of the 13 eligible studies
regarding MTDH and prostate, bladder and kidney cancer.
| First author | Publication year | Language | Cancer type | Contents | Specimens | Main detecting
method(s) | Main conclusions | Reference |
|---|
| Kikuno | 2007 | English | Prostate | MTDH expression;
signal pathway of FOXO3a | Tissue samples; PC-3,
DU145 and LNCapP cells | Immunostaining;
RT-PCR, western blot; MTDH knockown | Overepression in
prostate cancer, MTDH activates AKT and suppresses FOXO3a | 23 |
| Ash | 2008 | English | Prostate | MTDH and its
associated protein, BCCIPα | DU145 cells | Yeast two-hybrid;
Western blot; IP and IIF | MTDH is a negative
regulator of BCCIPα and NF-κB may be involved | 27 |
| Thirkettle | 2009 | English | Prostate | MTDH is targeted to
different subcellular compartment | Tissue samples; | Microarray | A significant
difference among MTDH distribution, Gleason grade and survival | 10 |
| Lee | 2012 | English | Prostate | Molecular mechanism
of anticancer drug cryptotanshinone | PC-3, DU145 and
LNCaP cells | siRNA transfection,
western blot, IIF array, immunohistochemistry; PC-3 xenograft
model | Inhibition of MTDH
is one of the molecular targets for cryptotanshinone exerting
anticancer effects. | 24 |
| Zhang | 2012 | Chinese | Prostate | Observation of
MTDH-based gene vaccine in prostate cancer models in mice | Mice models |
Immunohistochemistry, flow cytometry; RM-1
xenograft model survival periods | Mice with prostate
cancer in situ with MTDH gene vaccine exhibit prolonged
tumor-bearing | 30 |
| Erdem | 2013 | English | Prostate | Association of the
prognostic factors in prostate carcinoma | Tissue samples | Tissue
microarray | bFGF and MTDH is
one of the independent prognostic parameters in prostate
carcinoma | 22 |
| Zhang | 2013 | Chinese | Prostate | Evaluation efficacy
of GM-CSF adjuvant in prostate cancer models in mice | Mice models |
Immunohistochemistry, flow cytometry; RM-1
xenograft model | Combining with
GM-CSF adjuvant shows stronger anticancer effects, no prolonged
tumor-bearing survival period | 28 |
| Hu | 2013 | Chinese | Prostate | Evaluation efficacy
of MTDH gene vaccine on paclitaxel in prostate cancer models in
mice | Mice models |
Immunohistochemistry, flow cytometry; RM-1
xenograft model | Sensitivity of
paclitaxel was enhanced in prostate cancer models in mice | 29 |
| Zhou | 2012 | English | Bladder | Investigating the
association of MTDH expression and bladder cancer clinical stage;
and MTDH as a biomarker in bladder cancer | Tissue samples |
Immunohistochemistry, RT-PCR | MTDH may be a
biomarker of bladder cancer progression, associated with patient
survival | 19 |
| Nikpour | 2014 | English | Bladder | Exploring
functional role of MTDH in bladder cancer cells | Tissue samples; 17
bladder cancer cell lines | RT-PCR, western
blot, immunohistochemistry; RNA interference; viability and caspase
assay | MTDH is
overexpressed in bladder cancer. Downregulation of MTDH reduced
cell viability and cell migration and increased apoptosis in
bladder cancer cell lines | 31 |
| Chen | 2010 | English | Kidney | Detecting MTDH
expression in renal cell carcinoma, revealing the association among
MTDH, histopathological features and survival | Tissue samples;
786-0, OS-RC-1, Caki-2, ACHN cells | RT-PCR, western
blot; immunohistochemistry | MTDH is
overexpressed in kidney cancer and is associated with tumor
differetiation, progression and prognosis | 32 |
| Erdem | 2013 | English | Kidney | Investigating the
association of MTDH and p53 in renal cell carcinoma | Tissue samples |
immunohistochemistry | MTDH is
overexpressed in renal cell carcinoma and may be one of the
prognostic parameters in renal cell cancer patients | 33 |
| Wu | 2013 | English | Kidney | Exploring pathways
of miR-30d in renal cell cancer | Tissue samples;
ACHN and 786-0 RCC cell lines | RT-PCR, western
blot; flow cytometry, ChIP, RNA interference, nuclear run-on
assay | Identified a novel
Akt/FOXO/miR-30d/MTDH signaling transduction pathway in kidney
cancer | 34 |
Results and Discussion
MTDH and prostate cancer
A total of 8 eligible studies reported the
association between MTDH and prostate cancer. High MTDH expression
was detected in tissue specimens and prostate cancer cell lines,
and was confirmed to be associated with prognosis and patient
survival. Studies also investigated the possible signal
transduction pathways in prostate cancer cell lines mediated by
MTDH, which may be potential therapeutic targets for
anticancer.
