Introduction
Cancers of the gallbladder (GBCa) and biliary tract
are highly lethal, as they are usually detected at an advanced
stage and effective chemotherapy agents have not yet been
developed. The 5-year survival rate for patients with advanced
biliary tract cancer (BTC) is <5% (1). These aggressive malignancies are
uncommon in the USA, accounting for an estimated 7,480 new cases
and 3,340 deaths in 2005 (2,3); however, they are endemic in India,
Pakistan, Korea, Japan, Eastern Europe and certain South American
countries. In Chile, GBCa is the leading cause of cancer-related
mortality in women (4–6).
One of the major clinical issues in BTC treatment is
the identification of prognostic factors that affect patient
survival in order to establish effective treatment strategies. It
would be beneficial if clinicians were able to predict, from
surgical specimens obtained at initial surgery, the malignant
potential and post-surgical survival in order to determine the role
for adjuvant therapy. However, the commonly used indicators of
prognosis, including pathological stage and histological grade, do
not adequately predict the clinical course of the majority of
biliary malignancies or the biological characteristics of specific
tumors. Therefore, novel prognostic biomarkers are required for
predicting the aggressiveness of BTC.
The details of tumorigenesis, growth and progression
of this disease are complex and have not been fully elucidated.
Certain predisposing factors, such as chronic cholecystitis,
cholelithiasis, obesity and the presence of an anomalous
pancreaticobiliary junction have been associated with the risk of
this disease (7). Several signaling
pathways have been shown to be involved in the carcinogenesis of
BTC. We previously reported that positive expression of epidermal
growth factor receptor (EGFR) and human epidermal growth factor
receptor 2 (Her2) was detected in 11.7 and 31.6% of BTC cases,
respectively, indicating the possibility of anti-HER2 therapies
against BTC (8). Genetic alterations
in Kras may also contribute to the development of certain types of
GBCa (9). However, the published data
vary and there have been no systematic studies to evaluate other
proteins that may be useful biomarkers.
In this study, we focused on insulin-like growth
factor type I receptor (IGF-IR), mammalian target of rapamycin
(mTOR) and rapidly accelerated fibrosarcoma-1 (Raf-1), as the roles
of these proteins in BTC have not been fully elucidated. IGF-IR is
a cell membrane receptor that participates in cell proliferation,
differentiation and prevention of apoptosis (10). IGF-IR has been reported to be
associated with clinical outcome in breast and gastric cancer
(11,12). The mTOR and Raf-1 genes are also
involved in the regulation of cell growth and proliferation in
carcinogenesis. In the present study, the immunohistochemical
expression of these proteins was investigated in formalin-fixed,
paraffin-embedded surgical specimens from patients with BTC at
different pathological stages and the clinical outcomes were
compared. This study was based on the knowledge that the IGF-IR
family of oncogenes is important in several tumor types and that
several therapeutic agents targeting this gene family are
clinically available. In order to establish a patient selection
strategy for targeted therapy and to identify a useful predictive
marker in BTC for improving the therapeutic options for these
patients, we analyzed the baseline expression profiles of these
gene targets in a large number of patient tumor specimens using
immunohistochemistry (IHC).
Patients and methods
Tissue specimens
A total of 40 patients with GBCa, 12 with
extrahepatic bile duct carcinoma (EHBDCa) and 26 with intrahepatic
bile duct carcinoma (IHBDCa) from Tsukuba University Hospital in
Japan and the University of Texas MD Anderson Cancer Center in the
United States undergoing surgical tumor resection between 1991 and
2006 were selected for this study. The research protocol was
approved by the Institutional Review Board of Tsukuba University
Hospital. Prior to surgery, written informed consent was obtained
from all the patients regarding use of their residual tissue for
future research, including this study.
The mean age of the patients was 63 years (range,
34–89 years) and the patients included 41 men and 37 women. The
demographic characteristics, pathological staging and histological
findings (grade and type) according to the American Joint Committee
on Cancer (AJCC) criteria are summarized in Table I. The surgically resected tumor
specimens were fixed in 10% buffered formalin for 24 h, embedded in
paraffin and 4-µm sections were obtained. The tissue sections were
placed on silane-coated slides and used for hematoxylin and eosin
(H&E) staining and detection of IGF-IR, mTOR and Raf-1 by
IHC.
 | Table I.Characteristics of patients with
biliary tract cancer. |
Table I.
