Introduction
While accurate preoperative staging of mediastinal
and hilar lymph nodes is essential in determining the treatment
strategy for patients with non-small-cell lung cancer (NSCLC)
(1,2),
the accuracy of a preoperative diagnosis for N1 disease is
problematic. Clinically diagnosed N1 (cN1) patients have been
reported to comprise 19–30% pathologically N0 (pN0), 44–47% pN1 and
17–60% pN2-pN3 cases (3–5). Partly due to the high rate of occult pN2
patients, the cN1 cohort was associated with an unsatisfactory
surgical prognosis. Furthermore, unexpected extracapsular invasion
of the metastatic hilar lymph nodes often results in extensive
surgical resection more often than standard lobectomy, such as
pneumonectomy, bilobectomy, or lobectomy with bronchoplasty and
angioplasty. Diagnostic clues for the preoperative prediction of
extranodal invasion and subsequent extensive surgical resection may
be useful in determining the treatment strategy in surgical
candidates, particularly those with certain surgical risks or with
poor cardiopulmonary reserve. Positron emission tomography (PET)
with 2-deoxy-2-(18F)fluoro-D-glucose as a tracer
(18F-FDG-PET) was recently reported to be more effective
in detecting tumor involvement of mediastinal and hilar lymph nodes
compared with computed tomography (CT) (6–8). However,
those reports did not refer to the correlation between the PET
findings and the type of lymph node metastasis, i.e., intracapsular
or extracapsular, and the required surgical procedure, which was
standard lobectomy or an extended resection. The aim of this study
was to investigate the ability of fusion PET/CT to predict
extracapsular invasion of hilar lymph node metastasis.
Patients and methods
Patients
Between April, 2007 and April 2013, 509 consecutive
patients with primary lung cancer underwent surgical resection at
our institution. Among these patients, 28 with pathologically
proven hilar lymph node metastasis (at stations 10 and 11), without
mediastinal lymph node metastasis and without having received
induction therapy, were retrospectively reviewed in this study. All
the patients underwent chest and abdominal CT, brain magnetic
resonance imaging (MRI) and PET/CT for clinical staging, and were
pathologically diagnosed with hilar lymph node metastasis
postoperatively. The following parameters were assessed from the
medical records: patient gender, age, smoking habits, histological
type, tumor location, tumor stage, surgical procedure and
prognosis.
This study was reviewed and approved by the
Institutional Review Board of Toho University (Tokyo, Japan).
Treatments and evaluation
The routine preoperative workup included pulmonary
function tests, CT scans of the chest and abdomen, PET/CT, flexible
bronchoscopy and brain MRI. To evaluate lymph node metastasis,
enhanced chest CT and PET/CT were performed. A team of experienced
radiologists reviewed the integrated PET/CT images independently. A
lymph node maximum standardized uptake value (SUVmax) of
>2.5 was interpreted as positive (9,10). All
integrated PET/CT imaging was performed within 4 weeks of surgery.
The surgical procedures included standard lobectomy or extensive
pulmonary resection, such as bilobectomy, sleeve resection, or
pneumonectomy. Systematic lymph node dissection was mandatory for
all the patients in this study. Mediastinal lymph node dissection
consisted of en bloc resection of all nodes at stations 2R, 4R, 7,
8, 9, 10R and 11R for right-sided tumors, and at stations 4L, 5, 6,
7, 8, 9, 10L and 11L for left-sided tumors. The designation of
dissected nodal status was based on the International Association
for the Study of Lung Cancer (IASLC) lymph node map (11) and the seventh edition of the TNM
staging system (12). The
histological classification of NSCLC was based on the WHO
classification. The dissected lymph nodes were histologically
examined using hematoxylin and eosin staining.
