Introduction
Keloids are characterized by excessive deposition of
collagen in the dermis beyond the boundaries of a wound and they
often grow beyond the extent of a cutaneous injury, such as an area
of inflammation, a burn or a surgical incision. Spontaneous keloids
sometimes develop without a preceding trauma. Although benign,
keloids are often aesthetically unpleasant, painful and/or
pruritic. Keloids may also be asymptomatic (1). Surgical resection of the keloids alone
has been shown to result in a recurrence in 50–80% of cases
(2). Adjuvant treatments, such as
radiation therapy, have been utilized to reduce the rate of keloid
formation (3). Post-operative
radiation therapy is one of the valuable treatment modalities,
which substantially reduces the keloid formation (4).
External beam radiation therapy and brachytherapy
have been used in the post-operative radiation therapy of keloids.
However, to the best of our knowledge, no consensus has been
reached on the total dosage, fractionation or optimal timing of the
delivery of radiotherapy. The purpose of the present study was to
evaluate the efficacy and toxicity of radiotherapy (brachytherapy
and electron beam irradiation) following keloid surgery.
Materials and methods
Patient characteristics
Subsequent to receiving the approval of the Research
Ethics Board, the tumor database and radiotherapy records of the
Department of Oncology, Taihe Hospital (Shiyan, China) were
retrospectively searched and reviewed. Patients were selected with
the following characteristics: 15–60 years, Eastern Cooperative
Oncology Group performance status of 0 to 2, pathologically
confirmed keloids, and treated with surgical excision and
radiotherapy. Of the 431 patients, 116 patients were enrolled at
the Department of Clinical Oncology, Taihe Hospital, Hubei
University of Medicine between January 1, 2002 and June 30, 2012.
The characteristics of the 116 patients recruited for the
retrospective study are summarized in Table I.
 | Table I.Characteristics of the 116
patients. |
Table I.
Characteristics of the 116
patients.
| Characteristic | Patients, n (%) |
|---|
| Age, years |
|
|
10–20 | 11 (9.5) |
|
21–30 | 44 (37.9) |
|
31–40 | 39 (33.6) |
|
41–50 | 16 (13.8) |
|
51–60 | 6 (5.2) |
| Gender |
|
|
Female | 84 (72.4) |
| Male | 32 (27.6) |
| Localization of
keloids |
|
|
Sternum | 26 (22.4) |
| Ear
auricle | 3 (2.6) |
| Haired
occiput | 15 (12.9) |
|
Back/scapulae | 24 (20.7) |
| Neck | 18 (15.5) |
|
Shoulder | 21 (18.1) |
| Skin of
chin | 1 (0.9) |
|
Instep | 3 (2.6) |
| Dosage and type of
radiation, dose/fractions |
|
|
192Ir HDR: 8
Gy/1F+9 Gy/3F | 22 (19.0) |
|
192Ir HDR: 20
Gy/4F | 22 (19.0) |
|
60Co HDR: 20
Gy/4F | 17 (14.7) |
|
60Co HDR: 18
Gy/6F | 17 (14.7) |
|
Electrons: 26 Gy/13F | 18 (15.5) |
|
Electrons: 30 Gy/15F | 20 (17.2) |
Radiotherapy
Due to the changes in radiation equipment during the
past ten years, different radiation techniques were used in the
treatment for keloids. Between January 1, 2002 and July 19, 2005,
high-dose rate (HDR) brachytherapy using Iridium-192 was performed
in our center; between August 1,2005 and September 2009, HDR
brachytherapy using Cobalt-60 was performed; and between October 1,
2009 and June 30, 2012, a linear accelerator (operating at 4, 6 or
9 Mev) was used in postoperative radiotherapy of keloids.
For the analyses, the patients treated with each
different radiotherapy technique were identified, and the dose and
fractionation regimen used for each patient were examined. The
patients were subsequently grouped by radiation type and also by
dose and fractionation pattern. Only the groups that included
>15 patients were included in the analyses. Data on the patients
in the different groups are shown in detail in Table I.
Of the 116 patients, 44 had received
192Ir HDR brachytherapy, 34 had received 60Co
HDR brachytherapy and 38 had been treated with electron beam
external irradiation. Between January 1, 2002 and July 19, 2005,
when HDR 192Ir brachytherapy was performed, all the 44
patients enrolled were administered their first dose of radiation
within 12 h after surgery. Of these 44 patients who had received
192Ir HDR brachytherapy, 22 (19% of the total patients)
were treated with a dose of 8 Gy delivered in 10 fractions and 9 Gy
delivered in three fractions and 22 (19%) were treated with a dose
of 20 Gy delivered in four fractions. Between October 1, 2009 and
June 30, 2012, when HDR Cobalt-60 brachytherapy was used in our
center as a replacement for 192Ir in the treatment of
keloids, of the 34 patients who had received brachytherapy, 17
(15%) were treated with 60Co HDR brachytherapy at a
total dose of 20 Gy delivered in four fractions. In total, 17 (15%)
were treated with 60Co HDR brachytherapy at a dose of 18
Gy delivered in six fractions. Between October 1, 2009 and June 30,
2012, electron beams (4, 6 and 9 MeV) were used, as they were more
convenient for patients. The energy of the electron beams used was
decided on the basis of the depth and location of the keloids.
