Introduction
Approximately 30% of patients with renal cell
carcinoma (RCC) exhibit distant metastasis at initial presentation,
whereas a further 30% of the patients develop metastases following
nephrectomy (Nx) (1). RCC patients
develop metastases at various sites, including the lungs, lymph
nodes, bone, brain and liver and their prognosis depends on the
metastatic site. The frequency of liver metastasis (LM) in RCC
patients is lower compared to that of metastasis to other sites,
such as the lungs, lymph nodes and bone. The rate of LM was
reported to be 9.3–18% (2–5). The prognosis of RCC patients with LM
is poor and the median overall survival is 7.6–12 months, which is
shorter compared to that of patients with metastasis to other sites
(2–4). Patients with metastatic RCC were
treated with interferon and/or interleukin-2 during the cytokine
era; however, the response rate to cytokine therapy was reportedly
only 10–20% (4). Cytokine therapy
was occasionally effective for lung or lymph node metastases;
however, it was generally not effective for liver, brain and bone
metastases. Local treatments are reportedly effective in certain
RCC patients with bone and brain metastases (6, 7).
Combination therapy with radiation and zoledronic acid was shown to
decrease the rate of skeletal-related events in RCC patients;
reossification was also reported in some patients (8). γ-knife surgery achieves good local
control of brain metastasis from RCC. This procedure improves
peritumoral edema and the survival rate of patients with multiple
brain metastases (6, 9). A definitive treatment for LM in RCC
patients, however, has not been established. At present, tyrosine
kinase inhibitors (TKIs) are used to treat metastatic RCC and the
response is expected to be adequate when TKIs are used for organ
metastases such as LM and brain metastases, which are considered to
be extremely refractory to cytokine therapy (10, 11). LM from RCC may grow rapidly and
become life-threatening. Local treatments for LM may be beneficial
for RCC patients. Long-term survival following surgical resection
of a solitary LM was reported in RCC patients (12, 13), although the efficacy of local
treatments, such as surgical resection, radiofrequency ablation and
transarterial embolization, was not fully evaluated. To the best of
our knowledge, the number of studies focusing on the treatment of
LM from RCC is currently limited.
In the present study, we evaluated the clinical
characteristics, prognosis and prognostic factors in RCC patients
with LM. We also aimed to determine the characteristics of RCC
patients with LM who survived over a relatively long period, with
particular focus on the clinical results of local treatments for
RCC with LM.
Materials and methods
Patients and design
We retrospectively reviewed the records of all the
patients who underwent radical nephrectomy (RNx) or partial Nx for
RCC between November, 1980 and April, 2013 at the National Defense
Medical College, Tokorozawa, Saitama, Japan. Our cohort included 25
patients (21 men and 4 women; age at Nx, 59.4±12.4 years; range,
30–77 years) with LM at initial presentation or who developed LM
following Nx. The clinicopathological factors were assessed for
each patient using the patient database or clinical records. The
factors evaluated included gender, age, treatment after LM
presentation, Eastern Cooperative Oncology Group performance status
(ECOG PS), histological characteristics, tumor grade, Fuhrman
grade, microvascular invasion, histological tumor necrosis,
sarcomatoid differentiation and biochemical parameters, such as
hemoglobin level, platelet count, lactate dehydrogenase level,
corrected calcium (Ca) level and C-reactive protein (CRP) level.
All 25 patients underwent RNx. Local recurrence and metastases were
monitored by postoperative examination of each patient every 3–6
months for the first 5 years and every 6–12 months thereafter. The
follow-up included physical examination, laboratory tests, chest
radiography, abdominal and chest computed tomography (CT) and, if
indicated, radionuclide bone scanning. LM was confirmed using CT or
magnetic resonance imaging in all the patients. The median
follow-up duration was 28.9 months (range, 2.7–180.1 months).