MTDH overexpression in prostate
cancer
One study showed that MTDH overexpression in
prostate cancer was 66.7% (22),
whereas in another study, the total expression of MTDH was 100%
(23). The later case-control
study involved 20 prostate cancer samples from radical
prostatectomy and 20 benign prostatic hyperplasia (BPH) specimens
from transurethral resection. MTDH strongly positive expression in
prostate cancer was 80%, whereas it was 10% in BPH. Weakly positive
expression of MTDH in prostate cancer was reduced to only 20%, but
35% in BPH. The negative expression in BPH reached 55%.
A high expression of MTDH in prostate cancer cell
lines was also observed in the eligible studies. Quantitative
reverse transcription polymerase chain reaction (RT-PCR) and
western blotting showed that the expression level of MTDH in
prostate cancer cells was nearly three times higher compared to
those in non-cancerous human prostatic epithelial cell line (RWPE-1
cells) at the mRNA and protein level, respectively. High MTDH
expressions were found in three common prostate cancer cells
lines|: LNCaP, DU145 and PC-3 cell lines (23,24).
Thirkettle et al (10) reported the MTDH distribution in the
subcellular compartments of prostate cancer and benign samples. The
MTDH expression in the nucleus of luminal cells was much higher in
benign cases (82.5%) compared to in tumors (26.6%). The
distributions of MTDH in cytoplasm alone, cytoplasm plus nucleus
and global cells in prostate cancer samples were 33.6%, 42.9% and
8.8%, respectively. The distribution of MTDH was different in
subcellular compartments, which was also associated with tumor
grades (10).
These results show that an association exists
between the expression of MTDH and prostate cancer. MTDH
overexpression not only occurs in human tissue samples, but also in
prostate cancer cell lines. Studies have demonstrated that the high
expression of MTDH is similar in prostate cancer, but is not
distinctive in benign lesions (23). However, in another study, MTDH was
also proved to be highly expressed in benign samples (10).
Progression and survival of MTDH in
prostate cancer
A follow-up of 50 patients for <120 months
indicated that mean survival times in patients with MTDH in the
nucleus are longer than those losses of MTDH in the nucleus
(10). In patients with bone
metastasis, a high expression of MTDH was confirmed in prostate
bone metastases (81.8%) and it was mainly distributed in the
cytoplasm and membrane (10).
Following an investigation of 97 radical prostatectomy samples,
Erdem et al (22) showed
that MTDH combined with the basic fibroblast growth factor is one
of the independent prognostic parameters. These results indicate
that MTDH may be a potential prognostic factor of prostate
cancer.
MTDH and signal transduction in prostate
cancer
The pathogenesis of MTDH in tumors may be
interpreted as participating in signal transduction pathways,
including NF-κB, Ha-ras and AKT. These signaling pathways may be
involved in carcinogenesis, metastasis and progression in
malignancies, predicting that MTDH may be a biomarker in cancers
(7,25,26).
Ash et al (27) used the yeast two-hybrid assay to
identify that BRCA2 and CDKN1A interacting protein (BCCIP) is an
associated protein of MTDH. In DU145 cells, MTDH was found to be a
negative regulator of BCCIPα, which induces DU145-apparent
neuroendocrine differentiation. The NF-κB signaling pathway was
considered to be involved in the interactions of MTDH and BCCIP in
prostate cancer cells. Another study also verified that the
knockdown of MTDH downregulated the activity of NF-κB in PC-3 and
DU145 cells (23). In addition,
these results also prove that the inhibition of prostate cancer
progression by MTDH knockdown may be mediated by suppression of AKT
and upregulation of FOXO3a activity (23). In hypoxic PC-3 cells, MTDH may
participate in the downstream of the hypoxia-inducible factor 1α
(HIF-1α) or phosphoinositide 3-kinase pathways (24).