Radiotherapy schemes were 30 Gy delivered in 15 fractions (30
Gy/15F) or 26 Gy delivered in 13 fractions (26 Gy/13F).
The biologically effective dose (BED), as derived
from the linear quadratic concept (5), was calculated for the various radiation
regimens using the formula: BED = nd[1+d/(α/β)], where n is the
number of fractions, d is the dose per fraction, and α and β are
the parameters that determine the initial slope and degree of
curvature of the underlying cell-survival curve, respectively. As
keloids are considered to be acute-reacting tissues, it was assumed
that α/β = 10 Gy (6). According to
this linear quadratic concept, the BED of each scheme was
calculated, as shown in Table
II.
 | Table II.Treatment scheme and BED of
radiotherapy. |
Table II.
Treatment scheme and BED of
radiotherapy.
| Dosage and type of
radiation, dose/fractions | BED, Gy (α/β=10) |
|---|
| 192Ir HDR:
8 Gy/1F+9 Gy/3F | 26.1 |
| 192Ir HDR:
20 Gy/4F | 30.0 |
| 60Co HDR:
20 Gy/4F | 30.0 |
| 60Co HDR:
18 Gy/6F | 23.4 |
| Electrons: 26
Gy/13F | 31.2 |
| Electrons: 30
Gy/15F | 36.0 |
Toxicity was graded according to the Common
Terminology Criteria for Adverse Events [CTCAE v3.0 (http://ctep.info.nih.gov/CTC3/ctc.htm)].
An adverse effect >90 days after completion of radiotherapy was
defined as a late adverse effect.
Surgical techniques
Local anesthesia was administered during almost all
the surgical procedures. Excision outside the margin of the lesion
was performed; the wound was closed sub-cutaneously with vicryl on
which a plastic flexible tube was placed. The closed end of the
tube was placed >5 mm beyond the wound margin, and the open end
extended >10 cm beyond the wound margin to facilitate the
connection of the tube to the HDR brachytherapy afterloader. The
subcutaneous vicryl sutures act, among other effects, as a support
for the tube. The wound was closed using a monocryl intracutaneous
soluble suture. The scar, which is the junction of the two skin
sections following excision, formed the target volume for the
irradiation. The tube was placed as close as possible to the scar,
such that the scar would be enclosed by a radius of 5 mm within the
100% isodose line. In case of wound dehiscence, two tubes were
placed; the target volume was an oval enclosing the entire scar
