Introduction
Pancreatic ductal carcinoma is a highly aggressive
cancer, with one of the highest mortality rates among
gastrointestinal cancers. The survival of patients with pancreatic
ductal carcinoma has not improved significantly over the last 30
years; the 5-year survival rate was reported to be ∼6% (1).
Complete surgical resection for localized pancreatic
ductal carcinoma is recommended as the only curative treatment
option. However, due to the high incidence of locoregional
recurrence (mainly in the pancreatic bed) and liver metastasis, the
5-year survival rate is ≤20% following curative surgical resection
(2–6).
The clinical benefit of preoperative chemoradiation (CRT) for better
local control following surgical resection was recently reported
(7,8).
Moreover, in addition to preoperative CRT followed by curative
resection, postoperative liver perfusion therapy may be efficient
in reducing the incidence of liver metastasis (6,9). These
findings suggest that pancreatic ductal carcinoma is a type of
high-grade malignant tumor that requires multidisciplinary
treatment for a complete cure. The identification of clinically
useful predictive markers is necessary to maximize the therapeutic
effect.
As regards clinical pathology, pancreatic cancer
cells are mainly surrounded by a dense desmoplastic region
consisting primarily of myofibroblasts as the main cellular
component and extracellular matrix proteins (2). This desmoplastic change represents the
key characteristic of pancreatic ductal carcinoma. Although the
effect of the surrounding stromal cells on the malignant behavior
of pancreatic cancer cells is controversial (3–5), the
abundant fibrotic environment inhibits neovascularization.
Hypovascularity may lead to the insufficient delivery of oxygen and
nutrients to the tumor (6). Hypoxic
tumors are associated with poor patient prognosis, due to
hypoxia-mediated treatment resistance and hypoxia-induced
biological changes that promote malignancy, including metastasis
(7–10). Under such stressful hypoxic
microenvironment conditions, cancer cells undergo a shift in
cellular metabolism. This shift in energy production from oxidative
phosphorylation to glycolysis, known as the Warburg effect, is a
fundamental property of cancer cells (11).
Even under conditions of abundant oxygen supply,
cancer cells preferably produce large amounts of lactate;
therefore, cancer metabolism often involves aerobic glycolysis.
Despite the inefficient adenosine 5′-triphosphate (ATP) production
system in tumors, known as Warburg effect, cancer cells exhibit ATP
production ability equivalent to that of normal cells by utilizing
the glycolytic system, leading to the production of nucleic acids
and nicotinamide adenine dinucleotide phosphate (12). During aerobic glycolysis, glucose is
phosphorylated by hexokinase 2 (HK2) to form glucose-6-phosphate
and lactic acid is produced from pyruvic acid by pyruvate kinase
isoenzyme type M2 (PKM2).
Although the expression of HK2 and PKM2 were
reported to be correlated with cancer cell growth (13,14), their
role in pancreatic ductal carcinoma remains unclear. Several
histopathological factors have been shown to predict postoperative
prognosis in pancreatic ductal carcinoma (15–20). As a
key regulator of aerobic glycolysis, the expression of HK2 and PKM2
is likely significant for the progression and prognosis of
pancreatic ductal carcinoma, which is a classical hypoxic tumor. In
the present study, we investigated the expression of HK2 and PKM2
in surgically resected specimens from patients with pancreatic
ductal carcinoma using immunohistochemical staining. The
correlation of HK2 and PKM2 expression with clinicopathological
characteristics and prognosis was then investigated.
Patients and methods
Patients
Between 2007 and 2012, a total of 91 patients
underwent curative surgical resection for pancreatic ductal
carcinoma. The diagnosis was confirmed by a pathologist based on
the cytology of the pancreatic juice and/or endoscopic
ultrasound-guided fine-needle aspiration preoperatively. We have
been performing preoperative CRT since 2007 with the aim of
securing a curative margin to achieve better local control and
survival in selected patients. However, to avoid the effect of
preoperative treatment on immunohistochemical staining patterns,
this study included 36 patients who underwent curative surgical
resection without preoperative treatment, such as CRT or
chemotherapy.
Clinical data were collected from patient medical
records. Overall survival (OS), local recurrence-free survival
(LRFS) and distant metastasis-free survival (DMFS) were calculated
from the date of surgery to the occurrence of adverse events.
This study was approved by the ethics committee of
the Graduate School of Medicine, Osaka University, for use of
clinical samples. Prior to enrollment in this study, all the
patients provided written informed consent, as required by the
Osaka University Human Study Committee.
Immunohistochemical staining
All the samples were fixed using 10% neutral
formalin and embedded in paraffin wax. Immunohistochemistry was
performed on 3.5-µm paraffin-embedded sections cut from the main
block. The sections were deparaffinized in Hemo-De (FALMA, Tokyo,
Japan) and rehydrated in a graded ethanol series. Antigen retrieval
was performed using citrate buffer (10 mM, pH 6.0) heated in a
water bath for 40 min. The slides were blocked using goat serum for
20 min at room temperature, then incubated with monoclonal rabbit
anti-human HK2 antibody (1:400; cat. no. 2867; Cell Signaling
Technology, Danvers, MA, USA) or monoclonal anti-human PKM2
antibody (1:400; cat. no. 4053; Cell Signaling Technology)
overnight at 4˚C. The Vectastain ABC Peroxidase kit (Vector
Laboratories, Burlingame, CA, USA) was used to visualize the
antigen and counterstaining was performed with hematoxylin. The
staining intensity of HK2 and PKM2 was defined as negative (0),
weak (+1), or strong (+2). The distribution of positively stained
cells was scored on a scale of 0–5: 0, no staining; 1, <20%; 2,
20–40%; 3, 40–60%; 4, 60–80%; and 5, 80–100%. The total
histological score was calculated as the staining intensity ×
distribution (score <5, negative expression and ≥5, strong
expression). Parrafin-embedded sections of human normal liver and
colon cancer tissue served as positive controls for HK2 and PKM2,
respectively. Prognostic analyses were performed according for OS,
LRFS and DMFS.