Characteristics of patients with
biliary tract cancer.
| Characteristics | GBCa (n=40) | EHBDCa (n=12) | IHBDCa (n=26) |
|---|
| Sources |
|
|
|
| Tsukuba
University Hospital | 33 | 7 | 15 |
|
MDACC | 7 | 5 | 11 |
| Gender |
|
|
|
|
Male/female | 16/24 | 6/6 | 19/7 |
| Mean age, years
(range) | 64 (36–89) | 61 (34–84) | 62 (38–77) |
| Histological
grade |
|
|
|
| Well
differentiated adenocarcinoma | 17 | 2 | 4 |
|
Moderately differentiated
adenocarcinoma | 16 | 8 | 16 |
| Poorly
differentiated adenocarcinoma | 3 | 1 | 5 |
|
Unclassified
adenocarcinoma | 4 | 1 | 1 |
| pStage (AJCC, 6th
edition) |
|
|
|
| I | 10 | 2 | 2 |
| II | 6 | 2 | 5 |
|
III | 2 | 2 | 10 |
| IV | 22 | 5 | 8 |
|
Unknown | 0 | 1 | 1 |
Procedures of immunohistochemical
staining
Routine H&E staining was performed for
morphological investigation. Immunostaining for IGF-IR, mTOR and
Raf-1 using a Dako EnVision™+ dual link kit (Dako A/S, Glostrup,
Denmark) was performed according to the manufacturer's
instructions. Briefly, following deparaffinization and rehydration,
the sections were brought to the boil in 10 mmol/l sodium citrate
buffer (pH 6.0) at high power by microwave treatment for 10 min.
Endogenous peroxidase activity was blocked by incubation with Dual
Endogenous Enzyme Block (Dako A/S) for 10 min. After washing with
phosphate-buffered saline (PBS, pH 7.4), the sections were
incubated in blocking solution for 30 min to block non-specific
staining. The following antibodies were used: Rabbit anti-IGF-IR
(Cat. no. sc-713; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), rabbit anti-mTOR (Cat. no. NB100–240; Novus Biologicals,
Littleton, CO, USA) and mouse anti-Raf-1 (Cat. no. sc-713; Santa
Cruz Biotechnology). The sections were incubated overnight at 4°C
with the respective primary antibodies at the optimized dilutions
of 1:200 for IGF-IR, 1:100 for mTOR and 1:50 for Raf-1. After
washing in PBS, the sections were incubated at room temperature
with labeled polymer-horseradish peroxidase (Dako A/S) for 60 min
and washed in PBS, followed by treatment with diaminobenzidine
(KPL, Inc., Gaithersburg, MD, USA) solution for 30 sec. After
stopping the reaction with PBS, the sections were counterstained
with hematoxylin.
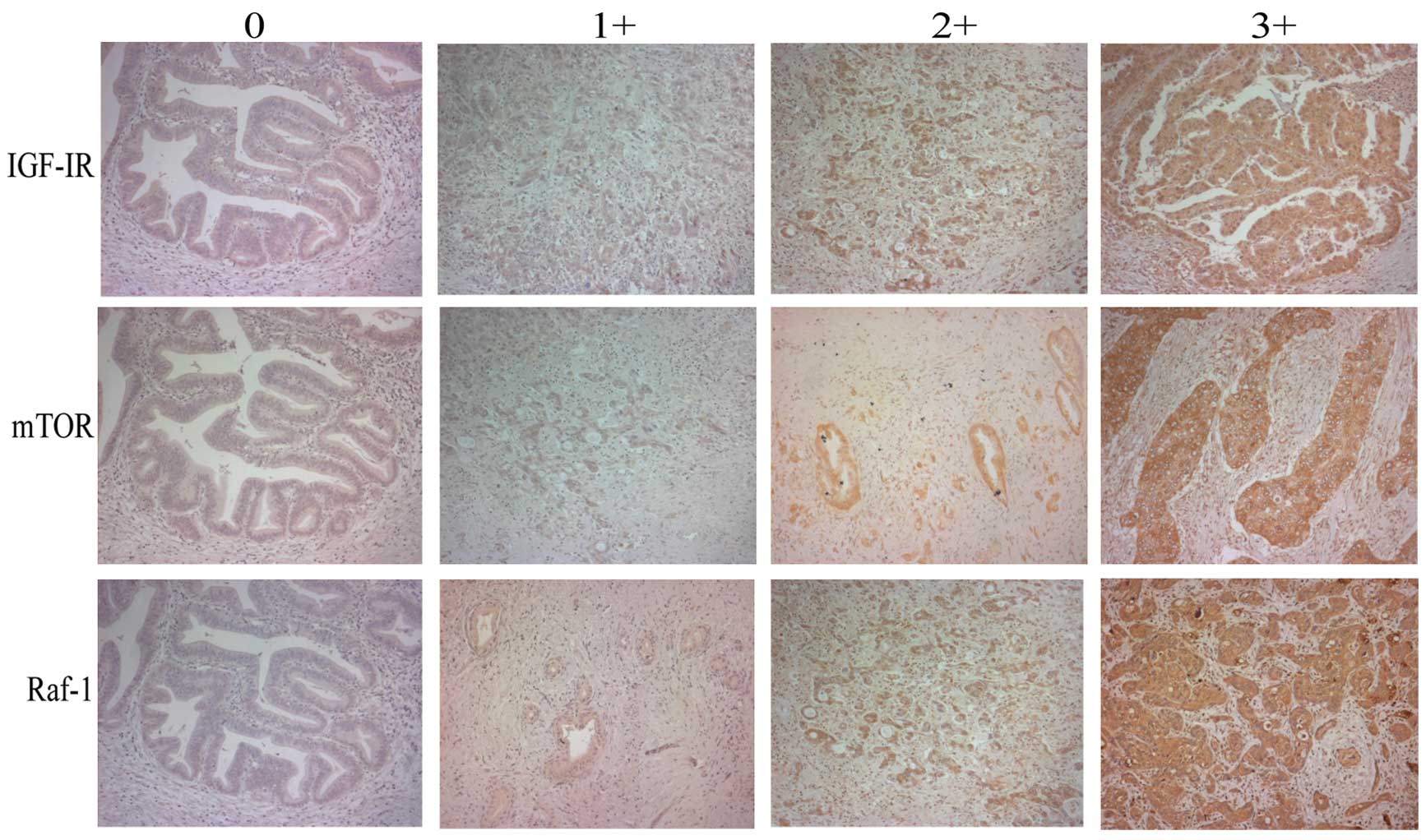
Evaluation of immunohistochemical
staining
The evaluation of the sections was performed by two
independent observers (H.S. and J.C.R) with respect to the
histopathological characteristics and specific immunoreactivity.
For the scoring of each protein expression, the membrane staining