area. Subsequently, in the Department of Radiation Therapy, the
irradiation was performed with an HDR afterloader using an
Iridium-192 or Cobalt-60 source, which was slid into the tube for
the irradiation. Upon administration of the last brachytherapy
fraction, the tube was removed and the wound was covered with a
gauze pad.
Statistical analysis
Patient data (patient characteristics and keloid
characteristics) were collected using Excel. The recurrent time was
defined as the days from the first day of radiotherapy to the date
of recurrence. The patients who were known to remain with no
recurrence at the time of the data update were censored at the
dates of their last follow-up. The median follow-up time was
calculated using the reverse Kaplan-Meier approach. The control
rate was estimated using the Kaplan-Meier method, and the log-rank
test was used to compare the differences between patient groups or
sub-groups. The hazard ratio (HR) among groups was estimated using
the proportional hazards regression with a 95% Wald confidence
interval (95% CI). Data analysis was performed with SPSS version
19.0 (IBM Corp, Armonk, NY, USA), and all P-values were two-sided.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient follow-up
The median follow-up period was 46.5 months (range,
10–120 months) for all the patients. At the last observation date,
18 of the 116 patients had undergone relapse, and the median
recurrent periods for the recurrent patients was 16.7 months
(range, 10–30 months). The control rates for the different
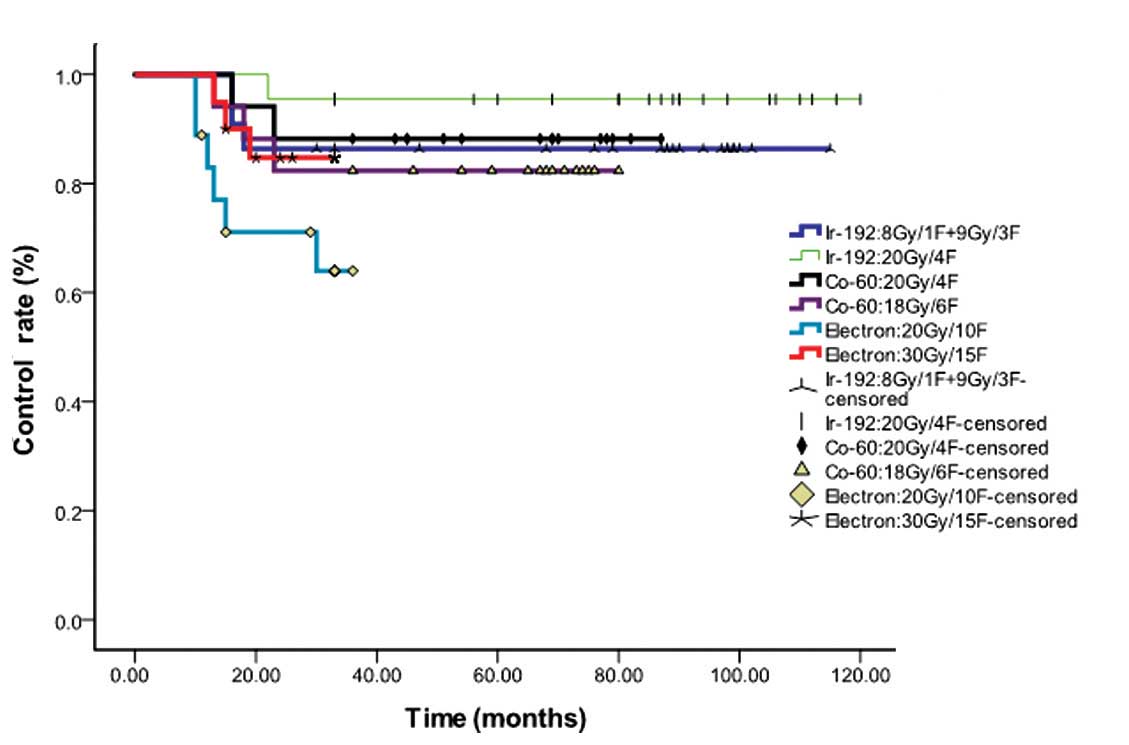
radiation regimens being compared are shown in Fig. 1. The median overall control rate for
all the different radiotherapy techniques was 84.5%. Although
several differences could be identified from the curve of Fig. 1, no statistical difference occurred
(P=0.094) for all the types of dosage and radiation.
Comparisons between subgroups
However, analyses comparing between the specific
subgroups revealed certain differences. The control rate for the
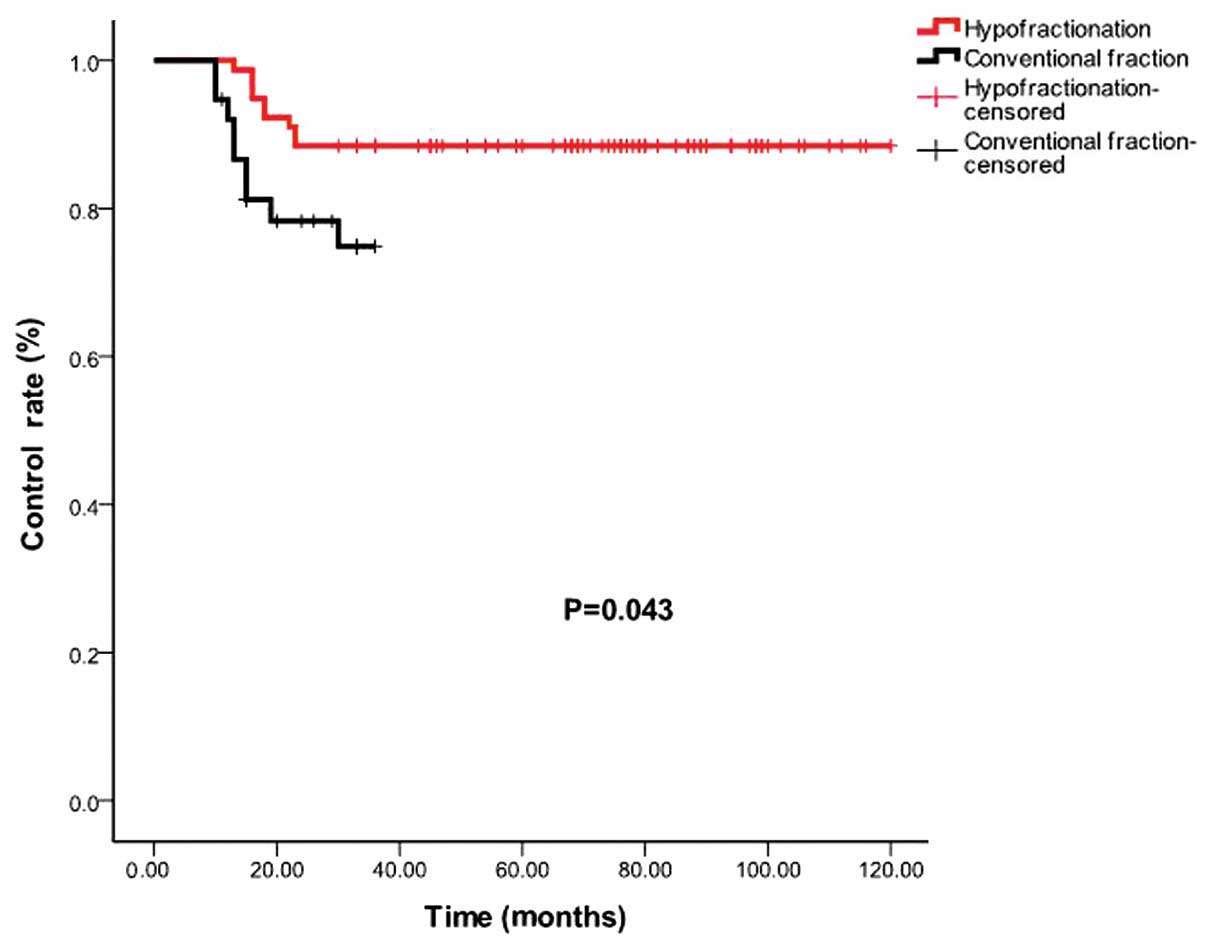
patients who received hypofractionated regimens (>2 Gy/1F) and
conventional fractionation (2 Gy/1F) were 88.5 and 76.3%,
respectively (P=0.043; Fig. 2).