Statistical analysis
The Fisher's exact test and χ2 test were
used to evaluate the association of HK2 and PKM2 expression with
clinicopathological variables. The Kaplan-Meier method and the
log-rank test were used to compare the survival rates among groups.
The Cox proportional hazard model was used for univariate and
multivariate survival analyses. P<0.05 was considered to
indicate a statistically significant difference. All the analyses
were performed using the JMP statistical software package, version
11 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
A total of 36 patients who underwent curative
resection for pancreatic ductal carcinoma between 2007 and 2012
were included in this study. The patient demographics and clinical
and surgical characteristics are summarized in Table I.
 | Table I.Demographics, clinical and surgical
characteristics of patients with pancreatic adenocarcinoma. |
Table I.
Demographics, clinical and surgical
characteristics of patients with pancreatic adenocarcinoma.
|
Characteristics | Patient no.
(n=36) | (%) |
|---|
| Age (years) | | |
|
Median | 70 | |
|
Range | (47–83) | |
| Gender | | |
|
Male | 21 | 58 |
|
Female | 15 | 42 |
| Location | | |
|
Head | 21 | 28 |
|
Body | 10 | 28 |
|
Tail | 5 | 14 |
| Surgical
procedure | | |
| PD | 22 | 61 |
| DP | 14 | 39 |
| Tumor size
(mm) | | |
|
≥25 | 19 | 53 |
|
<25 | 17 | 47 |
|
Differentiation | | |
|
High | 2 | 6 |
|
Moderate | 30 | 83 |
|
Poor | 3 | 8 |
|
Mucinous | 1 | 3 |
| pT stage | | |
|
pT1 | 4 | 11 |
|
pT2 | 4 | 11 |
|
pT3 | 28 | 78 |
|
pT4 | 0 | 0 |
| Nodal
metastasis | | |
|
Positive | 16 | 44 |
|
Negative | 20 | 56 |
| Stage (UICC) | | |
| IA | 4 | 11 |
| IB | 4 | 11 |
|
IIA | 12 | 33 |
|
IIB | 16 | 45 |
|
III | 0 | 0 |
| Portal vein
involvement | | |
|
Positive | 11 | 31 |
|
Negative | 25 | 69 |
| Arterial
involvement | | |
|
Positive | 3 | 8 |
|
Negative | 33 | 92 |
| Ly | | |
|
Positive | 26 | 72 |
|
Negative | 10 | 28 |
| V | | |
|
Positive | 15 | 42 |
|
Negative | 21 | 58 |
| Ne | | |
|
Positive | 29 | 81 |
|
Negative | 7 | 19 |
| HK2 | | |
|
Positive | 21 | 58 |
|
Negative | 15 | 42 |
| PKM2 | | |
|
Positive | 16 | 44 |
|
Negative | 20 | 56 |
HK2
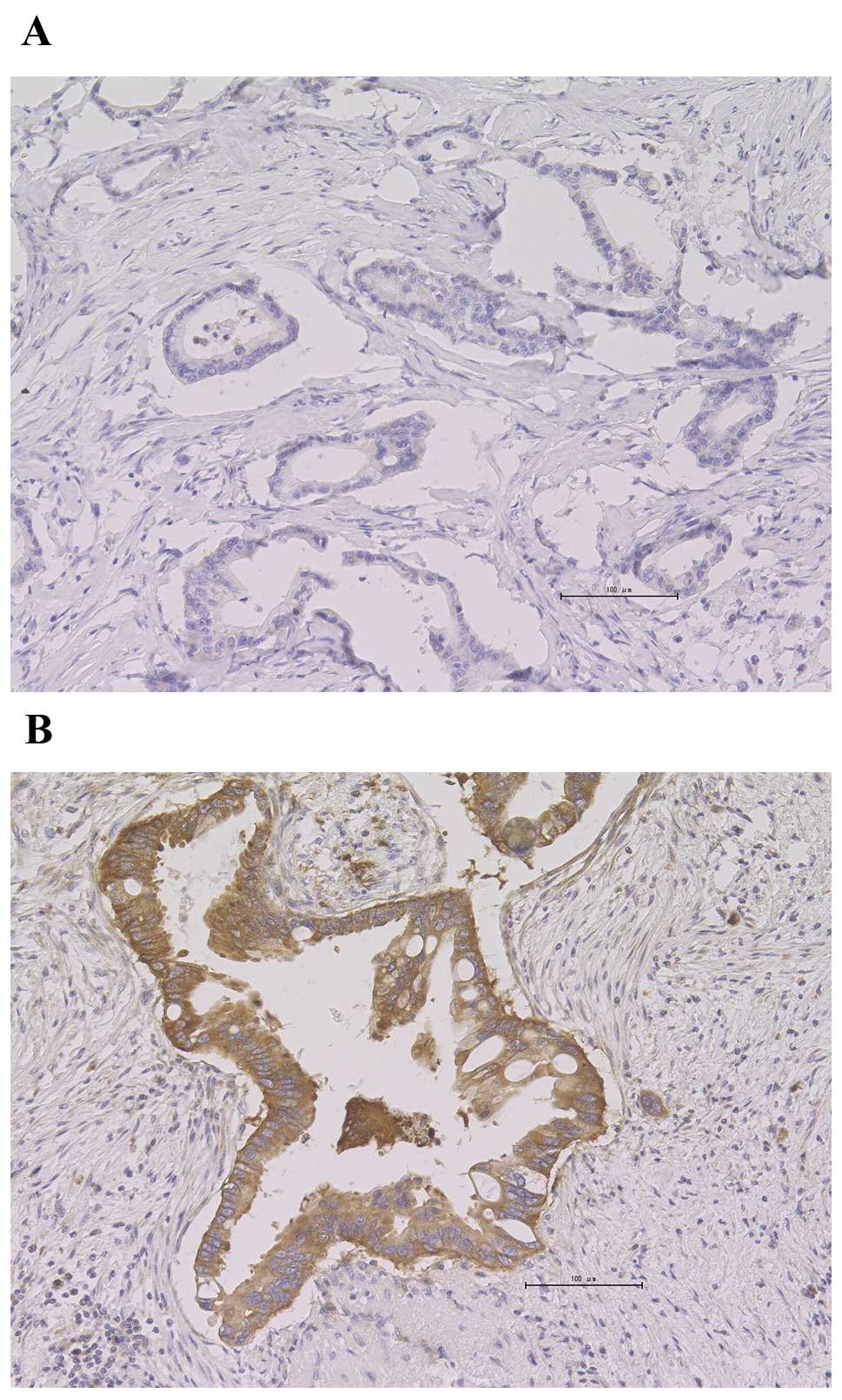
HK2-positive staining was detected in 58% (21/36) of
the patients. HK2-positive tumor specimens exhibited predominantly
cytoplasmic staining patterns, whereas the adjacent fibrotic tissue
was stained negative (Fig. 1).