Statistical analysis
Univariate data analysis was conducted using
Pearson's Chi-squared test and multivariate analysis was conducted
using logistic regression (backward stepwise). Differences were
considered to be statistically significant when P<0.05. Overall
survival (OS) was defined as the time from the date of surgery
until the date of the last follow-up or death. Disease-specific
survival was defined as the time from the date of surgery until the
date of the last follow-up. Patients who remained alive or who had
succumbed to a cause other than lung cancer, were censored for
disease-specific survival analysis. Survival curves were prepared
using the Kaplan-Meier method and were compared univariately using
the log-rank test. All the statistical analyses were performed
using JMP 11.0 software (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The 28 patients with hilar lymph node metastasis
were predominantly male (71%) and smokers (86%). The most common
tumor types were squamous cell carcinoma in 11 (39%) and
adenocarcinoma in 12 patients (43%). The surgical procedures
performed were standard lobectomy in 12 (43%) and extensive
pulmonary resection in 16 patients (57%). Of the patients receiving
extensive pulmonary resection, 2 underwent plasty of the bronchus
or pulmonary artery (Table I).
 | Table I.Characteristics of the patients with
hilar lymph node metastasis (n=28). |
Table I.
Characteristics of the patients with
hilar lymph node metastasis (n=28).
| Characteristics | Number |
|---|
| Age, years |
|
| Mean
(range) | 68 (44–81) |
| Gender |
|
| Male | 20 |
|
Female | 8 |
| Smoking habits |
|
|
Non-smoker | 4 |
|
Current/former smoker | 24 |
| Histology |
|
| Squamous
cell carcinoma | 11 |
|
Adenocarcinoma | 12 |
|
Othersa | 5 |
| Tumor location |
|
|
Right | 13 |
| Left | 15 |
| Pathological T
stage |
|
| T1 | 11 |
| T2 | 14 |
| T3 | 3 |
| Surgical
procedures |
|
|
Lobectomy | 12 |
| Lobectomy
with bronchoplasty | 1 |
| Lobectomy
with vascular plasty | 1 |
|
Bilobectomy | 5 |
|
Pneumonectomy | 9 |
Characteristics of hilar lymph
nodes
The metastatic N1 station was station 10 in 5 (18%)
and station 11 in 23 patients (82%). Extracapsular invasion of the
hilar lymph node was detected in 14 patients (50%) (Table II).
 | Table II.Characteristics of the metastatic
hilar lymph nodes. |
Table II.
Characteristics of the metastatic
hilar lymph nodes.
| Variables | Number |
|---|
| Pathological N1
station |
|
| 10L | 5 |
| 10R | 0 |
| 11L | 10 |
| 11R | 13 |
| Extracapsular nodal
invasion |
|
|
Absent | 14 |
|
Present | 14 |
| Preoperative hilar
lymph node |
|
| PET/CT findings |
|
|
Positive | 17 |
|
Negative | 11 |
PET/CT visual assessment analysis
A total of 17 patients (60%) had positive hilar
lymph node findings on PET/CT and the remaining 11 had negative
findings (Table III). The rate of
extracapsular invasion was significantly higher in the patient
group with positive PET/CT findings (13/17, 76%) compared with that
in the group with negative PET/CT findings (1/11, 9%) (P=0.0005).
Extensive pulmonary resection was performed more frequently in the
patient group with positive PET/CT findings (13/17, 76%) compared
with the group with negative PET/CT findings (3/11, 27%)
(P=0.01).
 | Table III.Univariate analysis for factors
associated with PET/CT findings in hilar lymph node metastasis. |
Table III.
Univariate analysis for factors
associated with PET/CT findings in hilar lymph node metastasis.
|
| Hilar lymph node
PET/CT findings |
|
|---|
|
|
|
|
|---|
| Variables | Positive | Negative | P-value |
|---|
| Mean age, years | 69 | 66.5 | 0.47 |
| Gender |
|
| 0.11 |
| Male | 14 | 6 |
|
|
Female | 3 | 5 |
|
| Smoking habits |
|
| 0.12 |
|
Non-smoker | 1 | 3 |
|
|
Current/former smoker | 16 | 8 |
|
| Tumor location |
|
| 0.93 |
|
Right | 9 | 6 |
|
|
Left | 8 | 5 |
|
| Pathological T
stage |
|
| 0.59 |
| T1 | 6 | 5 |
|
|
T2-3 | 11 | 6 |
|
| Pathological N1
station |
|
| 0.29 |
| No.
10 | 2 | 3 |
|
| No.