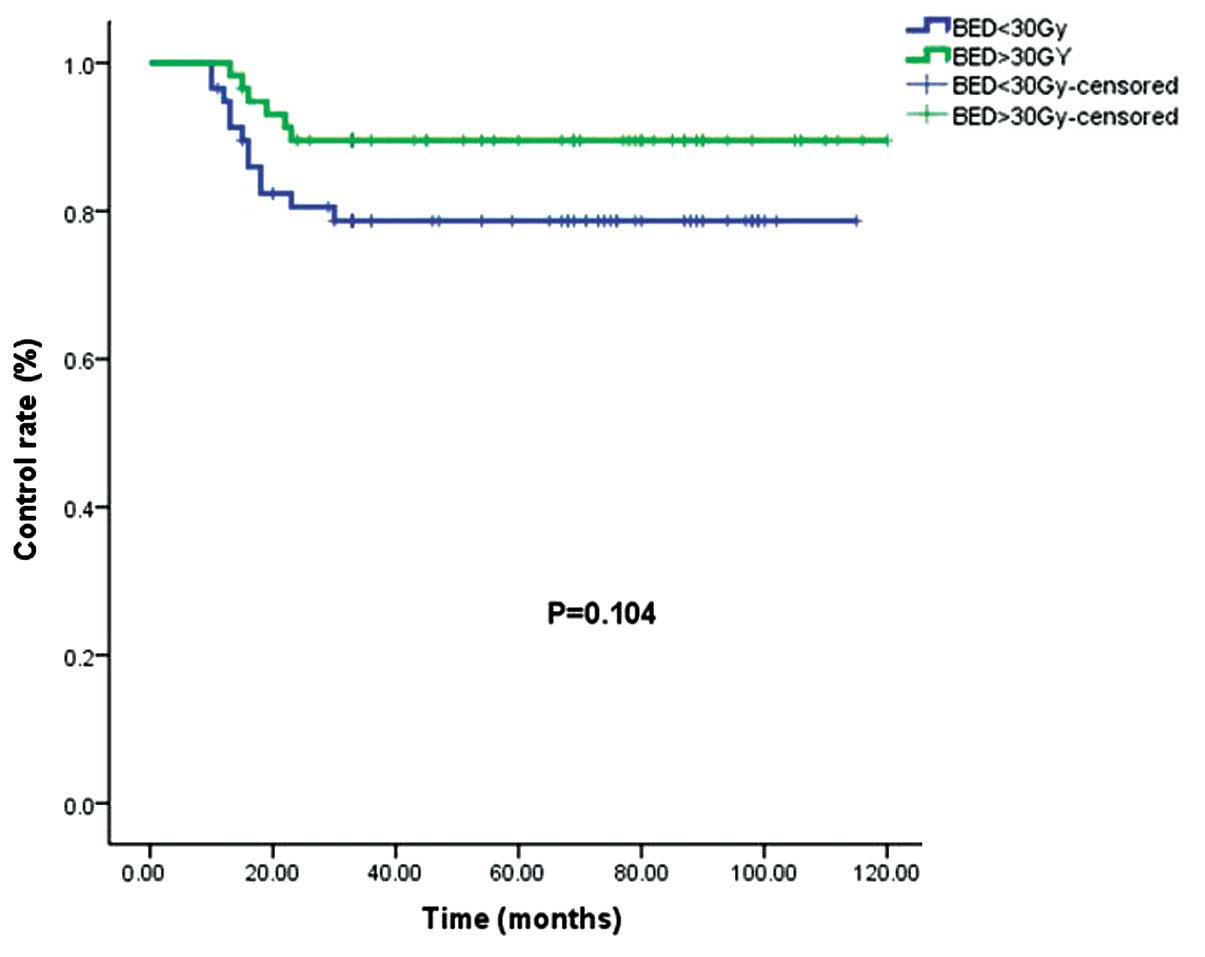
The control rates for the patients who received a
BED of >30 Gy and <30 Gy were 89.7 and 79.3%, respectively
(P=0.104; Fig. 3).