MTDH and prostate cancer therapies
The signaling pathways of MTDH were reviewed
systematically in prostate cancer previously in this study and it
was concluded that MTDH may be a target in the treatment of
prostate cancer. In order to investigate the molecular mechanism of
cryptotanshinone in anticancer, several genes were analyzed in the
study by Lee et al (24),
including MTDH and HIF-1α. Inhibition of MTDH expression was found
to be one of the molecular targets for cryptotanshinone exerting
anticancer effects (24).
Previous studies have examined MTDH-based DNA
vaccines in prostate cancer using mice models (28–30).
The results have shown that a significant enhancement of humoral
and cellular immune responses was detected in MTDH-immunized mice.
With the MTDH gene vaccine, mice with prostate cancer in
situ exhibited prolonged tumor-bearing survival times (28). This was considered to be due to the
inhibition of the proliferation and invasion of prostate cancer.
The MTDH gene vaccine combined with granulocyte-macrophage
colony-stimulating factor (GM-CSF) adjuvant shows stronger
anticancer effects, except that there was no significant difference
in tumor-bearing survival time (29). The MTDH gene vaccine combined with
paclitaxel exhibits significant inhibition of prostate cancer
growth and prolonged tumor-bearing survival times (30).
MTDH and bladder cancer
The first study of MTDH expression in bladder cancer
was in 2012 by Zho et al (19). The study involved 15 benign cases
and 60 cancer samples. The results showed a significant difference
of MTDH expression between bladder benign specimens and cancers
(19). This result was also proved
by another study by detection of MTDH mRNA expression in bladder
benign and cancer samples (31),
and MTDH expression has also been verified in 17 bladder cancer
cell lines (31). RT112 cell lines
have the highest expression, which is higher than that of DU145 and
LNCaP as the controls, whereas HT1376 showed the lowest expression
in the 17 cell lines (31).
Another study indicated that MTDH is a potential
prognostic factor of bladder cancer, as a significant association
was demonstrated between the expression of MTDH and
clinicopathological stage, tumor classification, tumor multiplicity
and survival time (19). However,
no significant correlations were reported in the study by Nikpour
et al (31) between the
expression of MTDH mRNA and bladder cancer grade, tumor category
and lymph node metastasis.
As a positive correlation between MTDH and Ki67 has
been demonstrated, MTDH may promote bladder cancer tissue growth
(19). The study by Nikpour et
al (31) also confirmed that
MTDH promoted bladder cancer cell survival, clonogenicity and
migration, which were verified by downregulation of MTDH via small
interfering RNA. In addition to increased apoptosis, reductions of
cell viability and migration have also been observed in bladder
cancer (31).
MTDH and kidney cancer
MTDH overexpression in primary kidney cancer has
been confirmed by immunohistochemistry, RT-PCR and western blot.
Two studies found that the expression of MTDH in kidney carcinoma
specimens was high compared to normal tissues (32,33).
An in vitro study by Chen et al (32) investigated the expression of MTDH
in four kidney cancer cell lines; 786-0, OS-RC-1, Caki-2 and ACHN.
A significantly higher level of MTDH was expressed in these four
cell lines in the cytoplasm compared to the HK-2 cells as the
control (32).
The results of Chen et al (32) indicated that the MTDH expression
level is associated with the differentiation degree of renal cell
carcinoma and clinical stage, with tumor and metastasis
classifications, but not node classifications. Survival analysis
confirmed a shorter survival time when the MTDH level was higher
(32). The conclusion was also
verified by another study showing that MTDH may be one of the
prognostic parameters in renal cell cancer patients (33). A novel signal transduction pathway
Akt/FOXO/miR-30d/MTDH in renal cell carcinoma was reported by the
study by Wu et al (34),
providing a new insight into the anticancer strategy of kidney
cancer.
Limitations of the present studies and
prospects
MTDH, as one of numerous oncogenes, has been studied
in urogenital cancers for <10 years. Limitations of the current
studies may exist in this area and are as follows.
Firstly, a larger tissue sample size should be
analyzed to measure the association between MTDH expression and
urogenital cancers in progression, metastasis and prognosis.
Regarding MTDH and progression of bladder cancer, conflicting
conclusions were reported in two studies (19,31)
possibly due to their limited samples. Secondly, follow-up times
and repeated studies are required to investigate the higher
expression of MTDH as an influence of prognostic factors. Only one
study performed a 120-month follow-up of prostate cancer, which may
not be enough (10). Follow-up
information is not available in bladder and kidney cancers.