However, there was heterogeneity regarding the HK2 staining
pattern. HK2-positive staining was homogenous in 67% of the
positive samples (14/21) and heterogeneous in 33% (7/21). The
association between positive staining for HK2 and
clinicopathological characteristics was investigated (Table II) and significant differences in HK2
staining were identified according to pathological tumor stage
(P=0.017).
 | Table II.Association between HK2 expression
and the clinicopathological characteristics. |
Table II.
Association between HK2 expression
and the clinicopathological characteristics.
|
| HK2 |
|
|---|
|
|
|
|
|---|
|
Characteristics | Positive
(n=21) | Negative
(n=15) | P-value |
|---|
| Age (years) |
|
| 0.74 |
|
≥70 | 11 | 9 |
|
<70 | 10 | 6 |
| Gender |
|
| 1.00 |
|
Male | 12 | 9 |
|
Female | 9 | 6 |
| Tumor size
(mm) |
|
| 1.0 |
|
≥25 | 11 | 7 |
|
<25 | 10 | 8 |
| Location |
|
| 0.31 |
|
Head | 14 | 7 |
|
Body | 5 | 5 |
|
Tail | 2 | 3 |
|
Differentiation |
|
| 0.63 |
|
High/moderate | 18 | 14 |
|
Poor/mucinous | 3 | 1 |
| pT STAGE |
|
| 0.017 |
| pT1,
T2 | 2 | 6 |
|
pT3 | 19 | 9 |
| Nodal
metastasis |
|
| 0.32 |
|
Positive | 11 | 5 |
|
Negative | 10 | 10 |
| Portal vein
involvement |
|
| 0.67 |
|
Positive | 7 | 4 |
|
Negative | 14 | 11 |
| Arterial
involvement |
|
| 1.00 |
|
Positive | 2 | 1 |
|
Negative | 19 | 14 |
|
Microinvolvement |
|
|
|
| Ly |
|
| 0.71 |
|
Positive | 16 | 10 |
|
Negative | 5 | 5 |
| V |
|
| 0.18 |
|
Positive | 11 | 4 |
|
Negative | 10 | 11 |
| Ne |
|
| 1.00 |
|
Positive | 17 | 12 |
|
Negative | 4 | 3 |
PKM2
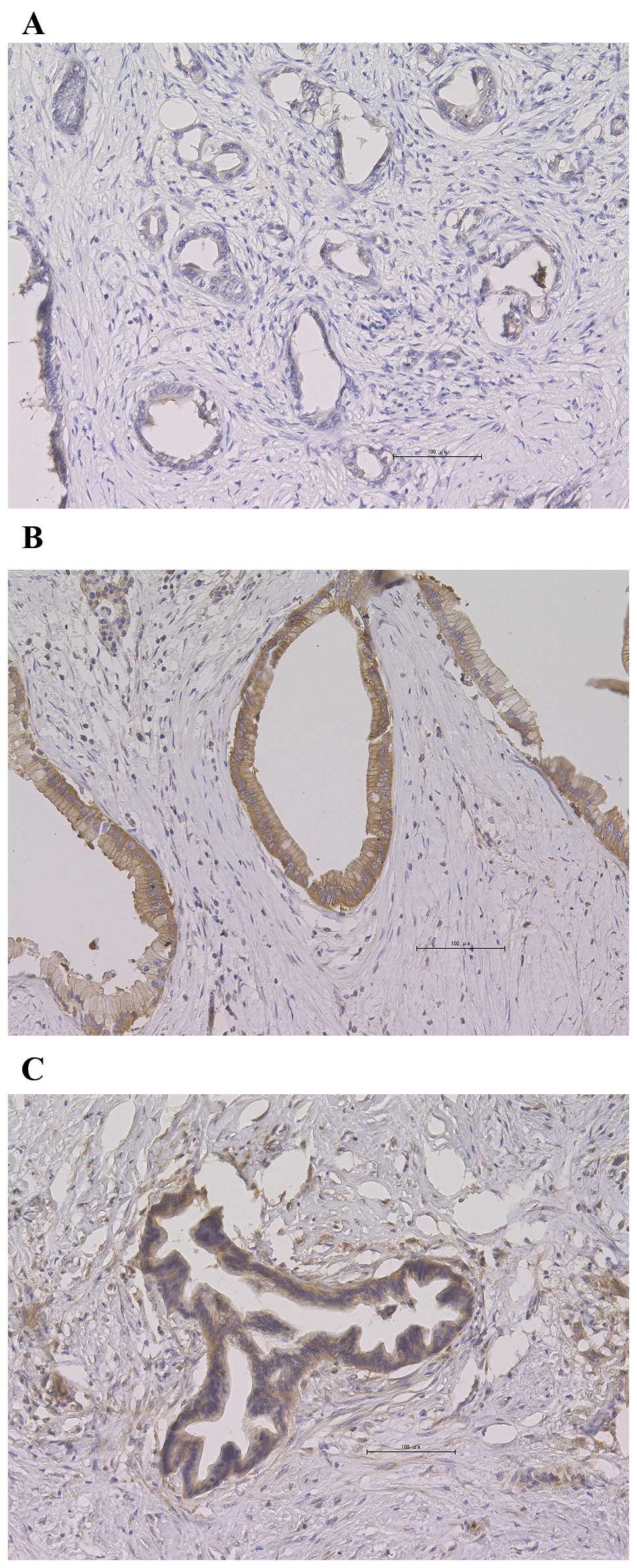
PKM2-positive staining was detected in 44% (16/36)
of the patients and was localized to the cytoplasm in 44% of the
samples (7/16), whereas in the remaining samples it was localized
in the nucleus as well as the cytoplasm. Representative staining
images are shown in Fig. 2.