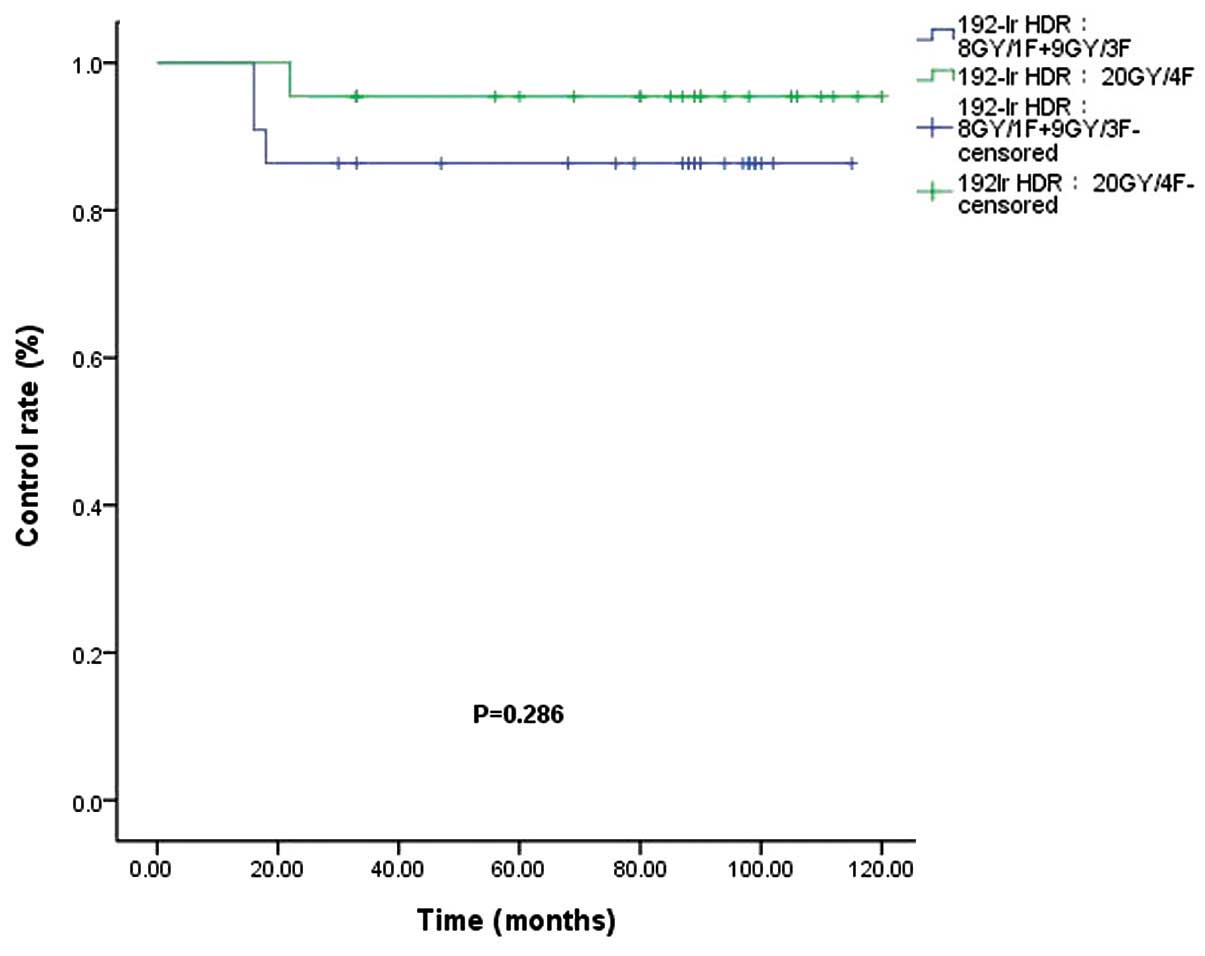
The control rates (86.4 vs. 95.5%) for the
192Ir HDR (8 Gy/1F+9 Gy/3F) and 192Ir HDR (20
Gy/4F) groups were not significantly different (P=0.286; Fig. 4). Of the 34 patients who received
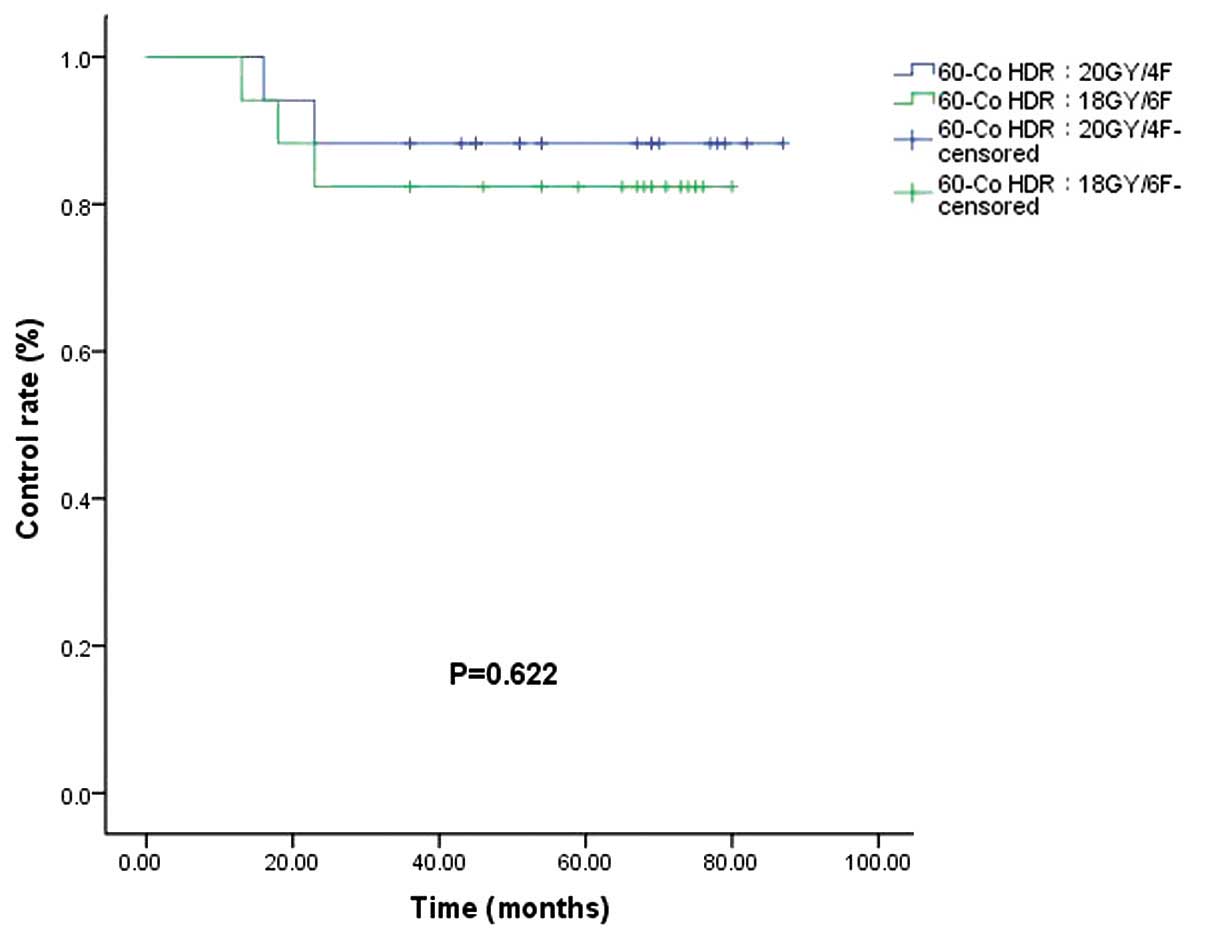
60Co HDR brachytherapy, a recurrence rate was observed
in 11.7% of patients in the 60Co HDR 20 Gy/4F group and
17.6% of patients in the 60Co HDR 18 Gy/6F group
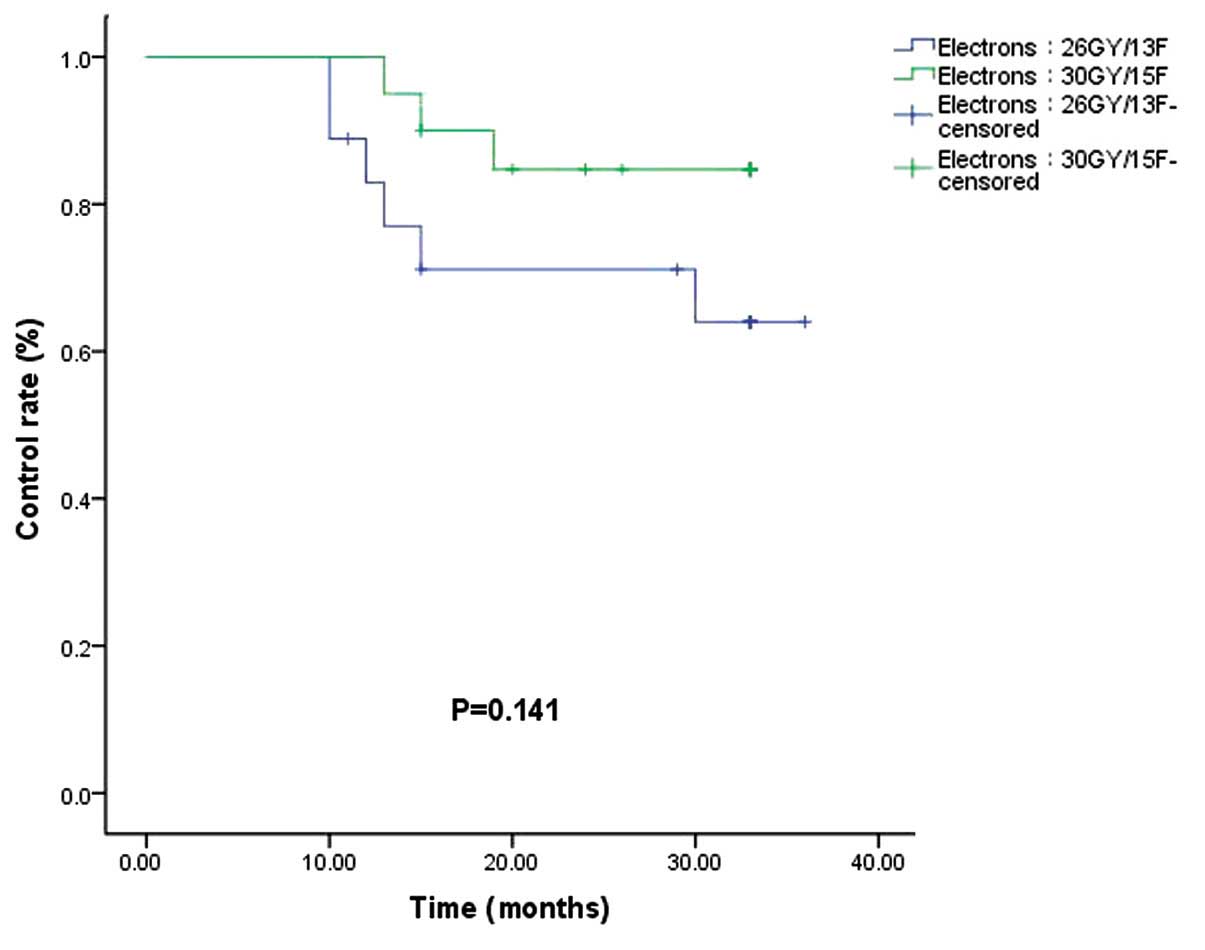
(P=0.622; Fig. 5). In the electron
beam irradiation groups, the control rates for 66.7 vs. 85.0% of
the electron 26 Gy/13F and 30 Gy/15F groups, respectively, were not
significantly different (P=0.141; Fig.
6).
There were no grade 2 or higher adverse effects
based on the CTCAE v3.0 in the late phase. Of all the 116 patients
studied, two patients were diagnosed with middle esophageal cancer,
one 5 years after treatment and another 6 years after. The
locations of the keloids were the neck, and the irradiated areas
were a distance away from the middle esophagus. Therefore, whether
there is any association between radiotherapy and cancer cannot be
confirmed, due to the high incidence rate of esophageal cancer in
northern China.
Discussion
Keloids have been treated using radiation for more
than a century, and the value of radiotherapy in the treatment of
keloids has been established for almost a century. The mechanism by
which radiation prevents regrowth of keloids is unknown. A previous
study showed that fibroblasts were destroyed by radiation and were