Cancer-specific survival (CSS) was calculated from the date of Nx
to the date of death or the date of the last follow-up. Tumor
staging was performed according to the 2009 TNM classification of
the Union for International Cancer Control (14). Tumor grades were assigned according
to the General Rules for Clinical and Pathological Studies on Renal
Cell Carcinoma in Japan (3-grade system) (15). Furmann nucleolar grading was also
performed (16). This study was
approved by the Ethics Committee of the National Defense Medical
College (no. e-253). Consent was obtained for use of patient
data.
Statistical analysis
All the calculations were performed using JMP 9.0
software for Windows (SAS Institute Inc., Cary, NC, USA). The
results are expressed as means ± standard deviation. The CSS rates
were calculated using the Kaplan-Meier method and compared using
the log-rank test. P<0.05 was considered to indicate a
statistically significant difference. The associations of
clinicopathological parameters with death from RCC were assessed
using the Cox proportional hazards regression model and summarized
as hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
Patient characteristics
The clinicopathological characteristics of the
patients are listed in Table I.
The histopathological types were clear cell RCC in 24 cases and
chromophobe RCC in 1 case. A total of 21 patients (84%) had
histological grade 3 disease, while 24 (96%) had grade ≥3 disease
according to the Fuhrman classification. The mean age at LM
diagnosis was 62.6±12.1 years (range, 30–79 years). The interval
from Nx to LM appearance was 38.1±55.9 months (range, 0–175.3
months) and the follow-up period between LM appearance and outcome
was 16.6±17.6 months (range, 0.4–60.2 months). LM was present at
the time of Nx in 8 and developed following Nx in 17 patients. The
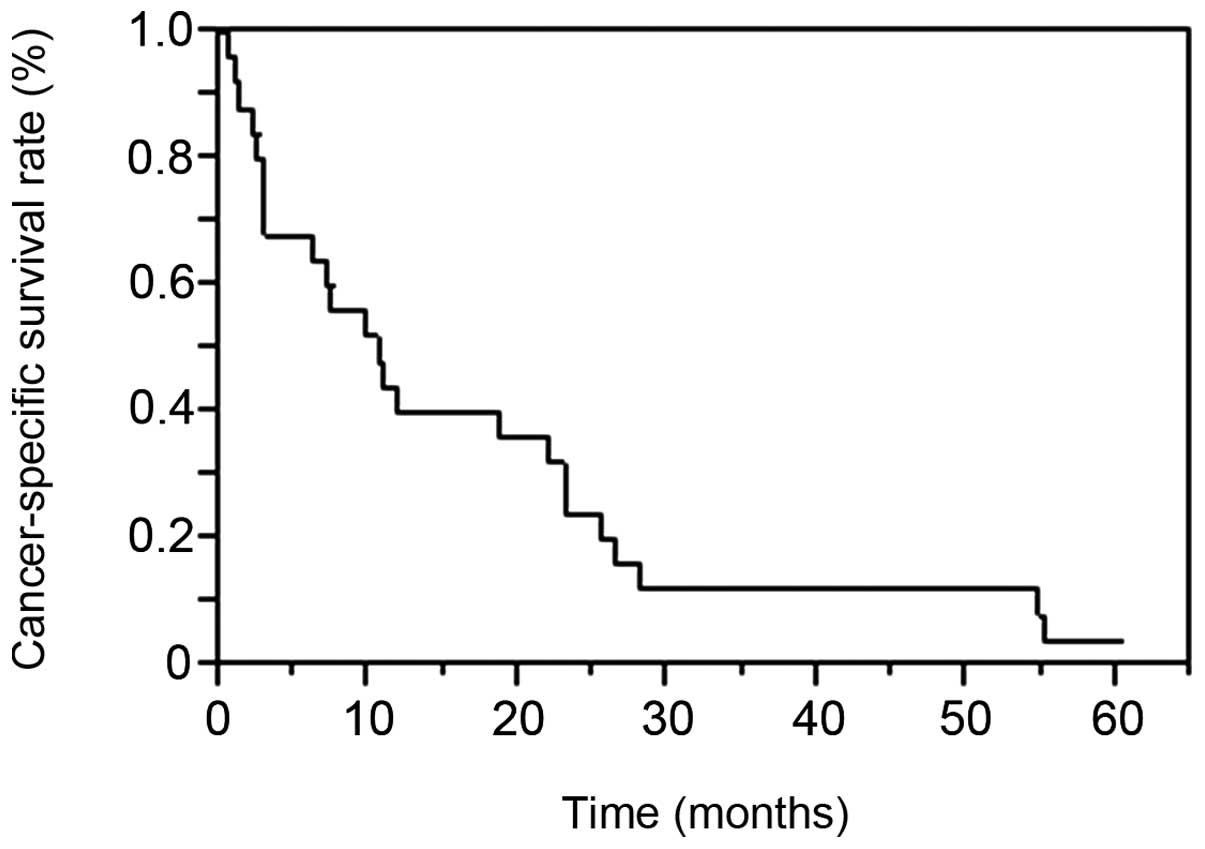
median CSS following LM diagnosis was 10.6 months. The patient
survival rates at 1, 2 and 3 years were 40, 24 and 12%,
respectively (Fig. 1).