PKM2-positive staining was predominantly distributed homogeneously.
The association between staining for PKM2 and
clinicohistopathological characteristics is presented in Table III. There was no significant
difference in the clinicopathological variables between
PKM2-positive and -negative patients.
 | Table III.Association between PKM2 expression
and clinicopathological Characteristics. |
Table III.
Association between PKM2 expression
and clinicopathological Characteristics.
|
| PKM2 |
|
|---|
|
|
|
|
|---|
|
Characteristics | Positive
(n=16) | Negative
(n=20) | P-value |
|---|
| Age (years) |
|
| 1.00 |
|
≥70 | 9 | 11 |
|
<70 | 7 | 9 |
| Gender |
|
| 0.32 |
|
Male | 11 | 10 |
|
Female | 5 | 10 |
| Tumor size
(mm) |
|
| 1.00 |
|
≥25 | 8 | 10 |
|
<25 | 8 | 10 |
| Location |
|
| 0.32 |
|
Head | 11 | 10 |
|
Body | 4 | 6 |
|
Tail | 1 | 4 |
|
Differentiation |
|
| 0.61 |
|
High/moderate | 15 | 17 |
|
Poor/mucinous | 1 | 3 |
| pT stage |
|
| 0.26 |
| pT1,
T2 | 2 | 6 |
|
pT3 | 14 | 14 |