not replaced by blood-borne cells from distant tissues (7). By destroying a sufficient number of
cells, a balance could be created between collagen synthesis and
degradation.
In the present study, the recurrence rates of
keloids following postoperative radiation therapy using HDR
Iridium-192, HDR Cobalt-60 and electron beams were analyzed. As
mentioned previously, the shapes of the curves suggest differences
in the various groups, but no significant differences were observed
when the curves were analyzed and compared using log-rank tests.
However, compared to the three different techniques, the pooled
data for all 116 patients showed that the control rate for patients
who received hypofractionated (>2 Gy/1F) radiotherapy was
significantly higher than that for the patients who received
conventional fractionated (2 Gy/1F) radiotherapy.
This result indicated that hypofractionated
radiotherapy played an important role in the adjuvant therapy
following surgical excision of keloids. Flickinger et al
(8) used a comprehensive literature
review and a database of 2,515 resected keloids to identify factors
that significantly affected recurrence rates following
postoperative external beam radiotherapy of keloids, and to
delineate any radiation dose response and effects of radiation dose
per fraction. The results showed that the relatively low α/β ratio
indicates that radiotherapy with a limited number of fractions and
high doses per fraction is the optimum strategy. The present
results support this conclusion.
It is widely accepted that the α/β ratio is equal to