intensity and pattern were scored as follows: 0, no staining; 1+,
faint staining; 2+, weak to moderate staining; and 3+, strong
staining, with reference to the HercepTest scoring system. Each
staining was interpreted as negative (0 or 1+) or positive (2+ or
3+) for each protein expression. Representative immunohistochemical
staining of each antibody is shown in Fig. 1.
Statistical analysis
A two-sided χ2 test or the Fisher's exact
test was used for comparison of immunohistochemical data between
groups. Survival curves were constructed using the Kaplan-Meier
method and differences among survival curves were compared using
the log-rank test. All the statistical analyses were performed
using Statcel software (OMS Publishing, Saitama, Japan). P<0.05
was considered to indicate a statistically significant
difference.
Results
IGF-IR, mTOR and Raf-1 IHC in BTC
The results of the analysis of the expression of
IGF-IR, mTOR and Raf-1 in carcinomas arising in different sites of
the biliary tract are summarized in Table II. Specimens from a total of 78 BTC
patients were used for immunohistochemical analysis. The positive
staining rate was ~70–80%, except for IGF-IR in IHBDCa (54%).
However, the differences in the positive expression rate between
cancer sites was not statistically significant.
 | Table II.Positive expression rates of IGF-IR,
mTOR and Raf-1 in biliary tract cancer (BTC). |
Table II.
Positive expression rates of IGF-IR,
mTOR and Raf-1 in biliary tract cancer (BTC).
| BTC | IGF-IR, n/total
(%) | mTOR, n/total
(%) | Raf-1, n/total
(%) |
|---|
| GBCa | 30/40 (75) | 28/40 (70) | 34/40 (85) |
| EHBDCa | 9/12 (75) | 8/12 (67) | 9/12 (75) |
| IHBDCa | 14/26 (54) | 21/26 (81) | 23/26 (88) |
| Total | 53/78 (68) | 57/78 (73) | 66/78 (85) |
Association of positive expression of
IGF-IR, mTOR and Raf-1 with histological grade of BTC
The frequency of protein expression according to the
AJCC histological grade classification is shown in Table III. For statistical analysis, the
specimens were grouped as AJCC histological grades of
well/moderately differentiated carcinoma vs. poorly differentiated
carcinoma. The results demonstrated that there was no association
between histological grade and the positive expression of these
proteins. Moreover, no statistical significance was found in a
subanalysis for IHBDCa, EHBDCa and GBCa (data not shown).
 | Table III.Immunohistochemical positive
expression rates according to histological differentiation and
pathological stage. |
Table III.