 | Table IClinicopathological characteristics of
25 patients with liver metastasis (LM). |
Table I
Clinicopathological characteristics of
25 patients with liver metastasis (LM).
| Variables | Values, mean ±
SD |
|---|
| Gender | |
| Male | 21 |
|
Female | 4 |
| Age at Nx, years
(range) | 59.4±12.4
(30–77) |
| Age at diagnosis of
LM, years (range) | 62.6±12.1
(30–79) |
| Tumor
sidea | |
|
Right | 9 |
| Left | 16 |
| Tumor
sizea, cm
(range) | 9.9±4.3 (3.5–18) |
| Cell
typea | |
| Clear
cell | 24 |
|
Chromophobe | 1 |
| Histological
gradea | |
| 1 | 0 |
| 2 | 4 |
| 3 | 21 |
| Fuhrman
gradea | |
| 1 | 0 |
| 2 | 0 |
| 3 | 6 |
| 4 | 18 |
| NA | 1 |
| pT stagea | |
| 1a | 0 |
| 1b | 8 |
| 2a | 1 |
| 2b | 4 |
| 3a | 3 |
| 3b | 4 |
| 3c | 0 |
| 4 | 5 |
| MVIa | |
| + | 20 |
| – | 5 |
| Tumor
necrosisa | |
| + | 14 |
| – | 11 |
| No. of
LMb | |
|
Solitary | 7 |
|
Multiple | 14 |
| NA | 4 |
| ECOG PSb | |
| 0 | 12 |
| 1 | 3 |
| 2 | 2 |
| 3 | 5 |
| 4 | 1 |
| NA | 2 |
|
Hemoglobinb, g/dl (range) | 10.3±2.2