11 | 15 | 8 |
|
| Histology |
|
| 0.29 |
|
Non-SCC | 9 | 8 |
|
|
SCC | 8 | 3 |
|
| Extracapsular
invasion |
|
| 0.001 |
|
Present | 13 | 1 |
|
|
Absent | 4 | 10 |
|
| Surgical
procedures |
|
| 0.01 |
|
Lobectomy | 4 | 8 |
|
|
Extensive pulmonary
resectiona | 13 | 3 |
|
Analysis of extracapsular invasion of
hilar lymph nodes
The univariate analysis identified two factors as
significant predictors of extracapsular nodal invasion, namely the
PET/CT and histological findings (P=0.0005 and 0.05, respectively)
(Table IV). In the multivariate
analysis, the PET/CT findings were the only independent predictor
(P=0.0004) (Table V).
 | Table IV.Univariate analysis for factors
predictive of extracapsular nodal invasion. |
Table IV.
Univariate analysis for factors
predictive of extracapsular nodal invasion.
|
| Extracapsular nodal
invasion |
|---|
|
|
|
|---|
| Variables | HR | 95% CI |
P-valuea |
|---|
| Age, years |
|
|
|
|
<70 | 1 |
|
|
|
≥70 | 0.42 | 0.09–1.91 | 0.25 |
| Gender |
|
|
|
|
Female | 1 |
|
|
|
Male | 2.03 | 0.38–10.9 | 0.4 |
| Smoking habits |
|
|
|
|
Non-smoker | 1 |
|
|
|
Current/former smoker | 3.5 | 0.32–39.1 | 0.28 |
| Tumor location |
|
|
|
|
Right | 1 |
|
|
|
Left | 1.33 | 0.30–5.91 | 0.14 |
| Hilar lymph node
PET/CT findings |
|
|
|
|
Negative | 1 |
|
|
|
Positive | 32.5 | 3.12–337 | 0.0005 |
| Pathological T
stage |
|
|
|
| T1 | 1 |
|
|
|
T2-3 | 1.35 | 0.29–6.18 | 0.7 |
| Pathological N1
station |
|
|
|
| No.