Immunohistochemical positive
expression rates according to histological differentiation and
pathological stage.
| Variables | IGF-IR, n/total
(%) | P-value | mTOR, n/total
(%) | P-value | Raf-1, n/total
(%) | P-value |
|---|
| All
(n=78a) |
|
|
|
|
|
|
|
Histological grade |
| 0.13 |
| 0.28 |
| 0.47 |
|
Well/moderate | 44/63 (70) |
| 46/63 (73) |
| 53/63 (84) |
|
|
Poor | 4/9 (44) |
| 8/9 (89) |
| 7/9 (78) |
|
Pathological stage |
| 0.72 |
| 0.46 |
| 0.96 |
|
I | 9/14 (64) |
| 11/14 (79) |
| 10/14 (71) |
|
|
II–IV | 42/62 (68) |
| 45/62 (73) |
| 54/62 (87) |
|
| GBCa (n=40) |
|
|
|
|
|
|
|
Pathological stage |
| 0.95 |
| 0.36 |
| 0.03 |
|
I | 6/10 (60) |
| 8/10 (80) |
| 6/10 (60) |
|
|
II–IV | 24/30 (80) |
| 20/30 (67) |
| 28/30 (93) |
|
Association of positive expression of
IGF-IR, mTOR and Raf-1 with clinicopathological staging of BTC
The comparison of these proteins with
clinicopathological staging is presented in Table III. To verify the association
between the expression of these proteins and pT stage, the
specimens were grouped as AJCC pathological stage I (early) vs.
II–IV (advanced). For all the proteins, no significant association
between early and advanced stage was observed in any of the BTC
patients. As regards GBCa, the frequency of Raf-1-positive staining
was significantly higher in advanced-stage compared to that in
early-stage disease (Table III, 93
vs. 60%, P=0.03), whereas the expression of Raf-1 did not differ
significantly by clinicopathological stage in IHBDCa and EHBDCa
(data not shown).
Correlation of IGF-IR, mTOR and Raf-1
in BTC
To elucidate the associations between IGF-IR, mTOR
and Raf-1 expressions, the positive frequency of any two of these
proteins was evaluated. As shown in Table IV, the expression of IGF-IR and mTOR
and the expression of Raf-1 and mTOR were correlated to some extent
in all the subjects, although these correlations were not found to
be statistically significant. Furthermore, there were no
correlations between these proteins when they were separately
analyzed in GBCa, IHBDCa and EHBDCa (data not shown).
 | Table IV.Immunohistochemical co-expression
rate and association between two proteins in biliary tract
cancer. |
Table IV.
Immunohistochemical co-expression
rate and association between two proteins in biliary tract
cancer.
| Protein
expression | mTOR | Raf-1 | Raf-1 |
|---|
|
|
|
|---|
| – |
| + | – |
| + | – |
| + |
|---|
| IGF-IR |
|
|
|
|
|
|
|
|
|
| – | 10 |
| 15 | 5 |
| 20 |
|
|
|
| + | 11 |
| 42 | 7 |
| 46 |
|
|
|
| mTOR |