(6.1–13.5) |
| Platelet
countb,
x104/mm3 (range) | 27.9±9.8
(5.8–43.8) |
| CRPb, mg/dl (range) | 6.8±8.3 (0.3–28) |
| LDHb, IU/l (range) | 323.9±286.3
(110–1,138) |
| Corrected
calciumb, mg/dl
(range) | 8.6±3.4
(8.4–12.7) |
Factors affecting prognosis
We next attempted to identify the clinical factors
affecting the prognosis of RCC patients with LM. The Kaplan-Meier
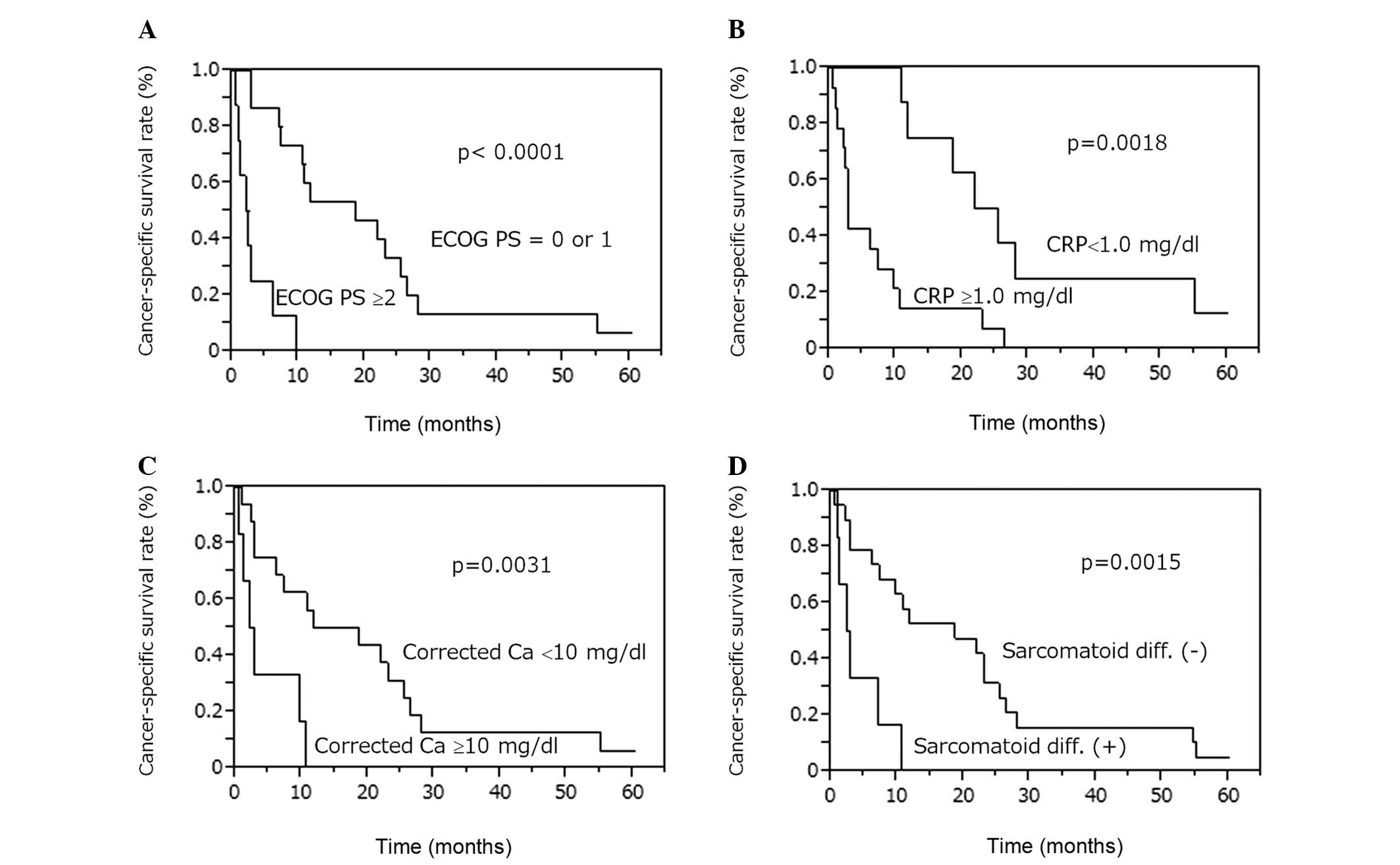
method revealed that the CSS rates were lower in patients with
sarcomatoid differentiation (P=0.0015), ECOG PS ≥2 (P<0.0001),
CRP levels ≥1.0 mg/dl (P=0.0018) and corrected Ca levels ≥10 mg/dl
(P=0.0031; Fig. 2). CSS was not
significantly different between patients with LM at presentation
and those who developed LM following Nx (P=0.1102), between
patients with LM alone and those with multiple organ metastases
(P=0.0578), between patients who were treated with TKIs and those
who were not (P=0.7848) and between patients who underwent hepatic
resection and those who did not (P=0.0912) (data not shown).
