Introduction
Locoregional control and treatment outcomes for
primary oral cancers and cervical lymph node metastases have
improved markedly with improvements in imaging diagnosis, advances
in multidisciplinary treatment applying surgical therapy,
radiotherapy and chemotherapy and the development of supportive
therapies for oral cancer treatment (1–3).
However, despite these advances, the primary lesion recurs in
several cases. Therefore, control of the primary lesion is a major
concern for oral surgeons, as recurrent lesions are difficult to
control and markedly compromise the quality of life of the
patients. In surgical therapy for oral cancers, the resection range
for the primary lesion is determined based on the TNM
classification following evaluation of the clinical findings and
images from contrast-enhanced computed tomography (CT),
contrast-enhanced magnetic resonance imaging (MRI), positron
emission tomography-CT and ultrasonography (1). The safety margins of the resected
primary lesion are confirmed during surgery by palpation and from
intraoperative frozen section histological analysis (FS). However,
the resection range varies among operators, the usefulness of FS
has not been verified and the primary lesion recurs in several
cases. As regards the methods used for evaluating the safety
margins of the resected primary lesions, the 2013 guidelines for
the treatment of oral cancer (1)
described vital Lugol staining as being useful for mucosal lesions
in cancer of the tongue. The recurrence rate of the primary lesions
was found to be lower among patients for whom the non-Lugol-stained
region was included in the resection field compared to those for
whom there was no vital Lugol staining in the resected lesions.
Although the examination of all the surgical margins of the
resected primary lesions in FS is difficult and the scope of
evaluation is limited, investigating the presence or absence of
residual tumor tissue in the resected margin appears to be useful.
Although actual methods for FS are not frequently reported, a
survey of the American Head and Neck Society by Meier et al
(4) stated that 76% of their
members collected samples for FS from the surgical bed, 14% from
the resected specimens and the remaining 10% from both sites. There
were no differences in the findings of FS regardless of the
sampling site. Black et al (5) reported the actual condition of FS
from the viewpoint of the pathologists, stating that the evaluation
of the margins was inaccurate, as the anatomical orientation was
not labeled in the resected specimens submitted to pathologists,
which requires cooperation with the surgeons. Another report stated
that FS is inappropriate for routine investigation of the margins
for resected oral cancers other than tongue cancer, as the
anatomical structure is complicated and anatomical limits mean that
surgical access to the tumor site is generally poor (6). However, Wang et al (7) histopathologically examined the
surgical margins of resected tumor specimens in FS using samples
obtained by excisional biopsy and reported that no patient required
additional treatment following surgery. Kurita et al
(8) observed cross-sectional
preparations of resected tumor specimens under a digital light
microscope and reported that evaluation of the deep margin of the
tumor was useful. Therefore, although FS was reported to be useful,
there is yet no established method. To achieve accurate FS, it is
important to share patient information with the pathologists,
indicate the anatomical orientation of the resected tumor specimens
and prepare samples from appropriate sites (9, 10).
The advantages of FS using samples collected from resected tumor
specimens are as follows: The anatomical orientation is readily
determined; the distance between the surgical margin and tumor is
macroscopically observed in the cross-sectional surface of the
resected specimen; reliable sampling from an appropriate region is
possible, as the anatomical orientation is readily determined; and
the anatomical position of additional tumor resection is accurately
reflected in the surgical field when the surgical margin is either
close to the tumor or positive (9,
10). Based on these advantages,
we collected samples from resected tumor specimens for FS.
To evaluate the usefulness of our FS system in the
control of primary lesions, using methods such as intraoperative
vital Lugol staining and FS of surgical specimens, the outcomes of
treatment for oral squamous cell carcinoma (OSCC) were
retrospectively investigated in patients treated prior to and after
the introduction of this FS method to Kagoshima University.
Materials and methods
Patient eligibility criteria
The subjects comprised 153 patients with OSCC who
underwent radical surgery at the Department of Oral and
Maxillofacial Surgery at Kagoshima University between January, 2000
and September, 2011. The patients were divided according to whether
they underwent surgery prior to or after adopting FS for the
control of primary lesions in October, 2005 as follows: Group 1 (52
patients), treated between January, 2001 and September, 2005; and
Group 2 (101 patients), treated from October, 2005 onwards. The
preservation of the morphological characteristics of the oral
cavity and functions such as mastication, swallowing, speech and
esthetics is crucial in the treatment of advanced OSCC (11). Several studies have reported the
effect of preoperative chemoradiotherapy plus radical surgery for
advanced squamous cell carcinoma of the oral cavity (11–14).
As a result, surgery was performed as the main treatment and
chemoradiotherapy was performed as preoperative treatment
throughout this period. Surgery comprised en bloc resection of the
primary site, with neck dissection in N1 or more advanced cases.
Chemoradiotherapy included external beam radiotherapy with a total
radiation dose of 30–40 Gy delivered in 10–20 fractions and
concurrent chemotherapy using either platinum-containing agents,
such as cisplatin or carboplatin, 5-fluorouracil, or oral S-1. The
clinical characteristics of the patients are summarized in Table I. There were no significant
differences according to gender, age, primary site, or distribution
of T or stage classification between the groups. However, more
patients were treated with surgery alone in Group 2 compared to
Group 1, as Group 1 included a higher number of advanced cases. The
duration of the follow-up ranged from 1 year to 10 years and 8
months (median, 2 years and 8 months).
 | Table IClinical characteristics of
patients. |
Table I
Clinical characteristics of
patients.
| Group 1, no. (%) | Group 2, no. (%) | Total patient no.