|
|
|
|
|
|
|
|
|
| – |
|
|
|
|
|
| 6 |
| 15 |
| + |
|
|
|
|
|
| 6 |
| 51 |
|
|
| P=0.07 |
|
| P=0.32 |
|
| P=0.05 |
|
Survival analysis
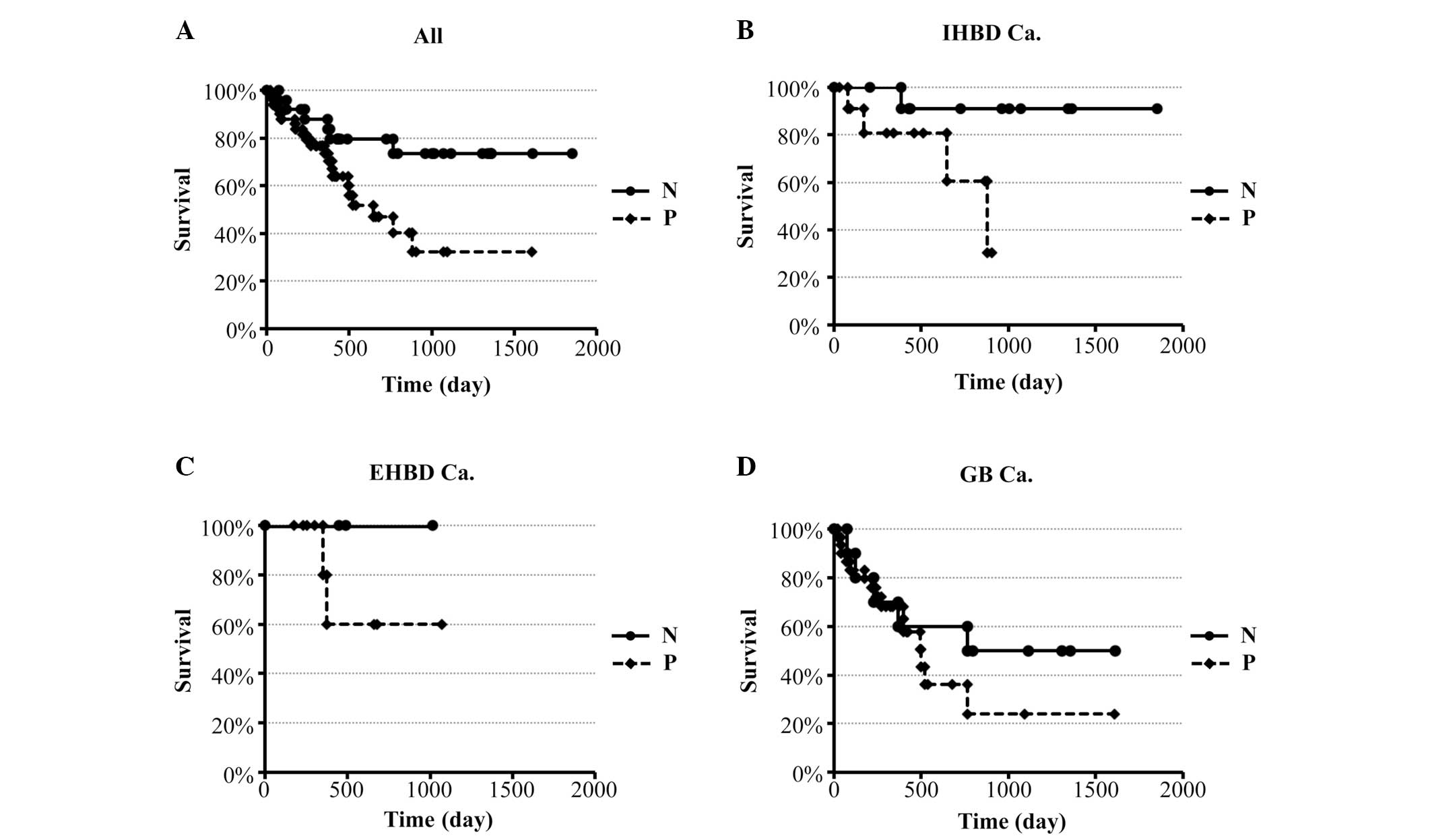
The expression of IGF-IR, mTOR and Raf-1 was taken
into consideration in the survival analysis. For IGF-IR, the 4-year
survival rate of patients with positive expression was 32%, which
was significantly lower compared to that of patients with negative
expression (73%, P=0.024) (Fig. 2A).
When each cancer site was analyzed individually, statistical
significance was only demonstrated in IHBDCa. Cases with positive
expression of IGF-IR tended to exhibit poorer prognosis compared to
cases with negative expression (Fig.
2B–D). As regards mTOR and Raf-1, the 4-year survival rates
were 52 and 49% in cases with positive expression vs. 50 and 64% in
cases with negative expression. However, there was no significant
association between survival rate and positive expression of these
two proteins when each cancer site was analyzed individually (data
not shown).
Discussion
In this study, we demonstrated that i) IGF-IR, mTOR
and Raf-1 are overexpressed in human BTC; ii) the expression of
these proteins was not correlated to the histological type of the
tumors; iii) the positive expression rate of Raf-1 was higher in
advanced-stage compared to that in early-stage GBCa; and iv) the
positive expression of IGF-IR was associated with poor prognosis in
patients with resected BTC.
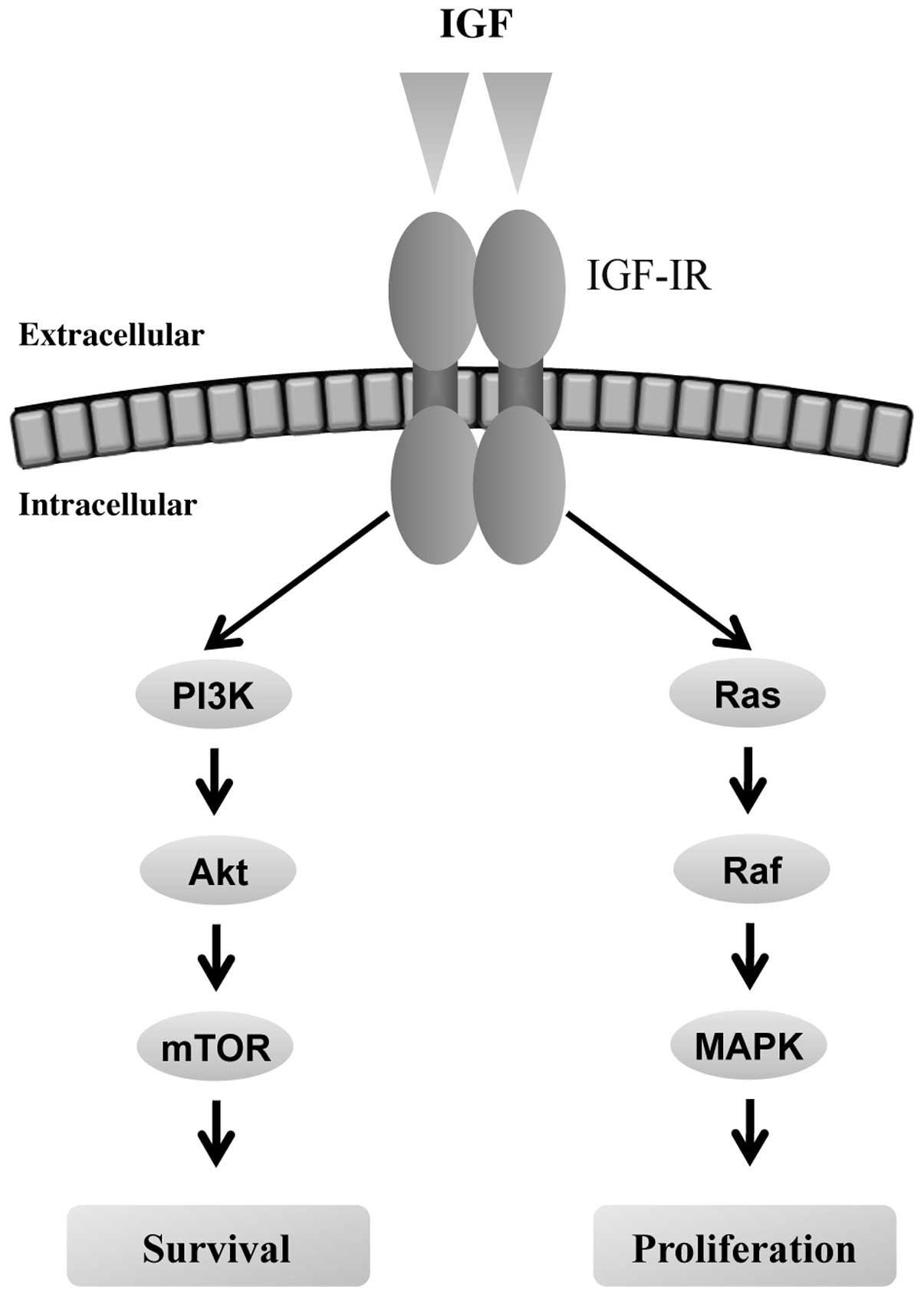
The insulin growth factor (IGF)-phosphoinositide 3
kinase (PI3K) pathway plays an important role in cell
proliferation, apoptosis, migration and differentiation (13) (Fig. 3).
This pathway is activated by interaction of IGF-IR, a transmembrane
tyrosine kinase and its ligands, IGF-1 and IGF-2, leading to
carcinogenesis and tumor progression by modulating cancer cell
motility, adhesion (14) and
angiogenesis (15). The expression
rate of IGF-IR in our study was 68%, which is similar to that
reported in other malignancies, including GBCa (16–18). If
this high rate of IGF-IR expression in BTC indicates activation of
the IGF-PI3K kinase pathway, there is the possibility of
IGF-IR-targeted therapy, such as the use of a receptor-specific
blocking monoclonal antibody and small-molecule tyrosine kinase
inhibitors. Indeed, a previous clinical trial using a monoclonal
antibody against IGF-IR in solid tumors indicated its safety and
efficacy (19). Our results
demonstrated that there was no association between IGF-IR and