Univariate analysis of
clinicopathological parameters and CSS
The results of the univariate analysis for the
association between clinicopathological parameters and CSS are
presented in Table II. The
presence of sarcomatoid differentiation (P=0.0067), ECOG PS ≥2
(P=0.0002), CRP levels ≥1.0 mg/dl (P=0.0019), corrected Ca levels
≥10 mg/dl (P=0.0100) and presence of multiple organ metastases
(P=0.0367) were identified as CSS predictors. The multivariate
analysis (Table III)
demonstrated that ECOG PS ≥2 (P=0.0063; HR=6.46; 95% CI: 1.67–32.8)
was an independent CSS predictor.
 | Table IIUnivariate analysis for
cancer-specific survival following development of liver metastasis
(LM). |
Table II
Univariate analysis for
cancer-specific survival following development of liver metastasis
(LM).
| Clinicopathological
factors | P-value |
|---|
| Agea, years (60> vs. 60≤) | 0.1172 |
| Sarcomatoid
differentiationb (+
vs. −) | 0.0067 |
| Histological grade
3b (+ vs. −) | 0.2898 |
| Fuhrman
gradeb (<3 vs.
4) | 0.4066 |
| MVIb (+ vs. −) | 0.9872 |
| Tumor
necrosisb (+ vs.
−) | 0.8618 |
| Tumor
sizeb (<10 vs. ≥10
cm) | 0.9160 |
| pT1 or 2 vs. pT3 or
4b | 0.3196 |
| Presence of LM at Nx
(yes vs. no) | 0.0992 |
| No. of LM at
presentation (1 vs. ≥2) | 0.4447 |
| ECOG
PSa (0 or 1 vs.
2≤) | 0.0002 |
| CRPa (<1.0 vs. ≥1.0 mg/dl) | 0.0019 |
| LDHa, IU/l (<338 vs. ≥338) | 0.9019 |
|
Hemoglobina (anemia vs. normal) | 0.1704 |
| Platelet
counta
(<35×104 vs. ≥35×104/mm3) | 0.3434 |
| Corrected
calciuma (<10 vs.
≥10 mg/dl) | 0.0100 |
| LM only (yes vs.
no) | 0.0367 |
| Tyrosine kinase
inhibitors (yes vs. no) | 0.8848 |
| Cytokine therapy
(yes vs. no) | 0.7278 |
| Local treatment
(yes vs. no) | 0.8373 |
| Hepatic resection
(yes vs. no) | 0.0528 |
| Interval from Nx to
LM, months (<24 vs. ≥24) | 0.4218 |
 | Table IIIUnivariate and multivariate analysis
for cancer-specific survival following liver metastasis (LM). |
Table III
Univariate and multivariate analysis
for cancer-specific survival following liver metastasis (LM).
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variables | P-value | P-value | Hazard ratio | 95% confidence
interval |
|---|
| Sarcomatoid
differentiation | 0.0067 | 0.1759 | | |
| ECOG PS
≥2a | 0.0002 | 0.0063 | 6.46 | 1.67–32.8 |
| Multiple organ
metastases | 0.0367 | 0.3526 | | |
| CRP ≥1.0
mg/dla | 0.0019 | 0.3704 | | |
| Corrected calcium
≥10 mg/dla | 0.0100 | 0.3339 | | |
Patients exhibiting long-term survival
following LM
The characteristics of the 9 patients who survived
for >20 months following LM diagnosis are summarized in Table IV. The longest survival time
following development of LM was 60.2 months. Two patients who
received treatment with TKIs for LM survived for ≥23 months. One
patient remains alive after 5 years on sunitinib treatment, with
prolonged stable disease. Another patient was treated with
sorafenib for multiple metastases, including LM; however, the LM
progressed. Two patients who received cytokine therapy for multiple
organ metastases survived for >2 years and their tumors did not
contain a high-grade component (both grade 2). Two patients who
underwent hepatic resection and had no metastases to other organs
survived for ≥22 months and one of these two patients survived for
55.1 months. One patient who received intra-arterial injection of
styrene-maleic acid neocarzinostatin (SMANCS) and lipiodol
(SMANCS/lipiodol) for LM treatment remained alive at 25.3 months.