| Nodal
metastasis |
|
| 0.31 |
|
Positive | 9 | 7 |
|
Negative | 7 | 13 |
| Portal vein
involvement |
|
| 0.52 |
|
Positive | 4 | 7 |
|
Negative | 12 | 13 |
| Arterial
involvement |
|
| 1.00 |
|
Positive | 1 | 2 |
|
Negative | 15 | 18 |
|
Microinvolvement |
|
|
|
| Ly |
|
| 0.46 |
|
Positive | 13 | 13 |
|
Negative | 3 | 7 |
| V |
|
| 1.00 |
|
Positive | 7 | 8 |
|
Negative | 9 | 12 |
| Ne |
|
| 0.10 |
|
Positive | 15 | 14 |
|
Negative | 1 | 6 |
Association between HK2 and PKM2 and
prognosis
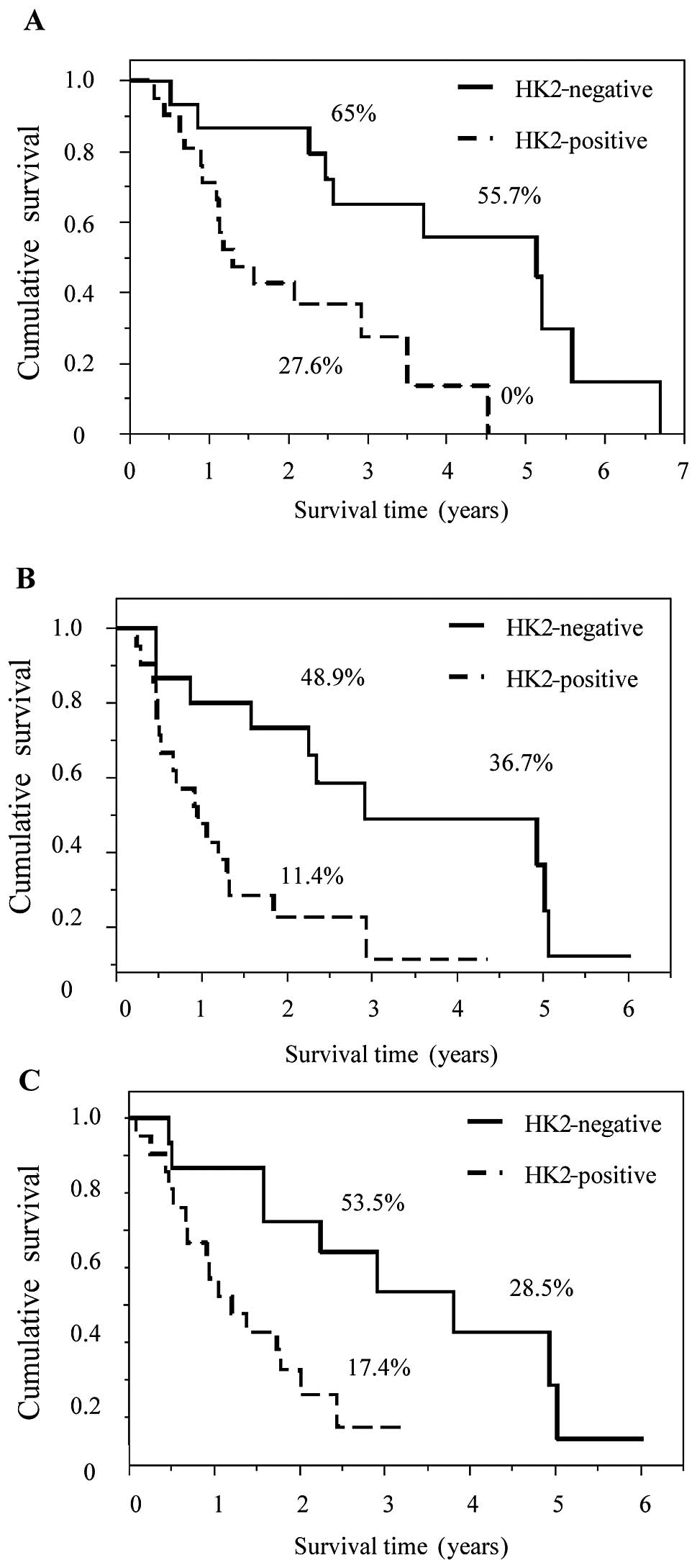
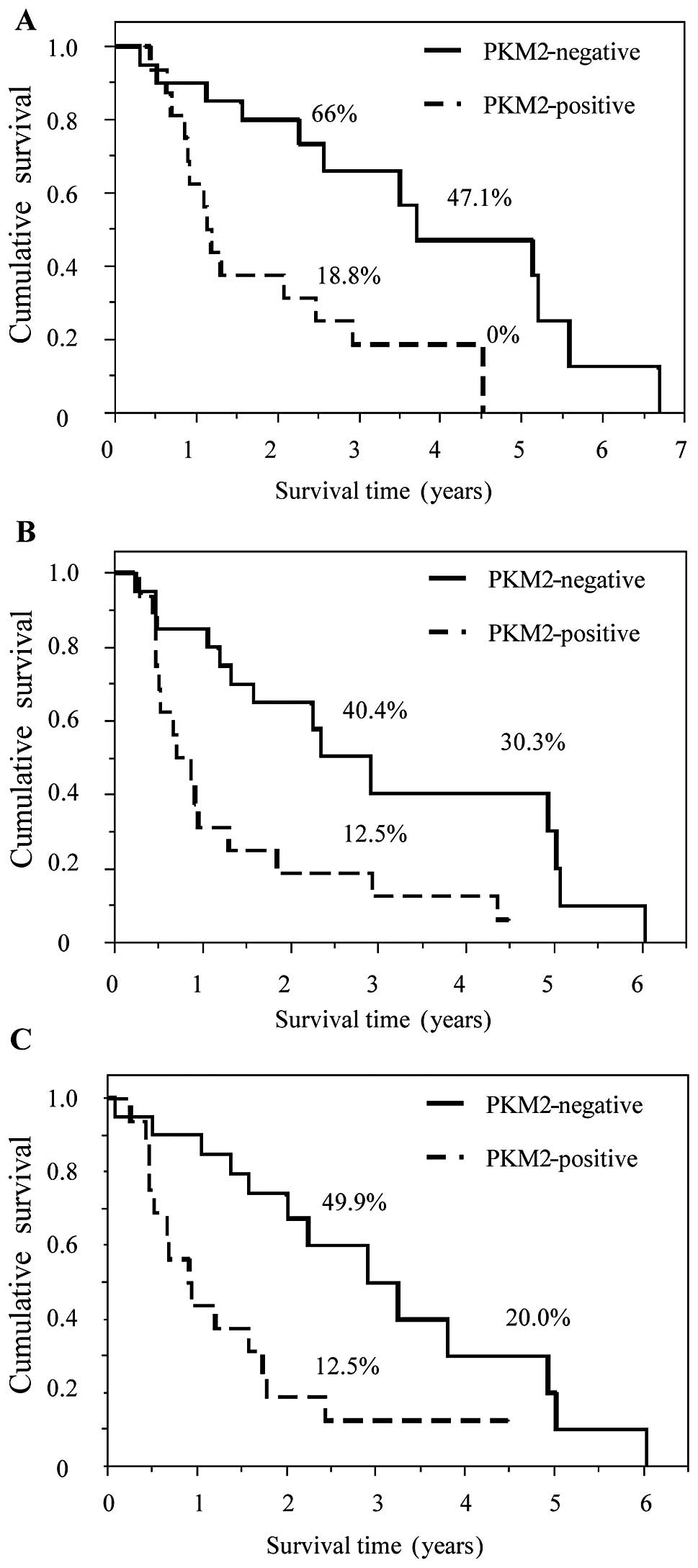
HK2- and PKM2-positive staining was significantly
associated with poor prognosis. OS, LRFS and DMFS in patients with
HK2- and PKM2-positive staining were statistically worse compared
to those with negative staining (Figs.
3 and 4). In addition, staining
for HK2 was significantly associated with staining for PKM2
(Table IV; P=0.01). The univariate
analysis revealed that progressive pathological T stage (pT3 vs.