(%) |
|---|
| Characteristics | (n=52) | (n=101) | (n=153) |
|---|
| Gender |
| Male | 32 (38.5) | 60 (40.6) | 92 (60.1) |
|
Female | 20 (61.5) | 41 (59.4) | 61 (39.9) |
| Age (years) |
|
<60 | 13 (25.0) | 33 (32.7) | 46 (30.0) |
| ≥61 | 39 (75.0) | 68 (67.3) | 107 (70.0) |
| Primary site |
| Upper
gingiva | 6 (11.5) | 10 (9.9) | 16 (10.5) |
|
Tongue | 23 (44.2) | 52 (51.5) | 75 (49.0) |
| Lower
gingiva | 16 (30.8) | 30 (29.7) | 46 (30.0) |
|
Other | 7 (13.5) | 9 (8.9) | 16 (10.5) |
| Clinical T
classification |
| T1/2 | 37 (71.2) | 83 (82.2) | 120 (78.4) |
| T3/4 | 15 (28.8) | 18 (17.8) | 33 (21.6) |
| Stage |
| I | 10 (19.2) | 18 (17.8) | 28 (18.3) |
| II | 12 (23.1) | 40 (39.6) | 52 (34.0) |
| III | 19 (36.5) | 24 (23.8) | 43 (28.1) |
| IV | 11 (21.2) | 19 (18.8) | 30 (19.6) |
| Treatment |
| S | 8 (15.4) | 54 (53.4) | 62 (40.5) |
| R→S | 21 (40.4) | 5 (5.0) | 26 (17.0) |
|
R+C→S | 23 (44.2) | 42 (41.6) | 65 (42.5) |
This study was approved by the Ethics Committee of
Kagoshima University and written informed consent was obtained from
all the included patients.
FS
To ensure reliable surgical margins, we have been
performing FS for the control of primary lesions since October,
2005 as follows: First, the orientation of the anatomical extent is
determined by oral pathologists and oral surgeons based on images
obtained by contrast-enhanced CT and MRI and pathological pictures
and the planned sampling site for FS is confirmed. Second, a tumor
team is organized and marks the tumor area, setting a reliable 1-cm
resection range from the mark to correct the setting errors of the
resection range by the operators. Third, only the presence or
absence of tumor in tissues collected from the surgical bed of the
tumor resection site is investigated in FS, but vital Lugol
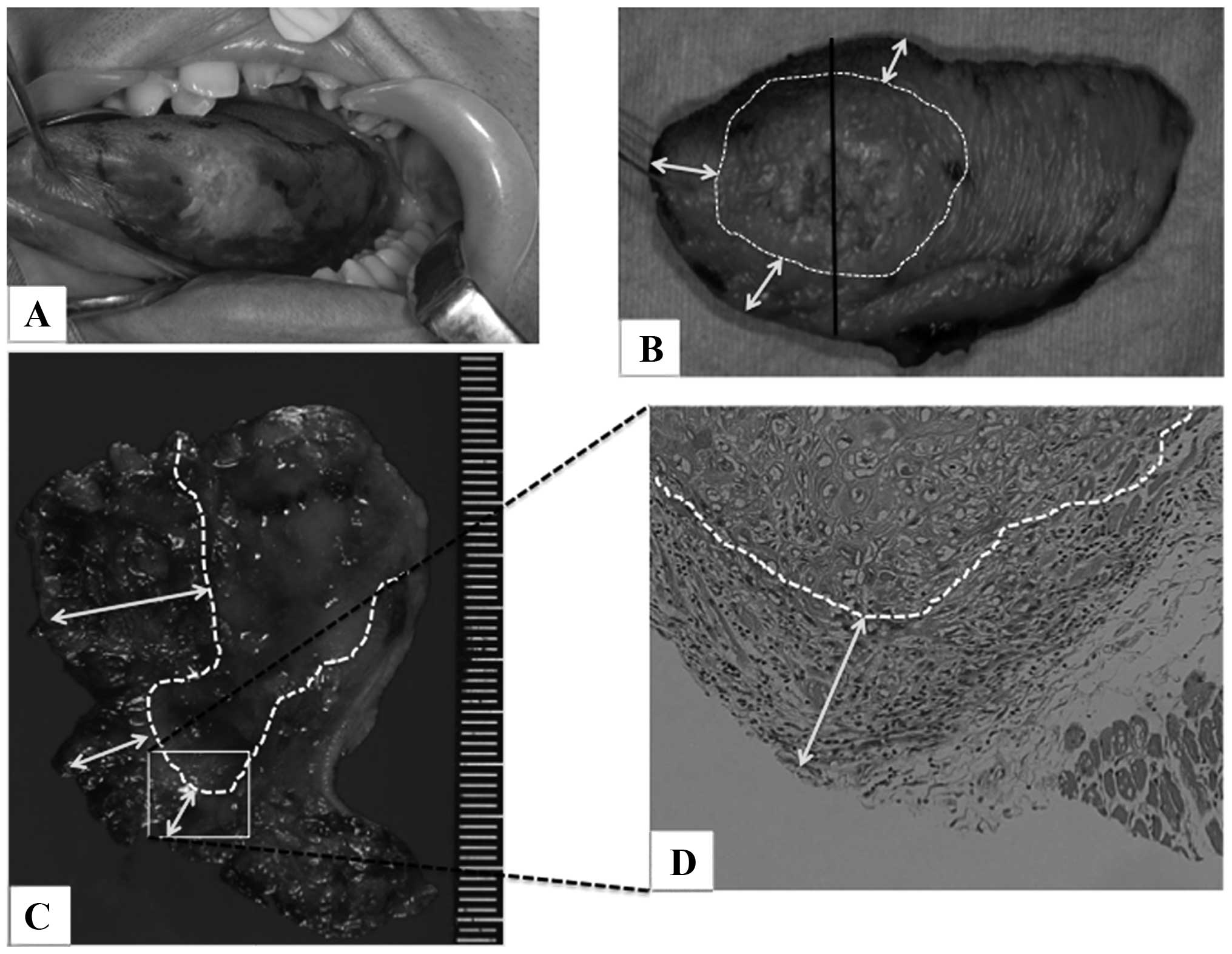
staining is applied (Fig. 1A) and
the surgical margin is set based on the non-stained region. The
distance from the tumor is macroscopically confirmed in the maximum
cross-sectional surface of the resected specimen by oral surgeons
and pathologists (Fig. 1B and C,
white arrows). Finally, FS is performed using a sample collected
from the resected specimen to confirm the mucoepithelium and safety
margin of the deep stump (Fig.