Thirdly, increasing detection methods and more comprehensive
sources of specimen should be tested in further studies. To confirm
MTDH as a potential biomarker in urogenital cancers, except for
body organs or tissues, samples of blood, plasma, urine, prostatic
and seminal fluid may also be taken into consideration to detect
the MTDH expression. Fourthly, in vitro and animal models
are required to reveal the function of MTDH as an oncogene and its
signaling transduction pathways. Signaling transduction pathways of
MTDH in prostate and kidney cancers is limited in the current
studies. An increase in the detailed signaling pathways should be
further investigated to define the role of MDTH in tumor
pathogenesis. Further studies in signaling may provide novel
targets for anticancer therapies.
MTDH is overexpressed in a number of urologic
cancers, including prostate, bladder and kidney cancers. MTDH may
be involved in urologic cancer progression, metastasis and
prognosis and may be associated with several signaling transduction
pathways in urologic cancers, indicating latent targets to develop
anticancer therapeutic strategies. Further studies are required to
confirm the aforementioned conclusions.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for the Central Universities of Central South
University in 2013 (no. 2013zzts095).
References
|
1
|
Sarkar D and Fisher PB: AEG-1/MTDH/LYRIC:
clinical significance. Adv Cancer Res. 120:39–74. 2013. View Article : Google Scholar
|
|
2
|
Lee SG, Kang DC, DeSalle R, et al:
AEG-1/MTDH/LYRIC, the beginning: initial cloning, structure,
expression profile, and regulation of expression. Adv Cancer Res.
120:1–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su ZZ, Kang DC, Chen Y, et al:
Identification and cloning of human astrocyte genes displaying
elevated expression after infection with HIV-1 or exposure to HIV-1
envelope glycoprotein by rapid subtraction hybridization, RaSH.
Oncogene. 21:3592–3602. 2002. View Article : Google Scholar
|
|
4
|
Kang DC, Su ZZ, Sarkar D, et al: Cloning
and characterization of HIV-1-inducible astrocyte elevated gene-1,
AEG-1. Gene. 353:8–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Britt DE, Yang DF, Yang DQ, et al:
Identification of a novel protein, LYRIC, localized to tight
junctions of polarized epithelial cells. Exp Cell Res. 300:134–148.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sutherland HG, Lam YW, Briers S, et al:
3D3/lyric: a novel transmembrane protein of the endoplasmic
reticulum and nuclear envelope, which is also present in the
nucleolus. Exp Cell Res. 294:94–105. 2004. View Article : Google Scholar
|
|
7
|
Emdad L, Sarkar D, Su ZZ, et al: Astrocyte
elevated gene-1: recent insights into a novel gene involved in
tumor progression, metastasis and neurodegeneration. Pharmacol
Ther. 114:155–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu G, Wei Y and Kang Y: The multifaceted
role of MTDH/AEG-1 in cancer progression. Clin Cancer Res.
15:5615–5620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang W, Yang L, Liang S, et al: AEG-1 is
a target of perifosine and is over-expressed in gastric dysplasia
and cancers. Dig Dis Sci. 58:2873–2880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thirkettle HJ, Girling J, Warren AY, et
al: LYRIC/AEG-1 is targeted to different subcellular compartments
by ubiquitinylation and intrinsic nuclear localization signals.
Clin Cancer Res. 15:3003–3013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Wu J, Guan H, et al: MiR-136
promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2.
FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Kong X, Li X, et al: Metadherin
mediates lipopolysaccharide-induced migration and invasion of
breast cancer cells. PLoS One. 6:e293632011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun S, Ke Z, Wang F, et al: Overexpression
of astrocyte-elevated gene-1 is closely correlated with poor
prognosis in human non-small cell lung cancer and mediates its
metastasis through up-regulation of matrix metalloproteinase-9
expression. Hum Pathol. 43:1051–1060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Wu J, Ying Z, et al: Astrocyte
elevated gene-1 upregulates matrix metalloproteinase-9 and induces
human glioma invasion. Cancer Res. 70:3750–3759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tokunaga E, Nakashima Y, Yamashita N, et
al: Overexpression of metadherin/MTDH is associated with an
aggressive phenotype and a poor prognosis in invasive breast
cancer. Breast Cancer. 21:341–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Su Z, Li G, et al: Increased
expression of metadherin protein predicts worse disease-free and
overall survival in laryngeal squamous cell carcinoma. Int J
Cancer. 133:671–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu DC and Yang ZL: MTDH and EphA7 are
markers for metastasis and poor prognosis of gallbladder
adenocarcinoma. Diagn Cytopathol. 41:199–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ke ZF, He S, Li S, et al: Expression
characteristics of astrocyte elevated gene-1 (AEG-1) in tongue
carcinoma and its correlation with poor prognosis. Cancer
Epidemiol. 37:179–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou J, Li J, Wang Z, et al: Metadherin is
a novel prognostic marker for bladder cancer progression and
overall patient survival. Asia Pac J Clin Oncol. 8:e42–e48. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Li Y, Wang X, et al: Elevated
expression of astrocyte elevated gene-1 (AEG-1) is correlated with
cisplatin-based chemoresistance and shortened outcome in patients
with stages III–IV serous ovarian carcinoma. Histopathology.