pT1 and pT2; P=0.02), positive HK2 staining (P=0.003), positive
PKM2 staining (P=0.004) and pathological nodal metastasis (P=0.003)
were significantly associated with poor OS. In the multivariate
analysis, pathological nodal metastasis was the only significant
factor for OS [hazard ratio = 2.76, 95% confidence interval:
1.08–7.73] (Table V). Moreover, the
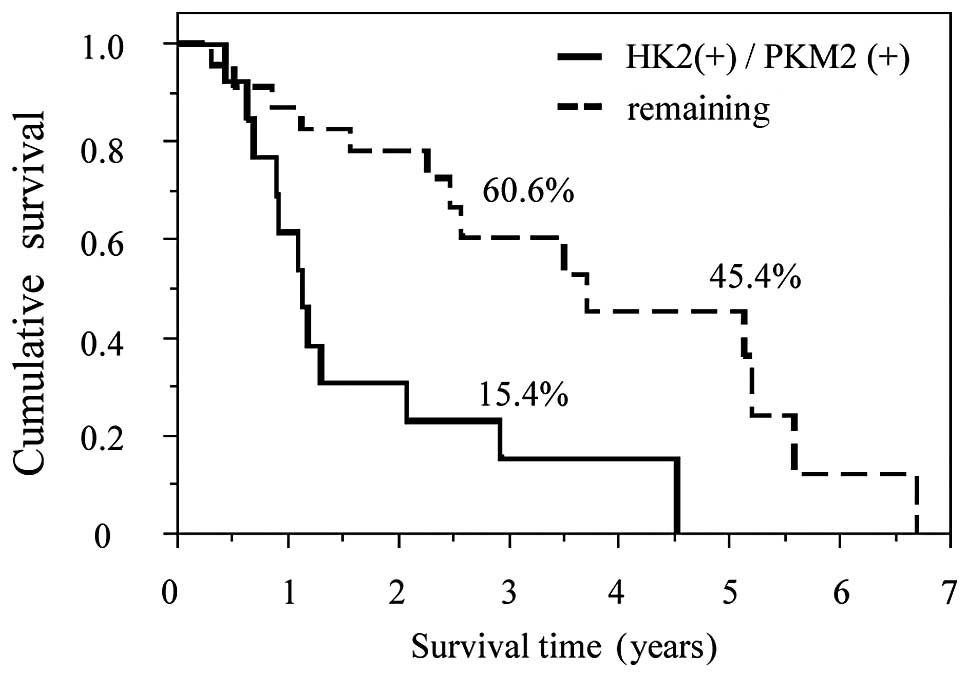
combination of HK2 and PKM2 had a clear prognostic effect. The high
expression of both HK2 and PKM2 was correlated with poor patient
survival compared to the remaining groups, including high HK2 and
low PKM2 expression, low HK2 and high PKM2 expression and low
expression of both HK2 and PKM2 (MST, 1.13 vs. 3.71 years;
P=0.0016). Among all groups, patients expressing high levels of HK2
as well as PKM2 exhibited the poorest prognosis (Fig. 5).
 | Table IV.Contingency table of HK2 and PKM2
expression. |
Table IV.
Contingency table of HK2 and PKM2
expression.
|
| HK2 expression |
|
|---|
|
|
|
|
|---|
| PKM2
expression | Positive | Negative | P-value |
|---|
| Positive | 13 | 13 | 0.01 |
| Negative | 8 | 12 |
|
 | Table V.Analysis of factors related to
overall survival after operation. |
Table V.
Analysis of factors related to
overall survival after operation.
| Factors | Patient no. | 5-year overall
survival (%) | Univariate
analysis, P-value | Multivariate
analysis, relative risk (CI) |
|---|
| Age (years) |
|
| 0.28 |
|
|
≥70 | 20 | 10.5 |
|
|
|
≤69 | 16 | 46.9 |
|
|
| Gender |
|
| 0.71 |
|
|
Male | 21 | 22.2 |
|
|
|
Female | 15 | 35.0 |
|
|
| pT stage |
|
| 0.02 |
|
|
pT3 | 28 | 16.8 |
| 1.66 |
| pT1 and
T2 | 8 | 62.5 |
| (0.48–6.14) |
| Tumor size
(mm) |
|
| 0.74 |
|
|
≥25 | 18 | 25.4 |
|
|
|
<25 | 18 | 30.4 |
|
|
| Nodal
metastasis |
|
| 0.003 |
|
|
Yes | 16 | 15.6 |
| 2.76 |
| No | 20 | 39.3 |
| (1.08–7.73) |
| Lymphatic
invasion |
|
| 0.22 |
|
|
Yes | 26 | 18.8 |
|
|
| No | 10 | 30.6 |
|
|
| Venous
invasion |
|
| 0.19 |
|
|
Yes | 15 | 17.8 |
|
|
| No | 21 | 34.1 |
|
|
| Perineural
invasion |
|
| 0.11 |
|
|
Yes | 28 | 24 |
|
|
| No | 7 | 37.5 |
|
|
| PV invasion |
|
| 0.39 |
|
|
Yes | 11 | 35.4 |
|
|
| No | 25 | 24.8 |
|
|
| Arterial
invasion |
|
| 0.74 |
|
|
Yes | 3 | 0.0 |
|
|
| No | 33 | 30.5 |
|
|
| HK2 staining |
|
| 0.003 |
|
|
Yes | 21 | 0.0 |
| 2.57 |
| No | 15 | 55.7 |
| (0.89–8.39) |
| PKM2 staining |
|
| 0.004 |
|
|
Yes | 16 | 0.0 |
| 2.16 |
| No | 20 | 47.1 |
| (0.82–6.1) |
Discussion
To the best of our knowledge, this is the first
study to describe the association between clinicopathological
characteristics and the expression of HK2 and PKM2 in pancreatic
ductal carcinoma. Pancreatic ductal carcinoma is characterized by
the presence of abundant fibrotic tissue consisting of stromal
cells, activated fibroblasts, stellate cells, immune cells and
extracellular matrix components (2,21). These
desmoplastic changes inhibit proper neovascularization, leading to
a severe shortage of oxygen and nutrients (6). Under such conditions, pancreatic ductal
carcinoma cells exhibit more aggressive malignant potential to
ensure their survival. The malignant shift depends mainly on
hypoxic-inducible factor-1 (HIF-1), which is the transcriptional
activator of various genes associated with cell immortalization,
genetic instability, glucose and energy metabolism,
vascularization, invasion, metastasis and resistance to
chemotherapy and radiotherapy (22–24). A
hypoxic environment induces the expression of HIF-1, which
subsequently activates the transcription of the glucose
transporters (GLUT)-1 and −3, as well as HK1 and HK2, which are the
first enzymes in glycolysis (23).