1D).
Items analyzed in the two groups
First, the rates of positive surgical margins,
recurrence of the primary lesion and disease-specific survival were
compared. Second, the clinicopathological factors associated with
recurrence of primary lesions were analyzed. The investigated
clinicopathological factors included age, gender, tumor location, T
classification, tumor properties, grade of differentiation,
invasion pattern, presence or absence of lymphatic, vascular, or
nerve invasion, condition of the surgical margins and histological
therapeutic effect. The patients were divided by age into those
aged ≥61 and those <60 years, by T classification into T2 or
lower and T3 or more advanced cases, by grade of differentiation
into moderately or poorly differentiated and well-differentiated
cases and by condition of the surgical margins into cases with
residual tumor (positive margins), without residual tumor but ≤3 mm
from the tumor, or without residual tumor and >3 mm from the
tumor (negative margins). The invasion pattern was classified as
YK3 or lower and YK4C or more advanced, according to the
classification reported by Yamamoto et al (15). As regards the histological
therapeutic effect, recurrence of the primary lesion was evaluated
in patients who received preoperative therapy by dividing them into
cases with Gr2a or lower and Gr2b or higher effects, according to
the classification reported by Shimosato et al (16). Third, disease-specific survival
rates were compared between the groups according to the condition
of the surgical margins. Finally, the primary site, condition of
the surgical margin, time of recurrence and prognosis were analyzed
in cases with recurrence of the primary lesion in Groups 1 and
2.
Statistical analysis
Statistical analysis was performed using
JMP® statistical analysis software, version 9 (SAS
Institute, Tokyo, Japan). The associations between recurrence rate
and clinicopathological factors were analyzed using the Pearson's
χ2 test. The survival rates were calculated using the
Kaplan-Meier method and analyzed using the log-rank test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Comparison of surgical margin
positivity, primary lesion recurrence and disease-specific survival
by the Kaplan-Meier method
The surgical margin positivity rates were 9.6 and