10 | 1 |
|
|
| No.
11 | 1.64 | 0.23–11.7 | 0.62 |
| Histology |
|
|
|
|
Non-SCC | 1 |
|
|
|
SCC | 4.88 | 0.93–25.6 | 0.05 |
 | Table V.Multivariate analysis for factors
predictive of extracapsular nodal invasion. |
Table V.
Multivariate analysis for factors
predictive of extracapsular nodal invasion.
| Variables | HR | 95% CI |
P-valuea |
|---|
| PET/CT
findings |
|
|
|
|
Negative | 1 |
|
|
|
Positive | 39 | 4.2–1217 | 0.0004 |
| Histology |
|
|
|
|
Non-SCC | 1 |
|
|
|
SCC | 6.5 | 0.75–142 | 0.09 |
Survival pattern analysis
A total of 3 patients (11%) succumbed during the
postoperative period (pneumonia, 2 patients; and acute exacerbation
of interstitial pneumonia, 1 patient). Follow-up was performed for
all the patients. The median follow-up time was 39.5 months (range,
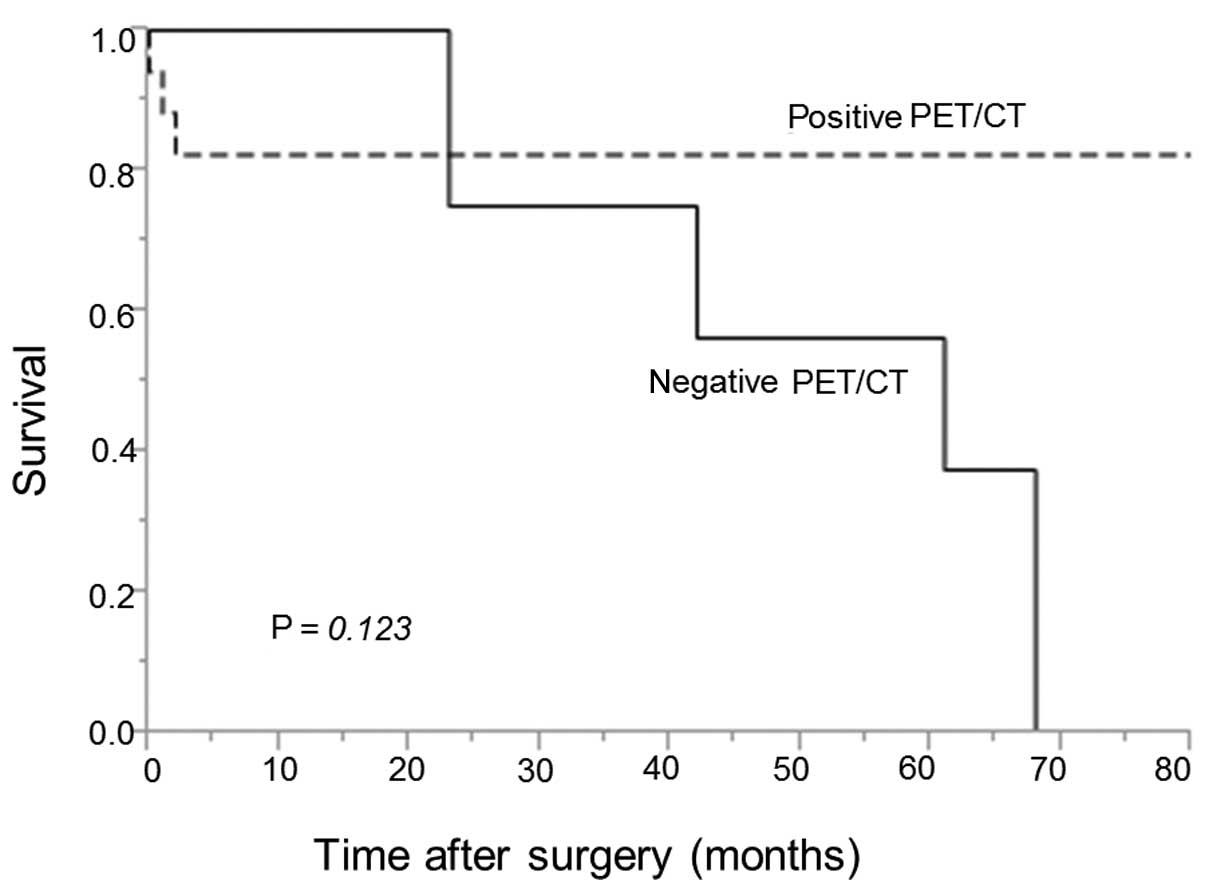
0–80 months). The 5-year OS rate was 82 vs. 38% in the patient
groups with positive vs. negative PET/CT findings, respectively
(Fig. 1). The difference between the
two groups was not statistically significant (P=0.123). The 5-year
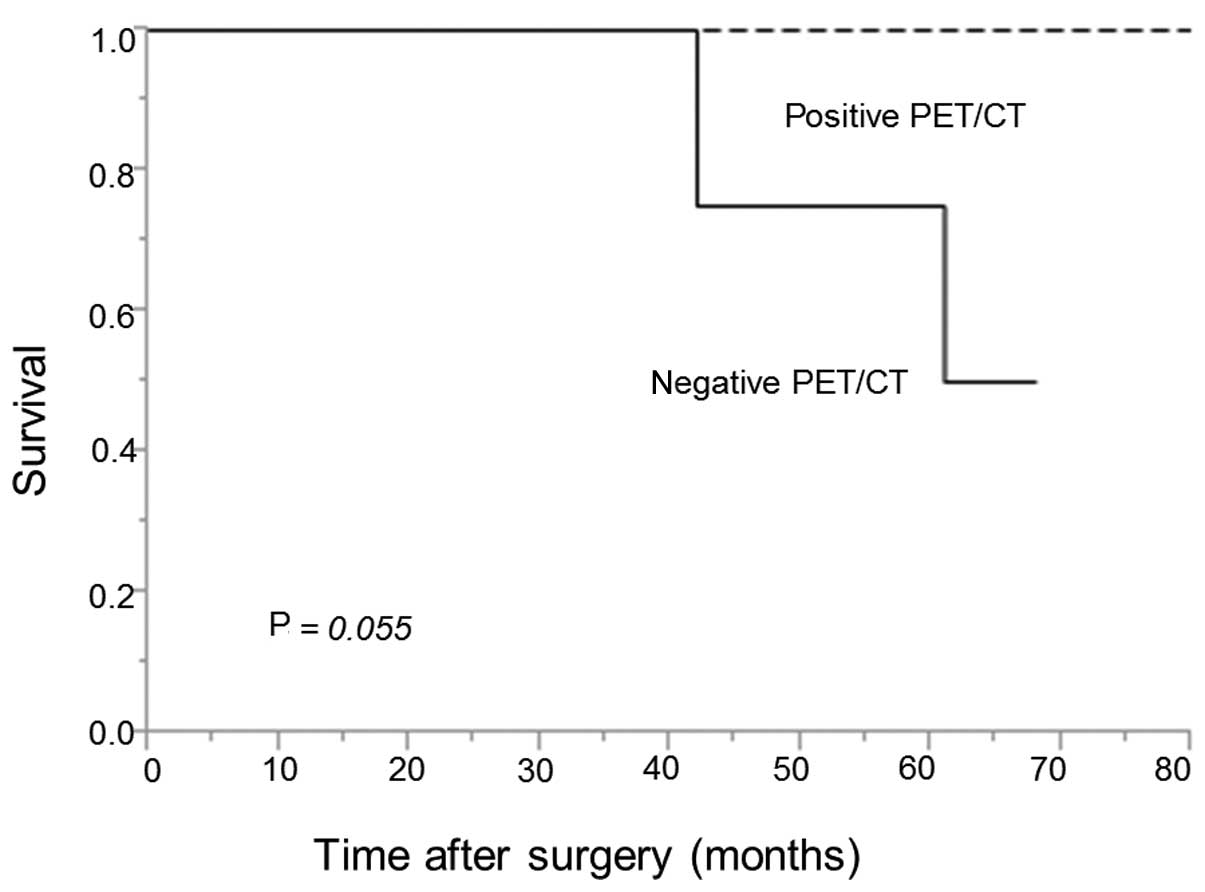
disease-specific survival rate was 100 vs. 50% in the patient
groups with positive vs. negative PET/CT findings, respectively
(Fig. 2). The difference between the
two groups was not statistically significant (P=0.055), although
the group with positive PET/CT findings tended to have a better
prognosis compared with the group with negative findings.