60:953–963. 2012.PubMed/NCBI
|
|
21
|
Zhao Y, Moran MS, Yang Q, et al:
Metadherin regulates radioresistance in cervical cancer cells.
Oncol Rep. 27:1520–1526. 2012.PubMed/NCBI
|
|
22
|
Erdem H, Yildirim U, Uzunlar AK, et al:
Relationship among expression of basic-fibroblast growth factor,
MTDH/astrocyte elevated gene-1, adenomatous polyposis coli, matrix
metalloproteinase 9, and COX-2 markers with prognostic factors in
prostate carcinomas. Niger J Clin Pract. 16:418–423. 2013.
View Article : Google Scholar
|
|
23
|
Kikuno N, Shiina H, Urakami S, et al:
Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer
progression through upregulation of FOXO3a activity. Oncogene.
26:7647–7655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HJ, Jung DB, Sohn EJ, et al:
Inhibition of hypoxia inducible factor alpha and astrocyte-elevated
gene-1 mediates cryptotanshinone exerted antitumor activity in
hypoxic PC-3 cells. Evid Based Complement Alternat Med.
2012:3909572012.
|
|
25
|
Zhang J, Zhang Y, Liu S, et al: Metadherin
confers chemoresistance of cervical cancer cells by inducing
autophagy and activating ERK/NF-kappaB pathway. Tumour Biol.
34:2433–2440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu C, Liu Y, Tan H, et al: Metadherin
regulates metastasis of squamous cell carcinoma of the head and
neck via AKT signalling pathway-mediated epithelial-mesenchymal
transition. Cancer Lett. 343:258–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ash SC, Yang DQ and Britt DE: LYRIC/AEG-1
overexpression modulates BCCIP alpha protein levels in prostate
tumor cells. Biochem Biophys Res Commun. 371:333–338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Li H, Qian B, et al:
Metadherin/astrocyte elevated gene-1-based DNA vaccine suppresses
progression and metastasis in prostate cancer of mice. Chin J Exp
Surg. 30:2148–2151. 2013.(In Chinese).
|
|
29
|
Hu W: GM-CSF combined with vaccine of MTDH
inhibiting prostate cancer growth and metastasis. Master Thesis.
Fujian Medical University; 2013, (In Chinese).
|
|
30
|
Zhang C: Vaccine of target gene MTDH/AEG-1
induced prostate cancer growth, metastasis and enhanced
chemotherapy sensitivity to paclitaxel. Master Thesis. Fujian
Medical University; 2012, (In Chinese).
|
|
31
|
Nikpour M, Emadi-Baygi M, Fischer U, et
al: MTDH/AEG-1 contributes to central features of the neoplastic
phenotype in bladder cancer. Urol Oncol. 32:670–676. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen W, Ke Z, Shi H, Yang S and Wang L:
Overexpression of AEG-1 in renal cell carcinoma and its correlation
with tumor nuclear grade and progression. Neoplasma. 57:522–529.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Erdem H, Oktay M, Yildirim U, Uzunlar AK
and Kayikci MA: Expression of AEG-1 and p53 and their
clinicopathological significance in malignant lesions of renal cell
carcinomas: a microarray study. Pol J Pathol. 64:28–32. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu C, Jin B, Chen L, Zhuo D, Zhang Z, Gong
K and Mao Z: MiR-30d induces apoptosis and is regulated by the
Akt/FOXO pathway in renal cell carcinoma. Cell Signal.
25:1212–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|