pathological stage or histological grade, as shown in other types
of cancer (13). By contrast, Ohashi
et al (18) reported that
IGF-IR expression was associated with pathological T stage. This
may be explained by the distribution of each stage, such as the
ratio of stage IV being 46% in our cases but only 7% in their
cases. Of note, despite the fact that there was no difference in
the rate of IGF-IR expression by pathological stage, patients with
positive expression of IGF-IR exhibited worse survival. These
results suggest that IGF-IR may be involved in cancer cell
proliferation at an early stage and that prolonged expression of
IGF-IR may contribute to rapid cancer growth and poor outcome.
To the best of our knowledge, this is the first
study indicating that IGF-IR expression may predict unfavorable
prognosis for the patients with BTC. Since surgery alone is rarely
curative, the prognosis of human BTC is generally poor. The
identification of a prognostic biomarker may help distinguish
patients who may benefit from additional treatments in order to
decrease the risk of recurrence. As mentioned above, an association
between IGF-IR expression and poor clinical outcome has been
demonstrated in other tumors. However, Kornprat et al
(17) reported that low IGF-IR
expression was an independent marker of poor prognosis. There is a
limit to the argument regarding the association between molecular
expression and patient prognosis, as prognosis depends on a number
of factors.
mTOR is a serine/threonine kinase regulated by
protein kinase B (Akt) and has been shown to integrate signaling
from growth factors and nutrients and to regulate cell growth and
cell cycle progression (20–22). Moreover, mTOR is overexpressed in a
significant number of human tumors, either through upregulation of
Akt or through alternative regulatory pathways (22). Activation of the Akt/mTOR signaling
pathway may also result from enhanced Her2, activation since a
significant percentage of human GB tumors have been shown to have
positive expression of Her2 and/or EGFR (23–26). Leal
et al (27,28) reported that phospho-mTOR was
associated with poor prognosis and the Akt/mTOR substrate P70S6K is
frequently phosphorylated in patients with GBCa. Our results
revealed that ~70% of BTC samples expressed mTOR, which did not
deviate from the IGF-IR expression rate. Indeed, we previously
demonstrated the therapeutic effect of rapamycin for GBCa in
BK5.erbB2 transgenic mice, which have an extremely high incidence
of GBCa (29).