All the patients who survived for >20 months had ECOG PS ≤1.
 | Table IVSummary of the 9 cases who survived
for >20 months following liver metastasis (LM). |
Table IV
Summary of the 9 cases who survived
for >20 months following liver metastasis (LM).
| CRPa | | Other metastatic
sites | | Survival after |
|---|
| Case | Agea/gender | ECOG
PSa | (mg/dl) | Grade | at the time of
LM | Treatment for
LM | LM (months) |
|---|
| 1 | 64/M | 0 | 0.3 | 3 | None | Sunitinib | 60.2 |
| 2 | 61/M | 1 | 0.6 | 3 | None | Hepatic recection,
Nx | 55.1 |
| 3 | 71/F | NA | ND | 3 | Bone, lung | NA | 54.6 |
| 4 | 54/M | 0 | 0.6 | 2 | Lung, pancreas,
stomach, duodenum | Interferon-α | 28.0 |
| 5 | 79/M | 0 | 5.9 | 2 | Lung | Interleukin-2 | 26.4 |
| 6 | 77/M | 1 | 0.3 | 3 | LR |
SMANCS/lipiodol | 25.3 |
| 7 | 57/F | 0 | 28 | 3 | Lung, bone, pleura,
LN, cerebellum | Interferon-α,
sorafenib | 23.0 |
| 8 | 78/M | NA | ND | 3 | Lung | NA | 23.0 |
| 9 | 77/M | 1 | 0.6 | 3 | None | Hepatic
recection | 22.0 |
Outcome with local treatment for
LM
The characteristics of the 9 patients who underwent
local treatment for LM are summarized in Table V. In patients 1 and 3, metastases
were identified only in the liver and were completely eliminated;
the survival duration was 55.1 and 22 months, respectively, as
described above. Three patients who received local treatments but
whose ECOG PS was >2 only survived for a short period of time.
These data suggest that local treatment may be ineffective in
patients with a poor ECOG PS. Four patients undergoing local
treatment survived for >18 months. In those patients, the number
of metastatic sites (patients 1, 2, 3 and 4) was relatively small.
Two patients had a solitary LM, 1 had LM and local recurrence and 1
had LM, as well as lung and lymph node metastases.
 | Table VSummary of the 9 cases who underwent
local treatment for liver metastasis (LM). |
Table V
Summary of the 9 cases who underwent
local treatment for liver metastasis (LM).
| Survival after |
|---|
| Case | Agea/gender | No. of
LMa | ECOG
PSa | Other metastatic
sites | Treatment | LM (months) |
|---|
| 1 | 61/M | 2 | 1 | None | Hepatic recection,
Nx | 55.1 |
| 2 | 77/M | 3 | 1 | LR |
SMANCS/lipiodol | 25.3 |
| 3 | 77/M | 1 | 0 | None | Hepatic
recection | 22.0 |
| 4 | 61/M | 1 | 0 | Lung, LN | SMANCS/lipiodol
RFA | 18.6 |
| 5 | 66/M | 2 | 0 | Pancreas, kidney,
lung, jejunum |
SMANCS/lipiodol | 11.7 |
| 6 | 46/M | 1 | 0 | Lung, pleura,
peritoneum, rib, pancreas, duodenum | TAE (lipiodol) | 7.2 |
| 7 | 54/M | 3 | 2 | Lung, bone | RFA | 2.8 |
| 8 | 45/F | 1 | 2 | Lung, bone, LN |
SMANCS/lipiodol | 2.3 |
| 9 | 64/M | 3 | 4 | LR, iliopsoas
muscle | RFA | 2.1 |
Discussion
The prognosis of RCC patients with LM is extremely
poor. Due to the poor prognosis, the clinical characteristics and
treatment for LM have not been extensively investigated. Long-term
survivors are rare among RCC patients with LM. However, it is
crucial to investigate patients who have benefited from treatments
such as local therapy and molecular-targeted therapy. During the
cytokine era, there was no effective drug therapy for LM from RCC.