Previous studies demonstrated that numerous cancers
display enhanced glucose uptake compared to normal tissues due to
the overexpression of various GLUTs (25,26).
Several studies have reported that the overexpression of GLUTs in
cancer is associated with an unfavorable prognosis (27,32). In
addition, abundant glucose uptake caused by GLUT overexpression in
malignant tumors is immediately metabolized by HK2 and is then used
for energy production (28,29). Tumor imaging using
18f-labeled 2-deoxyglucose positron emission tomography
(FDG-PET) was developed to assess cancer metabolism (30). FDG-PET imaging utilizes the
overexpression of HK2, which subsequently phosphorylates
18fdg to form fDG-6-phosphate in malignant cancer cells
(31). However, under aerobic
conditions, normal cells oxidize pyruvic acid, the end product of
glycolysis, via oxidative phosphorylation to achieve a high yield
of energy, thereby allowing tumors to be clinically diagnosed.
Unlike normal cells that use glycolysis only in the
hypoxic state, cancer cells depend exclusively on glycolysis for
energy production via PKM2, the rate-limiting glycolytic enzyme
that catalyses the conversion of phosphoenolpyruvate to pyruvate,
even in the presence of oxygen (32).
This cancer-specific aerobic glycolysis is a less efficient pathway
in terms of energy production.
Previous studies demonstrated that this metabolic
shift is driven by the activation of genetic mutations including
KRAS codon 12, INK4A/ARF, SMAD4/DPC4 and oncogenic signaling
pathway genes (33–35). To compensate for the inefficient
energy production system, cancer cells in a hypoxic environment
yield large amounts of ATP by the high flux between glycolysis and
oxidative phosphorylation in a feedback loop. Proliferating cancer
cells require nucleotides, fatty acids and membranous lipids and
proteins in addition to energy; PKM2 provides these substrates
during glycolysis. The glycolytic intermediate upstream of
phosphoenolpyruvate may then be used in synthetic processes. For
example, NADPH may be derived from glucose-6-phosphate in the
pentose phosphate pathway. NADPH contributes to fatty acid
synthesis and, together with ribose-5-phosphate, to nucleotide
synthesis (36). This enhanced
production of substrates may be beneficial to proliferative cancer
cells. Collectively, such aerobic metabolic flow may contribute to
the biological behavior of HK2 and PKM2 in pancreatic cancer. We
propose that the expression of these two markers has predictive
prognostic potential for curatively resected pancreatic ductal
carcinoma as part of a multidisciplinary treatment for a complete
cure.
Although a strong association between tumor
aggressiveness and the expression of HK2 (37–39) and
PKM2 (32,40,41) has
been reported in several tumors, only a limited number of studies
have investigated PKM2 in pancreatic cancer. In the present study,
we reported the prognostic value of PKM2 in patients with
pancreatic cancer and discussed the possible value of assessing the
combined expression of the HK2 and PKM2, as double-positive
staining was associated with a poorer prognosis compared to the
remaining groups. Cancer tissue highly expressing HK2 as well as
PKM2 may be more malignant due to the enhanced aerobic glycolysis
resulting from the hypoxic microenvironment (14,42).
In conclusion, immunohistochemical staining for HK2
and PKM2 in pancreatic ductal carcinoma was found to be associated
with poor survival. This may be due to the enhanced aerobic
glycolysis in more aggressive tumors.
Acknowledgements
We would like to thank the members of our laboratory
for their useful advice. This study received the following
financial support: a Grant-in-Aid for Scientific Research from the
Ministry of Education, Culture, Sports, Science and Technology, a
Grant-in-Aid from the Ministry of Health, Labor and Welfare, the
Kobayashi Cancer Research Foundation, the Princess Takamatsu Cancer
Research Fund, the Takeda Science and Medical Research Foundation,
the National Institute of Biomedical Innovation and the Osaka
University Drug Discovery Funds, Japan. Partial support was
received from Chugai Co., Ltd., Yakult Honsha Co., Ltd., Merck Co.,
Ltd., Unitech Co., Ltd., EBM Research Center Inc. and Taiho
Therapeutic Co., Ltd., via institutional endowments (to M.M. and
H.I.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. 2012.CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feig C, Gopinathan A, Neesse A, Chan DS,
Cook N and Tuveson DA: The pancreas cancer microenvironment. Clin
Cancer Res. 18:4266–4276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amakye D, Jagani Z and Dorsch M:
Unraveling the therapeutic potential of the Hedgehog pathway in
cancer. Nat Med. 19:1410–1422. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Özdemir BC, Pentcheva-Hoang T, Carstens
JL, et al: Depletion of carcinoma-associated fibroblasts and
fibrosis induces immunosuppression and accelerates pancreas cancer
with reduced survival. Cancer Cell. 25:719–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Provenzano PP, Cuevas C, Chang AE, Goel
VK, Von Hoff DD and Hingorani SR: Enzymatic targeting of the stroma
ablates physical barriers to treatment of pancreatic ductal
adenocarcinoma. Cancer Cell. 21:418–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koong AC, Mehta VK, Le QT, et al:
Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol
Biol Phys. 48:919–922. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Campbell PJ, Yachida S, Mudie LJ, et al:
The patterns and dynamics of genomic instability in metastatic
pancreatic cancer. Nature. 467:1109–1113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yachida S, Jones S, Bozic I, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamada S, Fuchs BC, Fujii T, et al:
Epithelial-to-mesenchymal transition predicts prognosis of
pancreatic cancer. Surgery. 154:946–954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan N, Milosevic M and Bristow RG: Tumor
hypoxia, DNA repair and prostate cancer progression: new targets
and new therapies. Future Oncol. 3:329–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Christofk HR, Vander Heiden MG, Harris MH,
et al: The M2 splice isoform of pyruvate kinase is important for
cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wolf A, Agnihotri S, Micallef J, et al:
Hexokinase 2 is a key mediator of aerobic glycolysis and promotes
tumor growth in human glioblastoma multiforme. J Exp Med.