3.9% in Groups 1 and 2, respectively, with a decreasing tendency,
although the difference was not statistically significant (Table II). The recurrence rate for
primary lesions was high (17.3%, 9/52) in Group 1, but improved
significantly to 6.9% (7/101) in Group 2 (Table II). Disease-specific survival
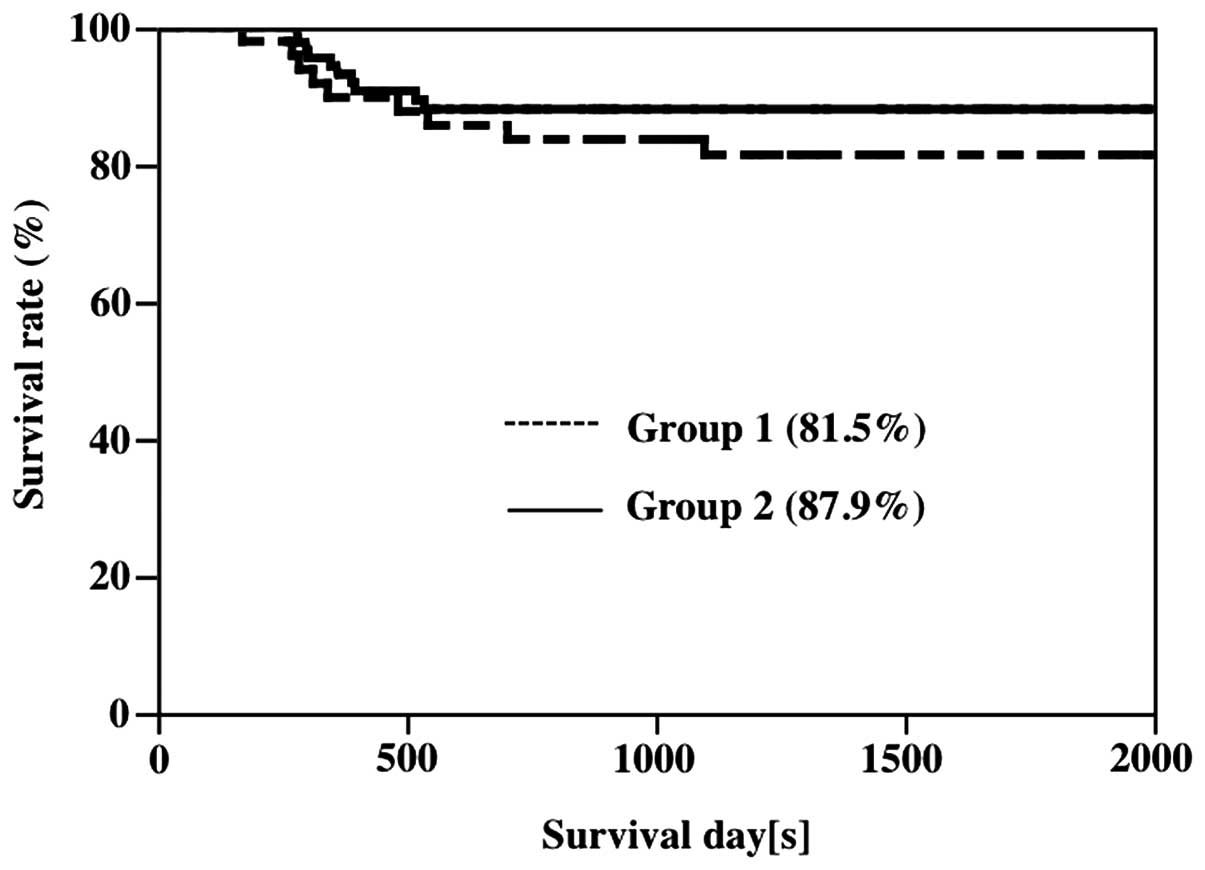
rates were 81.5 and 87.9% in Groups 1 and 2, respectively, showing
a slight but non-significant tendency toward improvement (Fig. 2).
 | Table IIRates of negative surgical margins and
recurrence at primary site in Groups 1 and 2. |
Table II
Rates of negative surgical margins and
recurrence at primary site in Groups 1 and 2.
| Variables | Group 1 | Group 2 | P-value |
|---|
| Margins |
|
Positive | 47 | 97 | |
| Negative
(%) | 5 (9.6) | 4 (3.9) | 0.16 |
| Recurrence |
| No | 43 | 94 | |
| Yes
(%) | 9 (17.3) | 7 (6.9) | 0.047a |
Clinicopathological factors associated
with recurrence of the primary lesions
The Pearson's χ2 test was performed
regarding the presence or absence of recurrence of the primary
lesion as a response variable and gender, age, location, T
classification, tumor properties, grade of differentiation,
invasion pattern, presence or absence of lymphatic, vascular, or
nerve invasions, condition of the surgical margins and histological
therapeutic effect as explanatory variables. In Group 1, factors
associated with recurrence of the primary lesion were the presence
or absence of nerve invasion and the condition of the surgical
margins; recurrence rate was found to be significantly higher among
cases with surgical margins close to the tumor or residual tumor in
the surgical margins (positive margins). In Group 2, none of the
explanatory factors were significantly associated with the presence
or absence of recurrence of the primary lesion. Regarding the
association between primary site and recurrence of the primary
lesion, primary lesions in the upper and lower gingiva frequently
recurred in both groups, but the incidence decreased in Group 2 and
cancer of the tongue recurred in only 1 patient (Table III).
 | Table IIIClinicopathological factors associated
with recurrence at primary site. |
Table III
Clinicopathological factors associated
with recurrence at primary site.
| Group 1 | Group 2 |
|---|
|
|
|
|---|