TKIs are currently used for patients with metastatic RCC and
patients with LM who have responded to TKIs have been reported
(10, 17). In the present study, 9 patients
(36%) survived for ≥22 months following LM diagnosis. Of those 9
patients, 5 were treated with local LM therapies or TKIs. Local
treatments and TKIs appeared to improve the prognosis of some RCC
patients with LM. The multivariate analysis demonstrated that only
ECOG PS ≥2 was an independent CSS predictor; therefore, patients
with LM and ECOG PS ≥2 have a poor prognosis, even if they are
treated with local and/or systemic therapies, including TKIs.
In this study, we observed that the median CSS
following LM appearance was 10.6 months. In previous studies, the
median CSS following LM was 7.6–12.6 months (2–4),
while the 1-year survival was 38.3% (2). Those results are similar to ours. In
the present study, however, we excluded patients who could not
undergo Nx due to their deteriorated general condition caused by
far-advanced disease, whereas the median CSS was only 10.6 months,
indicating that the prognosis of RCC patients with LM is poor.
According to the multivariate analysis, ECOG PS ≥2
at LM appearance was an independent predictor of a shorter CSS.
Among RCC patients with LM, those with a poor ECOG PS only survived
for a short period of time. As shown in Table V, the 3 patients with ECOG PS ≥2
survived for <3 months following LM presentation. According to
these results, all treatments appear to be ineffective for patients
with ECOG PS ≥2.
In a proportion of patients with LM alone or a
limited number of metastatic sites in addition to LM, local
treatment of LM may prolong survival. We administered local
treatments to 9 patients (36%) with LM (Table V) and their median CSS was 11.7
months. The survival duration of patients with LM alone (patients 1
and 3) and those with LM and a limited number of additional
metastases (patients 2 and 4) appeared to be longer compared to
that of patients with far-advanced disease. We compared patients
with LM alone to those with LM and metastases to other organ(s) and
observed that CSS was longer in the former (median, 38.6 months)
compared to that in the latter (median, 9.7 months), with the
difference being borderline significant (P=0.0578). Two of the 4
patients (50%) with LM alone underwent local resection and their
survival period following LM presentation was 55.1 and 22 months,
respectively. LM from RCC occasionally grows rapidly and the
patient's general condition deteriorates when LM becomes bulky.
Therefore, local treatment of LM should be considered for patients
with LM alone or those with LM and a limited number of additional
metastases.
Two patients (patients 2 and 9; Table IV) survived for >20 months
following hepatic resection for LM. Staehler et al (13) reported that the overall survival of
RCC patients with LM alone who underwent hepatic resection was
longer than that of those who did not undergo hepatic resection.
Therefore, in RCC patients with LM alone, prognosis may be improved
by hepatic resection. Furthermore, it was reported that in RCC
patients, metachronous hepatic resection for LM prolonged overall
survival compared to synchronous hepatic resection (18). Based on those reports, aggressive
hepatic resection should be recommended if a radiological
cancer-free status is achieved.
In the present study, the 2 patients who were
treated with TKIs survived for >20 months. TKIs were used by 6
of the 25 patients (24%) following LM diagnosis. CSS was not
significantly different between patients treated with TKIs and
those who were not. However, 1 patient (case 1 in Table IV) appeared to benefit from TKI
treatment, with the size of the LM remaining stable for 5 years. In
the 2 patients who were treated with TKIs and survived for >20
months, ECOG PS was 0. A proportion of RCC patients with LM may
indeed benefit from TKI treatment. Therefore, in patients with an
ECOG PS of <1, TKI treatment may be a viable option.
Two patients received cytokine therapy for multiple
metastases, including LM, and survived for >26 months. However,
such patients are a rare finding. The histological grade of the
primary lesions in those 2 patients was 2 (3-grade system), without
a high-grade component. As the growth rate of the metastatic
lesions is likely to be slow, such patients may survive over a long
period of time on cytokine therapy alone.
There were several limitations to this study. First,
this was a retrospective study conducted at a single institution
with a small number of RCC patients with LM. LM is relatively rare
in patients with RCC and it is difficult to collect a sufficient
sample size at a single institution. Therefore, a
multi-institutional joint study is required to verify our findings.