~10 Gy for acute reacting tissues, in the range of 1 to 3 Gy for
late-reacting tissues. The study by Kal and Veen (6) assumed keloids to be one of these
acute-reacting tissues, and applied an α/β rate of 10 Gy. However,
the review by Flickinger et al (8) showed that the radiobiological dose
response function for postoperative radiotherapy of keloids has a
low α/β ratio, with a mean value of 2.08 from the different models
(and maximum 95% confidence values of 3.72–5.40). The differences
in α/β values were mainly between those from the model database and
the higher values obtained in the expanded database. The low
overall average α/β ratio value of 2.08 identified for
postoperative keloid radiotherapy was comparable to values of 1.9
to 3.1 for late fibrosis and 2.8 to 3.7 for telangiectasia
following breast radiotherapy (5,9,10). The low α/β ratio for postoperative
keloid radiotherapy and the relatively high BED time/repopulation
correction factor indicate that there is no advantage of increased
fractionation in this treatment.
Surgical resection followed by radiotherapy, despite
a variety of doses administered, produced an acceptably low
recurrence rate. However, no consensus has been reached on the
total dosage, fractionation or optimal timing of the delivery of
radiotherapy. The present study analyzed the recurrence rates of
keloids following postoperative radiation therapy using the linear
quadratic-BED concept with the aim of normalizing the radiotherapy
doses into BEDs to be able to construct dose-effect associations.