| | Recurrence | | | Recurrence | |
|---|
| Variables | No recurrence | no. (%) | P-value | No recurrence | no. (%) | P-value |
|---|
| Gender |
|
Male | 28 | 4 | | 57 | 4 | |
|
Female | 15 | 5 | 0.25 | 37 | 3 | 0.36 |
| Age (years) |
|
≥61 | 12 | 1 | | 32 | 1 | |
|
<60 | 31 | 8 | 0.29 | 62 | 6 | 0.28 |
| Primary site |
| Upper
gingiva | 4 | 2 (33.3) | | 8 | 2 (20.0) | |
|
Tongue | 20 | 3 (13.0) | | 51 | 1 (1.9) | |
| Lower
gingiva | 13 | 3 (18.8) | | 26 | 4 (13.3) | |
|
Other | 6 | 1 | 0.56 | 9 | 0 | 0.12 |
| Clinical T
classification |
|
T1/2 | 32 | 5 | | 78 | 5 | |
|
T3/4 | 11 | 4 | 0.26 | 16 | 2 | 0.44 |
| Pattern of tumor
growth |
|
Superficial spreading | 6 | 0 | | 22 | 3 | |
|
Outgrowing | 2 | 0 | | 24 | 1 | |
|
Ingrowing | 35 | 9 | 0.37 | 48 | 3 | 0.49 |
|
Differentiation |
|
Moderate/poor | 29 | 7 | | 81 | 7 | |
|
High | 14 | 2 | 0.54 | 13 | 0 | 0.29 |
| Mode of
invasionb |
|
≤YK3 | 36 | 6 | | 76 | 4 | |
|
YK4C/4D | 7 | 3 | 0.24 | 18 | 3 | 0.16 |
| Lymphatic
invasion |
|
Negative | 39 | 7 | | 82 | 6 | |
|
Positive | 4 | 2 | 0.27 | 11 | 1 | 0.85 |
| Vascular
invasion |
|
Negative | 37 | 7 | | 76 | 6 | |
|
Positive | 6 | 2 | 0.53 | 17 | 1 | 0.79 |
| Nerve invasion |
|
Negative | 42 | 7 | | 86 | 7 | |
|
Positive | 1 | 2 | 0.02a | 7 | 0 | 0.45 |
| Surgical
margin |
|
Negative | 32 | 3 | | 73 | 4 | |
| Close
(<3 mm) | 9 | 3 | | 17 | 3 | |
|
Positive | 2 | 3 | 0.01a | 4 | 0 | 0.21 |
| Chemoradiation
effectc |
|
≤Gr2a | 11 | 5 | | 12 | 2 | |
|
≥Gr2b | 22 | 3 | 0.13 | 30 | 1 | 0.17 |
Disease-specific survival rate by
condition of the surgical margins in Groups 1 and 2
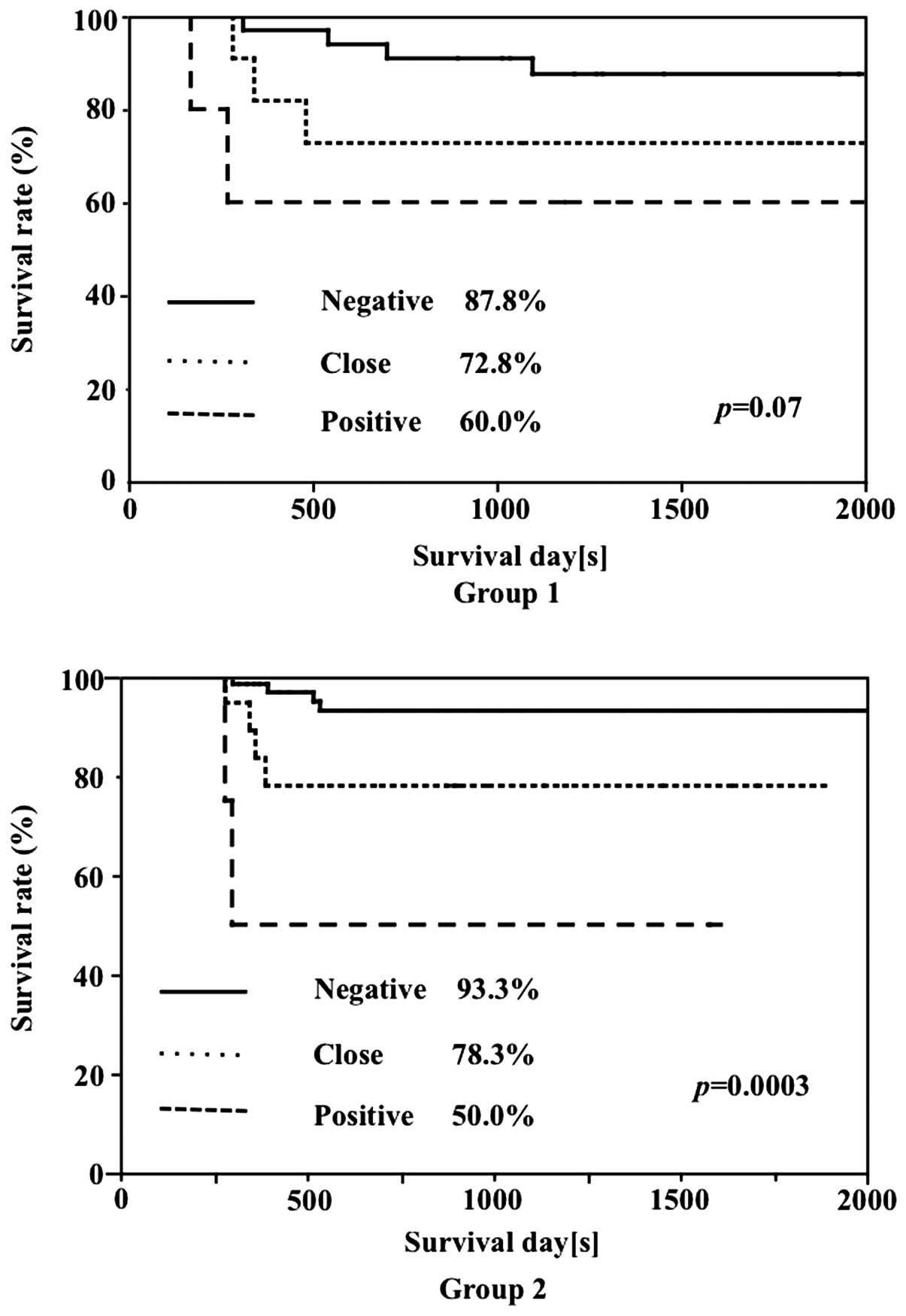
In Group 1, the survival rate was 87.8% in cases
with negative surgical margins, 72.8% in cases with margins close
to the tumor and 60.0% in cases with positive margins. In Group 2,
the survival rates of cases with negative margins and cases with
margins close to the tumor were 93.3 and 78.3%, respectively,
exhibiting a tendency toward higher rates compared to those in
Group 1, although the differences were not significant. The
disease-specific survival rate in positive-margin cases was 50.0%,
which was lower compared to that in Group 1. Significant
differences according to the condition of the surgical margins were
noted in the survival rates of both groups (Fig. 3).