208:313–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitsunaga S, Hasebe T, Iwasaki M,
Kinoshita T, Ochiai A and Shimizu N: Important prognostic
histological parameters for patients with invasive ductal carcinoma
of the pancreas. Cancer Sci. 96:858–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitsunaga S, Hasebe T, Kinoshita T, et al:
Detail histologic analysis of nerve plexus invasion in invasive
ductal carcinoma of the pancreas and its prognostic impact. Am J
Surg Pathol. 31:1636–1644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takahashi H, Ohigashi H, Ishikawa O, et
al: Perineural invasion and lymph node involvement as indicators of
surgical outcome and pattern of recurrence in the setting of
preoperative gemcitabine-based chemoradiation therapy for
resectable pancreatic cancer. Ann Surg. 255:95–102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakata B, Fukunaga S, Noda E, Amano R,
Yamada N and Hirakawa K: Chemokine receptor CCR7 expression
correlates with lymph node metastasis in pancreatic cancer.
Oncology. 74:69–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komoto M, Nakata B, Amano R, et al: HER2
overexpression correlates with survival after curative resection of
pancreatic cancer. Cancer Sci. 100:1243–1247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Javle MM, Gibbs JF, Iwata KK, et al:
Epithelial-mesenchymal transition (EMT) and activated extracellular
signal-regulated kinase (p-Erk) in surgically resected pancreatic
cancer. Ann Surg Oncol. 14:3527–3533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guillaumond F, Iovanna JL and Vasseur S:
Pancreatic tumor cell metabolism: focus on glycolysis and its
connected metabolic pathways. Arch Biochem Biophys. 545:69–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Semenza GL: HIF-1: upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peitzsch C, Perrin R, Hill RP, Dubrovska A
and Kurth I: Hypoxia as a biomarker for radioresistant cancer stem
cells. Int J Radiat Biol. 90:636–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Medina RA and Owen GI: Glucose
transporters: expression, regulation and cancer. Biol Res. 35:9–26.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Airley RE and Mobasheri A: Hypoxic
regulation of glucose transport, anaerobic metabolism and
angiogenesis in cancer: novel pathways and targets for anticancer
therapeutics. Chemotherapy. 53:233–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haber RS, Rathan A, Weiser KR, et al:
GLUT1 glucose transporter expression in colorectal carcinoma: a
marker for poor prognosis. Cancer. 83:34–40. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rempel A, Mathupala SP and Perdersen PL:
Glucose catabolism in cancer cells: regulation of the Type II
hexokinase promoter by glucose and cyclic AMP. FEBS Lett.
385:233–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mathupala SP, Rempel A and Pedersen PL:
Aberrant glycolytic metabolism of cancer cells: a remarkable
coordination of genetic, transcriptional, post-translational and
mutational events that lead to a critical role for type II
hexokinase. J Bioenerg Biomembr. 29:339–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Chiro G, DeLaPaz RL, Brooks RA, et al:
Glucose utilization of cerebral gliomas measured by
[18F] fluorodeoxyglucose and positron emission
tomography. Neurology. 32:1323–1329. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pedersen PL: Warburg, me and Hexokinase 2:
Multiple discoveries of key molecular events underlying one of
cancers' most common phenotypes, the ‘Warburg Effect’, i.e.,
elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr.
39:211–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mazurek S: Pyruvate kinase type M2: a key
regulator within the tumour metabolome and a tool for metabolic
profiling of tumours. Ernst Schering Found Symp Proc. 43:969–980.
2007.
|
|
33
|
Bardeesy N and DePinho RA: Pancreatic
cancer biology and genetics. Nat Rev Cancer. 2:897–909. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ramanathan A, Wang C and Schreiber SL:
Perturbational profiling of a cell-line model of tumorigenesis by
using metabolic measurements. Proc Natl Acad Sci USA.
102:5992–5997. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feron O: Pyruvate into lactate and back:
from the Warburg effect to symbiotic energy fuel exchange in cancer
cells. Radiother Oncol. 92:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lyshchik A, Higashi T, Hara T, et al:
Expression of glucose transporter-1, hexokinase-II, proliferating
cell nuclear antigen and survival of patients with pancreatic
cancer. Cancer Invest. 25:154–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mamede M, Higashi T, Kitaichi M, et al:
[18F] FDG uptake and PCNA, Glut-1 and Hexokinase-II
expressions in cancers and inflammatory lesions of the lung.
Neoplasia. 7:369–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sato-Tadano A, Suzuki T, Amari M, et al:
Hexokinase II in breast carcinoma: a potent prognostic factor
associated with hypoxia-inducible factor-1α and Ki-67. Cancer Sci.
104:1380–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Yang Z, Zou Q, et al: PKM2 and ACVR
1C are prognostic markers for poor prognosis of gallbladder cancer.
Clin Transl Oncol. 16:200–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lim JY, Yoon SO, Seol SY, et al:
Overexpression of the M2 isoform of pyruvate kinase is an adverse
prognostic factor for signet ring cell gastric cancer. World J
Gastroenterol. 18:4037–4043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo W and Semenza GL: Pyruvate kinase M2
regulates glucose metabolism by functioning as a coactivator for
hypoxia-inducible factor 1 in cancer cells. Oncotarget. 2:551–556.
2011.PubMed/NCBI
|