Raf-1 is a critical mediator of mitogenic signals
emanating from a variety of receptor tyrosine kinases, including
EGFR and Her2 (30). Raf-1 activation
of the mitogen-activated protein kinase kinase (MEK)/extracellular
signal-regulated kinase pathway inhibits apoptosis in cancer cells
and MEK inhibitors may abrogate the antiapoptotic function of Raf-1
(31,32). There are only episodic reports of
Raf-1-positive expression, particularly in ovarian and head and
neck cancer (33,34). Although the roles of Raf-1 in BTC
carcinogenesis and progression are not fully understood, our
results, demonstrating a high rate of Raf-1 expression, may
indicate activation of the IGFR-IR/Ras/Raf pathway.
There were several limitations to this study. First,
our results were from IHC of surgical specimens. The protein or
mRNA elevation was not investigated by western blotting or
polymerase chain reaction methods. Second, the phosphorylation of
IGF-IR, mTOR and Raf-1 was not analyzed. Finally, the methodology
of IHC and the interpretation of the results differ among
institutions. To solve these problems, further studies with
sufficient numbers of samples are required.
In summary, IGF-IR, mTOR and Raf-1 were found to be
highly expressed in BTC and targeted therapy against IGF-IR may be
effective in BTC patients. In addition, the high expression of
IGF-IR exhibited a significant association with poor prognosis,
indicating that IGF-IR may be a useful biomarker for predicting
prognosis.
Acknowledgements
This study was supported by Grants-in-Aid (no.
24390323) for scientific research from the Ministry of Education,
Culture, Sports, Science and Technology in Japan and the American
Society of Clinical Oncology Foundation Career Development Award
05-91-0325.
Glossary
Abbreviations
Abbreviations:
|
IGF-IR
|
insulin-like growth factor I
receptor
|
|
mTOR
|
mammalian target of rapamycin
|
|
Raf-1
|
rapidly accelerated fibrosarcoma-1
|
|
GBCa
|
gallbladder cancer
|
|
BTC
|
biliary tract cancer
|
|
IHC
|
immunohistochemistry
|
References
|
1
|
Jarnagin WR, Ruo L, Little SA, et al:
Patterns of initial disease recurrence after resection of
gallbladder carcinoma and hilar cholangiocarcinoma: implications
for adjuvant therapeutic strategies. Cancer. 98:1689–1700. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shaib YH, Davila JA, McGlynn K and
El-Serag HB: Rising incidence of intrahepatic cholangiocarcinoma in
the United States: a true increase? J Hepatol. 40:472–477. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics 2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Randi G, Franceschi S and La Vecchia C:
Gallbladder cancer worldwide: geographical distribution and risk
factors. Int J Cancer. 118:1591–1602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roa I, Araya JC, Villaseca M, Roa J, de
Aretxabala X and Ibacache G: Gallbladder cancer in a high risk
area: morphological features and spread patterns.
Hepatogastroenterology. 46:1540–1546. 1999.PubMed/NCBI
|
|
6
|
Medina E and Kaempffer AM: Cancer
mortality in Chile: epidemiological considerations. Rev Med Chil.
129:1195–1202. 2001.[(In Spanish)]. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kimura K, Ohto M, Saisho H, et al:
Association of gallbladder carcinoma and anomalous
pancreaticobiliary ductal union. Gastroenterology. 89:1258–1265.
1985.PubMed/NCBI
|
|
8
|
Kawamoto T, Krishnamurthy S, Tarco E, et
al: HER receptor family: novel candidate for targeted therapy for
gallbladder and extrahepatic bile duct cancer. Gastrointest Cancer
Res. 1:221–227. 2007.PubMed/NCBI
|
|
9
|
Watanabe H, Date K, Itoi T, et al:
Histological and genetic changes in malignant transformation of
gallbladder adenoma. Ann Oncol. 10 (Suppl 4):136–139. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furstenberger G and Senn HJ: Insulin-like
growth factors and cancer. Lancet Oncol. 3:298–302. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Papa V, Gliozzo B, Clark GM, et al:
Insulin-like growth factor-I receptors are overexpressed and
predict a low risk in human breast cancer. Cancer Res.