Patients with recurrence of primary
lesions in Groups 1 and 2 and outcome
In Group 1, the primary tumors recurred in 9 of the
52 patients (17.3%). By primary site, recurrence occurred in the
upper gingiva in 2 patients, tongue in 3, lower gingiva in 3 and
buccal mucosa in 1 patient. The T classification varied between T1
and T4 and the surgical margins were negative, close to the tumor
and positive in 3 patients each. The recurrence site was the
tongue, gingiva, buccal mucosa and retromolar mucosa around the
primary site in 6 patients and the tumor advanced into the skin and
recurred in 3 patients. The time to recurrence was between 1 and 3
months in cases with positive margins, after 5 months in 2 cases
with close margins and significantly later in cases with negative
margins (range, 1 year and 5 months to 3 years and 11 months).
The treatment comprised tumor resection or
chemotherapy in 8 patients and 5 patients (62.5%) survived, but the
outcomes were poor and 4 patients (37.5%) succumbed to the primary
tumor.
In Group 2, the primary lesions recurred in 7 of the
101 patients (6.9%). The primary site was located in the upper and
lower gingiva in 6 cases and in the tongue in 1 case. The T
classification was late T2 or more advanced and the surgical
margins were negative in 4 and close to the tumor in 3 cases;
however, no positive cases were recorded. The site of recurrence
was the tongue, gingiva and buccal mucosa around the primary lesion
in 4 patients and the skin in 3 patients. The time to tumor
recurrence was 4–7 months in cases with close margins, >1 year
in 2 cases with negative margins, but only 3 months after surgery
in 1 case with negative margins. The treatment comprised
radiotherapy or resection in 6 patients, of whom 3 (50%) survived
and 3 succumbed to the primary lesion. One patient with lower
gingival cancer was untreatable and eventually succumbed to the
disease. The characteristics of the cases with recurrence of the
primary tumor are summarized in Table
IV.
 | Table IVCases of recurrence at primary site
and prognosis. |
Table IV
Cases of recurrence at primary site
and prognosis.
| Age | | Primary | TN | Surgical | Site of | Time to | Salvage | |
|---|
| Case | (years) | Gender | site | stage | margins | recurrence | recurrence | treatment | Outcome |
|---|
| Group 1 |
| 1 | 52 |
Female | Upper gingiva | T2N1 | Close | Skin | 3y 2m | Excision | Alive |
| 2 | 63 |
Male | Upper gingiva | T3N0 | Close | Buccal mucosa | 5m | Excision | Alive |
| 3 | 70 |
Female | Tongue | T1N0 | Negative | Tongue | 3y 11m | Excision | Alive |
| 4 | 67 |
Male | Tongue | T2N0 | Negative | Tongue | 1y 5m | Excision | Deceased |
| 5 | 71 |
Male | Tongue | T2N1 | Negative | Skin | 3y | Excision | Alive |
| 6 | 62 |
Male | Lower gingiva | T4N2b | Positive | Retromolar | 3m | Chemotherapy | Alive |
| 7 | 68 |
Female | Lower gingiva | T2N0 | Positive | Skin | 1m | - | Deceased |
| 8 | 86 |
Female | Lower gingiva | T2N1 | Close | Gingiva | 5m | Chemotherapy | Deceased |
| 9 | 84 |
Female | Buccal mucosa | T3N0 | Positive | Buccal mucosa | 1m | Excision | Deceased |
| Group 2 |
| 10 | 66 |
Male | Upper gingiva | T2N2b | Negative | Buccal mucosa | 1y | Radiotherapy | Alive |
| 11 | 84 |
Female | Upper gingiva | T3N0 | Close | Skin | 7m | Excision | Deceased |
| 12 | 81 |
Female | Tongue | T4N0 | Negative | Tongue | 1y | Radiotherapy | Deceased |
| 13 | 72 |
Male | Lower gingiva | T4N1 | Close | Skin | 4m | Excision | Deceased |
| 14 | 81 |
Female | Lower gingiva | T2N0 | Negative | Skin | 3m | Excision | Alive |
| 15 | 84 |
Female | Lower gingiva | T4N0 | Close | Gingiva | 5m | - | Deceased |
| 16 | 60 |
Female | Lower gingiva | T2N0 | Negative | Gingiva | 1y 9m | Excision | Alive |
Discussion
The major clinical factor determining the prognosis
of patients with OSCC is cervical lymph node metastasis, whereas
the depth and pattern of invasion are important factors associated
with recurrence of the primary lesion and lymph node metastasis
(1). In addition to the depth and
invasion pattern of the tumor, the presence or absence of tumor
cells in the surgical margins is crucial for the surgical treatment
of OSCC (17, 18). Setting a safety margin ≥10 mm is
considered as appropriate for the resection of oral cancers,
although a clear basis for this distance is currently lacking
(19). We have attempted to
control primary lesions by following this criterion (10-mm safety
margin), confirming that the region remains unstained on vital
Lugol staining during surgery and including this region in the
resection field, confirming the macroscopic tumor extent in the
cross-sectional surface of the resected specimen and performing FS
for a sample collected from the resected specimen. Although the
disease-specific survival rate was not significantly affected, the
rate of positive surgical margins was decreased. The rate of
primary lesion recurrence was high (17.3%, 9/52) in Group 1, but
improved significantly to 6.9% (7/101) in Group 2. Among the
clinicopathological factors, the condition of the surgical margins
and the presence or absence of nerve invasion were associated with
recurrence of the primary lesion in Group 1, but no significant
association between the surgical margin status and recurrence of
the primary lesion was observed in Group 2. However, the prognosis
of patients with positive margins was poor in both groups and,
although the incidence of recurrent cancer of the tongue tended to
decrease, upper and lower gingival cancers recurred in a number of
patients, reflecting the limitations to our approach for the
control of primary lesions.