Second, this study excluded patients who did not undergo Nx. There
were certain patients with far-advanced RCC and LM who survived for
only a short period of time. In addition, the efficacy of
molecular-targeted therapies, including TKIs, for such patients mus
be evaluated in the future. However, despite these limitations, our
study may have generated useful clinical data on this understudied
type of cancer.
In conclusion, RCC patients with LM may benefit from
local treatment of LM, such as surgical resection, if they have a
limited number of metastatic sites in addition to LM and if their
ECOG PS is favorable and stable. Indeed, a proportion of RCC
patients with LM benefit from TKI therapy. By contrast, RCC
patients with LM and an ECOG PS ≥2 appear to have a poor prognosis,
regardless of any local or systemic treatment.
References
|
1
|
Linehan WM, Walther MM, Alexander RB and
Rosenberg SA: Adoptive immunotherapy of renal cell carcinoma:
studies from the Surgery Branch, National Cancer Institute. Semin
Urol. 11:41–43. 1993.PubMed/NCBI
|
|
2
|
Naito S, Yamamoto N, Takayama T, et al:
Prognosis of Japanese metastatic renal cell carcinoma patients in
the cytokine era: a cooperative group report of 1463 patients. Eur
Urol. 57:317–325. 2010. View Article : Google Scholar
|
|
3
|
Shinohara N, Nonomura K, Abe T, et al: A
new prognostic classification for overall survival in Asian
patients with previously untreated metastatic renal cell carcinoma.
Cancer Sci. 103:1695–1700. 2012. View Article : Google Scholar
|
|
4
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leibovich BC, Cheville JC, Lohse CM, et
al: A scoring algorithm to predict survival for patients with
metastatic clear cell renal cell carcinoma: a stratification tool
for prospective clinical trials. J Urol. 174:1759–1763. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shuto T, Inomori S, Fujino H and Nagano H:
Gamma knife surgery for metastatic brain tumors from renal cell
carcinoma. J Neurosurg. 105:555–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toyoda Y, Shinohara N, Harabayashi T, et
al: Survival and prognostic classification of patients with
metastatic renal cell carcinoma of bone. Eur Urol. 52:163–168.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yasuda Y, Fujii Y, Yuasa T, et al:
Possible improvement of survival with use of zoledronic acid in
patients with bone metastases from renal cell carcinoma. Int J Clin
Oncol. 18:877–883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoshi S, Jokura H, Nakamura H, et al:
Gamma-knife radiosurgery for brain metastasis of renal cell
carcinoma: results in 42 patients. Int J Urol. 9:618–627. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng H, Li X, Yao J, et al: Multifocal
brain metastases in clear cell renal cell carcinoma with complete
response to sunitinib. Urol Int. 83:482–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Molina AM, Ginsberg MS and Motzer RJ:
Long-term response with everolimus for metastatic renal cell
carcinoma refractory to sunitinib. Med Oncol. 28:1527–1529. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alves A, Adam R, Majno P, et al: Hepatic
resection for metastatic renal tumors: is it worthwhile? Ann Surg
Oncol. 10:705–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Staehler MD, Kruse J, Haseke N, et al:
Liver resection for metastatic disease prolongs survival in renal
cell carcinoma: 12-year results from a retrospective comparative
analysis. World J Urol. 28:543–547. 2010.PubMed/NCBI
|
|
14
|
Sobin LH, Gospodarowicz MK and Wittekind
Ch: TN. classification of Malignant Tumors7th. Wiley-Blackwell;
West Sussex, UK: 2009
|
|
15
|
Ito K, Yoshii H, Asakuma J, et al:
Clinical impact of the presence of the worst nucleolar grade in
renal cell carcinoma specimens. Jpn J Clin Oncol. 39:588–594. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic paramaters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gore ME, Hariharan S, Porta C, et al:
Sunitinib in metastatic renal cell carcinoma patients with brain
metastases. Cancer. 117:501–509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langan RC, Ripley RT, Davis JL, et al:
Liver directed therapy for renal cell carcinoma. J Cancer.
3:184–190. 2012. View
Article : Google Scholar : PubMed/NCBI
|