Discussion
The presence of pN1 disease may unavoidably lead to
changes in the surgical approach. A recent study reported that
extensive pulmonary resection was required in 41% of patients with
cN1 disease. Therefore, it is crucial to predict the potential
extracapsular invasion of hilar lymph node metastasis
preoperatively. The main findings of the present study were as
follows: i) PET/CT was a significant predictor of extracapsular
invasion in cases with hilar lymph node metastasis and ii)
extensive pulmonary resection was required in 76% of the patients
with positive hilar lymph node metastasis on PET/CT.
PET/CT is most beneficial for identifying the
presence of metastatic disease. PET/CT relies on the hypermetabolic
nature of cancer cells for preferential uptake of the radiolabeled
glucose analogue 18F-FDG. The fusion of PET and CT
simultaneously provides anatomical and functional information, thus
improving localization accuracy. Preferential uptake of
18F-FDG in the lymph nodes may be quantified by the SUV.
In the mediastinum, high SUV cut-off values identifying malignant
lymph nodes increase the chances of false-negative results, thus
leading to the current recommendation of an SUV of 2.5 as the
criterion for the classification of a node as positive (9,13). Using
an SUV of 2.5 as the threshold, the resulting sensitivity,
specificity and negative predictive value were reported to be 89,
84 and 96%, respectively (9).