53:3736–3740. 1993.PubMed/NCBI
|
|
12
|
Matsubara J, Yamada Y, Hirashima Y, et al:
Impact of insulin-like growth factor type 1 receptor, epidermal
growth factor receptor, and HER2 expressions on outcomes of
patients with gastric cancer. Clin Cancer Res. 14:3022–3029. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mauro L and Surmacz E: IGF-I receptor,
cell-cell adhesion, tumor development and progression. J Mol
Histol. 35:247–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reinmuth N, Fan F, Liu W, et al: Impact of
insulin-like growth factor receptor-I function on angiogenesis,
growth, and metastasis of colon cancer. Lab Invest. 82:1377–1389.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imsumran A, Adachi Y, Yamamoto H, et al:
Insulin-like growth factor-I receptor as a marker for prognosis and
a therapeutic target in human esophageal squamous cell carcinoma.
Carcinogenesis. 28:947–956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kornprat P, Rehak P, Ruschoff J and
Langner C: Expression of IGF-I, IGF-II, and IGF-IR in gallbladder
carcinoma. A systematic analysis including primary and
corresponding metastatic tumors. J Clin Pathol. 59:202–206. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohashi H, Adachi Y, Yamamoto H, et al:
Insulin-like growth factor receptor expression is associated with
aggressive phenotypes and has therapeutic activity in biliary tract
cancers. Cancer Sci. 103:252–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karp DD, Paz-Ares LG, Novello S, et al:
Phase II study of the anti-insulin-like growth factor type 1
receptor antibody CP-751,871 in combination with paclitaxel and
carboplatin in previously untreated, locally advanced, or
metastatic non-small-cell lung cancer. J Clin Oncol. 27:2516–2522.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asnaghi L, Bruno P, Priulla M and Nicolin
A: mTOR: a protein kinase switching between life and death.
Pharmacol Res. 50:545–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Richardson CJ, Schalm SS and Blenis J:
PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol.
15:147–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito Y, Takeda T, Sasaki Y, et al:
Expression and clinical significance of the erbB family in
intrahepatic cholangiocellular carcinoma. Pathol Res Pract.
197:95–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yukawa M, Fujimori T, Hirayama D, et al:
Expression of oncogene products and growth factors in early
gallbladder cancer, advanced gallbladder cancer, and chronic
cholecystitis. Hum Pathol. 24:37–40. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki H, Isaji S, Pairojkul C and
Uttaravichien T: Comparative clinicopathological study of resected
intrahepatic cholangiocarcinoma in northeast Thailand and Japan. J
Hepatobiliary Pancreat Surg. 7:206–211. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ukita Y, Kato M and Terada T: Gene
amplification and mRNA and protein overexpression of c-erbB-2
(HER-2/neu) in human intrahepatic cholangiocarcinoma as detected by
fluorescence in situ hybridization, in situ hybridization, and
immunohistochemistry. J Hepatol. 36:780–785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leal P, Garcia P, Sandoval A, et al:
Immunohistochemical expression of phospho-mTOR is associated with
poor prognosis in patients with gallbladder adenocarcinoma. Arch
Pathol Lab Med. 137:552–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leal P, Garcia P, Sandoval A, et al:
AKT/mTOR substrate P70S6K is frequently phosphorylated in
gallbladder cancer tissue and cell lines. Onco Targets Ther.
6:1373–1384. 2013.PubMed/NCBI
|
|
29
|
Wu Q, Kiguchi K, Kawamoto T, et al:
Therapeutic effect of rapamycin on gallbladder cancer in a
transgenic mouse model. Cancer Res. 67:3794–3800. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kolch W: Meaningful relationships: the
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J. 351:289–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cleveland JL, Troppmair J, Packham G, et
al: v-raf suppresses apoptosis and promotes growth of
interleukin-3-dependent myeloid cells. Oncogene. 9:2217–2226.
1994.PubMed/NCBI
|
|
32
|
Erhardt P, Schremser EJ and Cooper GM:
B-Raf inhibits programmed cell death downstream of cytochrome c
release from mitochondria by activating the MEK/Erk pathway. Mol
Cell Biol. 19:5308–5315. 1999.PubMed/NCBI
|
|
33
|
McPhillips F, Mullen P, Monia BP, et al:
Association of c-Raf expression with survival and its targeting
with antisense oligonucleotides in ovarian cancer. Br J Cancer.
85:1753–1758. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Riva C, Lavieille JP, Reyt E, Brambilla E,
Lunardi J and Brambilla C: Differential c-myc, c-jun, c-raf and p53
expression in squamous cell carcinoma of the head and neck:
implication in drug and radioresistance. Eur J Cancer B Oral Oncol.
31B:384–391. 1995. View Article : Google Scholar : PubMed/NCBI
|