The number of studies reporting the recurrence rate
of primary lesions in detail is limited. Although the rates vary
depending on the primary site, Yamamoto et al (18) reported a rate of 10.3% in patients
with T1/2 cancer of the tongue, whereas that of oral cancers of
other regions, including the tongue, was reported to be 9–18% by
other studies (18, 20–22).
Although a simple comparison with these reports is not feasible due
to the differences in patient background and treatment strategy,
the rate of primary lesion recurrence was 17.3% in Group 1, which
was similar to the previously reported rates, and decreased to 6.9%
in Group 2, which was lower compared to the rates reported
elsewhere. In addition, among the clinicopathological factors, the
condition of the surgical margins and nerve invasion were
associated with recurrence of the primary lesion in Group 1, while
no significant correlation was noted between surgical margin status
and recurrence of the primary lesion in Group 2. Surgical margin
positivity represents a significant factor associated with
decreased survival rate and a high risk of postoperative recurrence
(1, 22). The condition of the surgical
margins was significantly associated with survival rate in both
groups (Fig. 3), suggesting that
our approach for the control of primary lesions contributes to
decreasing the risk of recurrence and our FS method appears to be
useful for the evaluation of the surgical margins. However, the
survival rate did not significantly improve in Group 2 compared to
that in Group 1, although a tendency towards an increase was
observed. The poor prognosis of patients with cervical lymph node
metastasis, including secondary cervical lymph node metastasis in
Group 2 (data not shown), may have affected our results.
The recurrence rate of the primary lesions varies
depending on the primary site. The oral cavity has a complex
structure, comprising mixed hard and soft tissues and the invasion
pattern varies depending on the direction of tumor advancement.
Such factors may contribute to the difficulties in the
determination of the resection range with adequate safety margins
(1). Recurrence of the primary
lesion was frequently noted in the upper and lower gingiva in both
groups. This tendency persisted in Group 2, but the incidence was
decreased in all the primary sites. As regards cancer of the
tongue, a low rate of primary lesion recurrence (3.8%) has been
reported (15). In our patients
with cancer of the tongue, the rate of primary site recurrence was
13.0% in Group 1, but decreased to 1.9% in Group 2. In Group 2,
recurrence occurred in the upper and lower gingiva in 2 and 4
patients, respectively (Table
IV), but recurrence in the tongue occurred in only 1 case. The
advances in imaging diagnosis may also be a decisive factor when
determining the resection range, but the advantages of our FS
method (i.e., the cross-sectional surface of tumors is readily
observed macroscopically, the distance between the surgical margin
and tumor is readily determined and the anatomical orientation is
readily identified) is evident in tissues retaining anatomical
continuity, such as the tongue, which may facilitate determining a
reliable resection range for cancer of the tongue. In Group 2,
although recurrence was negative on intraoperative rapid
pathological diagnosis, upper and lower gingival cancers recurred
in the surrounding tissue relatively early after surgery (3–7
months) in 4 of the 6 patients. These cases reflect the limitations
of our FS method in assisting with determining a reliable tumor
resection range, in addition to the difficulties involved in
imaging diagnosis of tumors located in regions with a complex
anatomical structure, such as advanced upper and lower gingival
cancers containing hard as well as soft tissues. The prognosis for
cases with recurrence is very poor (23, 24). To determine the resection range for
the primary lesion in such cases, further improvements are required
in the imaging evaluation of jaw bone infiltration, tumor invasion
pattern and infiltration into the surrounding soft tissues in
consideration of the direction of tumor advancement (25).
In conclusion, our FS method appears to be useful
for resecting tumors with reliable safety margins for tissues
retaining anatomical continuity, such as the tongue. The
macroscopic observation of cross-sections of the resected tumor
specimens is easy and the surgical margins may be readily
investigated. However, this method is insufficient for determining
a resection range in tissues containing soft tissue and jaw bone,
such as upper and lower gingival tumors, and other methods to
control primary lesions must be investigated.
Acknowledgements
The authors would like to thank the members of the
Department of Oral and Maxillofacial Surgery, Field of Oral and
Maxillofacial Rehabilitation, Advanced Therapeutics Course,
Graduate School of Medical and Dental Sciences, Kagoshima
University, for their assistance with additional data
collection.
References
|
1
|
Joint Committee of Guidelines for
Treatment of Oral Cancers Working Group of the Japan Society for
Oral Tumors and Oral Cancer Clinical Practice Guidelines
Development Committee of the Japan Society of Oral and
Maxillofacial Surgeons. 2013 evidence-based oral cancer clinical
practice guidelines. Kanahara & Co.; Tokyo: pp. 37–39. pp.
65–87. pp. 85pp. 912013, (In Japanese).
|
|
2
|
Pfister DG, Ang KK, Brizel DM, et al
National Comprehensive Cancer Network: Head and neck cancers,
version 2.2013. Featured update to the NCCN guidelines. J Natl
Compr Canc Netw. 11:917–923. 2013.PubMed/NCBI
|
|
3
|
Balasundaram I, AI-Hadad I and Parmar S:
Recent advances in reconstructive oral and maxillofacial surgery.