The study by Kal and Veen (6)
reviewed the literature for the recurrence rates of keloids
subsequent to surgical excision followed by radiotherapy. In total,
18 studies were identified that provided data on the recurrence
rate and administered dose. Their results indicated a dose-effect
association for the recurrence rate of keloids as a function of the
BED, and they suggested that a BED of >30 Gy must be applied
within 1–2 days after surgery. The present data (Fig. 3) were consistent with the conclusions
of Kal and Veen (6), but the
differences between the <30 Gy and >30 Gy groups in the
present study were significantly different in the analysis.
Assessing whether this lack of significance reflects the limited
sample size in the present study, or whether other variable
factors, such as target volume, dose/fraction depth of
radiotherapy, surgical approach and location of the keloid, also
contributed is difficult.
The risk of a fatal tumor is of particular concern
to the majority of patients; plastic surgeons tend to avoid
radiation therapy for keloids for fear of inducing malignant
tumors. The International Commission on Radiological Protection
provided risk factors for a number of organs, but not for fat or
muscle tissues (11). Ogawa et
al (12) searched for previous
studies of carcinogenesis associated with radiation therapy for
keloids and examined the evidence-based opinions of radiation
oncologists regarding the acceptability of radiation therapy for
the treatment of keloids. This study noted that the volume
irradiated during the treatment of keloids is extremely small, and
as a consequence of the small volume irradiated, the risk of a
radiation-induced tumor is negligible. The study concluded that the
risk of carcinogenesis from keloid radiation therapy was extremely
low when performed with appropriate doses and under conditions that
provide adequate protection of surrounding tissues, including the
thyroid and the breast, particularly in children and infants, and
that radiation therapy was acceptable as a keloid treatment
modality. For the treatment of keloids in the present study, as a
result of the use of HDR brachytherapy and electron beams with a
rapid fall-off of the dose, the treated volume was extremely small
and the probability of tumor induction would also be negligible,
but further observations are required.
In conclusion, the results of the present study
indicate that hypofractionated radiotherapy can play an important
role in the adjuvant therapy following surgical excision of
keloids. A BED of >30 Gy appears to be sufficient. No definitive
evidence was found between radiotherapy and the occurrence of
cancer during the follow-up, but more cases and longer follow-up
periods are required.
Acknowledgements
The authors would like to thank Dr Sara Rockwell
(Department of Therapeutic Radiology, Yale University School of
Medicine, New Haven, CT, USA) for her modifications to the
language, comments and suggestions during the preparation of the
manuscript. The present study was supported by the Key Academic
Discipline Projects, Hubei University of Medicine (grant no.
2014XKJSXJ12 to Dr Zhiguo Luo).
References
|
1
|
Rubin P, Soni A and Williams JP: The
molecular and cellular biologic basis for the radiation treatment
of benign proliferative diseases. Semin Radiat Oncol. 9:203–214.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramakrishnan KM, Thomas KP and
Sundararajan CR: Study of 1,000 patients with keloids in South
India. Plast Reconstr Surg. 53:276–280. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakamoto T, Oya N, Nagata Y and Hiraoka M:
The outcome and the adverse effect of postoperative radiotherapy
for keloids: A prospective study with a total dose of 20 GY. Int J
Radiat Oncol Biol Phys 60 Suppl. (1): 549–550. 2004. View Article : Google Scholar
|
|
4
|
Leventhal D, Furr M and Reiter D:
Treatment of keloids and hypertrophic scars: a meta-analysis and
review of the literature. Arch Facial Plast Surg. 8:362–368. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barendsen GW: Dose fractionation, dose
rate and iso-effect relationships for normal tissue responses. Int
J Radiat Oncol Biol Phys. 8:1081–1997. 1982. View Article : Google Scholar
|
|
6
|
Kal HB and Veen RE: Biologically effective
doses of postoperative radiotherapy in the prevention of keloids.
Dose-effect relationship. Strahlenther Onkol. 181:717–723. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ragoowansi R, Cornes PG, Glees JP, Powell
BW and Moss AL: Ear-lobe keloids: treatment by a protocol of
surgical excision and immediate postoperative adjuvant
radiotherapy. Br J Plast Surg. 54:504–508. 2011. View Article : Google Scholar
|
|
8
|
Flickinger JC: A radiobiological analysis
of multicenter data for postoperative keloid radiotherapy. Int J
Radiat Oncol Biol Phys. 79:1164–1170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baltas D, Fehrentz D and Turesson I:
Analysis of late effects data using dose-response models:
application to human skin telangiectasia data. Radiother Oncol.
16:41–53. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chevray PM and Manson PN: Keloid scars are
formed by polyclonal fibroblasts. Ann Plast Surg. 52:605–608. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
ICRP, . International commission on
radiological protection. 1990 recommendations of the international
commission on radiological protection. ICRP Publication 60. Ann
ICRP. 21:1–3. 1991.
|
|
12
|
Ogawa R, Yoshitatsu S, Yoshida K and
Miyashita T: Is radiation therapy for keloids acceptable? The risk
of radiation-induced carcinogenesis. Plast Reconstr Surg.
124:1196–1201. 2009. View Article : Google Scholar : PubMed/NCBI
|