Br J Oral Maxillofac Surg. 50:695–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meier JD, Oliver DA and Varvares MA:
Surgical margin determination in head and neck oncology: current
clinical practice. The results of an International American Head
and Neck Society Member Survey. Head Neck. 27:952–958. 2005.
View Article : Google Scholar
|
|
5
|
Black C, Marotti J, Zarovnaya E, et al:
Critical evaluation of frozen section margins in head and neck
cancer resections. Cancer. 107:2792–2800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gerber S, Gengler C, Gratz KW, et al: The
impact of frozen sections on final surgical margins in squamous
cell carcinoma of the oral cavity and lips: a retrospective
analysis over an 11 years period. Head Neck Oncol.
3(56)2011.PubMed/NCBI
|
|
7
|
Wang YC, Fang KH, Jung SM, et al:
Excisional biopsy with margin control for oral cancers. Head Neck.
32:1528–1533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurita H, Uehara S, Funamoto S, et al:
Intraoperative digital microscopic assessment of the deep surgical
margins in oral carcinoma survey: a preliminary report. Am J Surg.
191:84–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gauthier P, Audet N, Guertin L, et al:
Complete frozen section margins (with measurable 1 or 5 mm thick
free margin) for cancer of the tongue: part 2: clinical experience.
J Otolaryngol Head Neck Surg. 39:20–27. 2010.
|
|
10
|
Hinni ML, Zarka MA and Hoxworth JM: Margin
mapping in transoral surgery for head and neck cancer.
Laryngoscope. 123:1190–1198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyawaki A, Ikeda R, Hijioka H, et al:
SUVmax of FDG-PET correlates with the effects of neoadjuvant
chemoradiotherapy for oral squamous cell carcinoma. Oncol Rep.
23:1205–1212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyawaki A, Hijioka H, Ikeda R, et al:
Analysis of the outcome of concurrent neoadjuvant chemoradiotherapy
with S-1 compared to super-selective intra-arterial infusion for
oral squamous cell carcinoma. Oncol Lett. 3:995–1001. 2012.
|
|
13
|
Kirita T, Ohgi K, Shimooka H, et al:
Preoperative concurrent chemoradiotherapy plus radical surgery for
advanced squamous cell carcinoma of the oral cavity: an analysis of
long-term results. Oral Oncol. 35:597–606. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohr C, Bohndorf W, Carstens J, et al:
Preoperative radiochemotherapy and radical surgery in comparison
with radical surgery alone. A prospective, multicentric, randomized
DOSAK study of advanced squamous cell carcinoma of the oral cavity
and the oropharynx (a 3-year follow up). Int J Oral Maxillofac
Surg. 23:140–148. 1994.
|
|
15
|
Yamamoto E, Kohama G, Sunakawa H, et al:
Mode of invasion, bleomycine sensitivity, and clinical course in
squamous cell carcinoma of the oral cavity. Cancer. 51:2175–2180.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimosato Y, Oboshi S and Baba K:
Histological evaluation of effects of radiotherapy and chemotherapy
for carcinoma. J Clin Oncol. 1:19–35. 1971.
|
|
17
|
Ling W, Mijiti A and Moming A: Survival
pattern and prognostic factors of patients with squamous cell
carcinoma of the tongue: a retrospective analysis of 210 cases. J
Oral Maxillofac Surg. 71:775–785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamamoto S, Yamada S, Takahasi S, et al:
Clinicopathological risk factors for local recurrence in oral
squamous carcinoma. Int J Oral Maxillofac Surg. 41:1195–1200. 2012.
View Article : Google Scholar
|
|
19
|
Nason RW, Binahmed A, Pathak KA, et al:
What is the adequate margin of surgical resection in oral cancer?
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 107:625–629.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yanamoto S, Yamada S, Takahashi H, et al:
Predictors of locoregional recurrence in T1-2N0 tongue cancer
patients. Pathol Oncol Res. 19:795–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Po Wing Yuen A, Lam KY, Lam LK, et al:
Prognostic factors of clinically stage I and II oral tongue
carcinoma - A comparative study of stage, thickness, shape, growth
pattern, invasive front malignancy grading, Martinez-Gimeno score,
and pathologic features. Head Neck. 24:513–520. 2002.
|
|
22
|
Woolgar JA, Rogers S, West CR, et al:
Survival and patterns of recurrence in 200 oral cancer patients
treated by radical surgery and neck dissection. Oral Oncol.
35:257–265. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jones AS, Bin Hanafi Z, Nadapalan V, et
al: Do positive resection margins after ablative surgery for head
and neck cancer adversely affect prognosis? A study of 352 patients
with recurrent carcinoma following radiotherapy treated by salvage
surgery. Br J Cancer. 74:128–132. 1996. View Article : Google Scholar
|
|
24
|
Kemohan MD, Clark JR, Gao K, et al:
Predicting the prognosis of oral squamous cell carcinoma after
first recurrence. Arch Otolaryngol Head Neck Surg. 136:1235–1239.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feichtinger M, Pau M, Zemann W, et al:
Intraoperative control of resection margins in advanced head and
neck cancer using a 3D-navigation system based on PET/CT image
fusion. J Craniomaxillofac Surg. 38:589–594. 2010. View Article : Google Scholar : PubMed/NCBI
|