Imaging tests, such as PET-CT, have been used for
the clinical diagnosis of the nodal status during staging. Previous
randomized trials have demonstrated that PET/CT is significantly
more accurate and more sensitive for the staging of NSCLC compared
with the conventional staging regimen (14). The pooled sensitivity and specificity
of PET for identifying mediastinal lymph node metastasis were
reported to be 74 and 85%, respectively (15). However, the sensitivity and
specificity of PET for identifying hilar lymph node metastasis were
only 48.5 and 80.2%, respectively (10). Furthermore, it is more difficult to
discriminate N1 involvement and primary tumors per se using
imaging alone, particularly when the primary tumor is very close in
proximity to the N1 nodes involved. Therefore, tissue confirmation
is recommended to determine whether lymph node metastasis is truly
present. Hilar lymph nodes have recently become accessible by means
of endobronchial ultrasound (16).
However, extracapsular invasion of hilar lymph node metastasis is
difficult to diagnose using endobronchial ultrasound.
The present study identified PET/CT as a significant
predictor of extracapsular invasion of hilar lymph node metastasis
in the multivariate analysis (P=0.0005). Shin et al
(17) reported that the presence of
extracapsular nodal invasion detected by both CT and PET/CT was
more frequent in the cN1-pN1 group compared with the cN0-pN1 group,
resulting in a poorer surgical outcome. Although PET/CT is reported
to be less sensitive for identifying hilar lymph node metastasis,
we found it to be useful for identifying extracapsular invasion of
hilar lymph node metastasis.
Regarding surgery, Watanabe et al (3) reported that extensive pulmonary
resection was required in 41% of patients with cN1 disease. Moreno
et al (18) reported that
pneumonectomy was required in 40% of surgically treated
T3>7 cm N1 NSCLC patients. The postoperative
mortality rate for surgical resections in lung cancer was recently
found to have significantly improved (19); thus, preoperative detailed
cardiopulmonary function tests should be mandatory to reduce
surgical morbidity and mortality. In the present study, extensive
pulmonary resection was required in 53% of the patients with hilar
lymph node metastasis. Furthermore, extensive pulmonary resection
was required in 76% of the patients with positive hilar lymph node
metastasis on PET/CT (P=0.003). It may be deduced from our findings
that such patients are more likely to require extensive pulmonary
resection, as hilar lymph node metastasis with extracapsular
invasion is very close in proximity to the bronchus and pulmonary
arteries.
As regards prognosis, there was no significant
difference in OS or disease-specific survival rates between the
positive and negative PET/CT groups in our study. However, the
positive PET/CT group tended to have a better prognosis in terms of
disease-specific survival (P=0.055), exhibiting a 100%
disease-specific survival rate at 5 years. The positive PET/CT
group displayed a high rate of extracapsular invasion (76%) and
required extended surgical resection. While the presence of
extracapsular invasion may indicate poor survival (17), the positive PET/CT group exhibited
favorable outcomes, probably due to the beneficial effect of
extended surgical resection on curability. Even with extracapsular
involvement, sufficient therapy by extensive surgical resection may
result in an acceptable surgical outcome. Preoperative meticulous
evaluation of pulmonary and cardiac function tests should be
mandatory for patients with positive hilar lymph node findings on
PET/CT to assess the possibility of extended resection.
This study had several limitations. As our data were
retrospectively collected and reviewed, there were some intrinsic
drawbacks. In addition, although all the patients had pN1 disease,
the study population comprised a heterogeneous group of subjects.
In this study, the CT size criterion for metastatic lymph nodes,
i.e., a short-axis diameter of >1 cm on a transverse CT image,
was not implemented, the reason being that the hilar lymph nodes
were difficult to distinguish from blood vessels in certain
patients examined without the use of intravenous contrast
medium.
In conclusion, we retrospectively reviewed the
clinical and pathological characteristics of patients with hilar
lymph node metastasis following surgical resection for NSCLC. The
PET/CT findings were a significant predictor of extracapsular
invasion of hilar lymph node metastasis. Extensive pulmonary
resection was required in patients with positive hilar lymph node
metastasis on PET/CT, resulting in acceptable surgical outcomes,
despite a relatively high postoperative mortality rate. Thus,
meticulous preoperative evaluation of pulmonary and cardiac
function test should be mandatory for patients with PET/CT positive
hilar lymph node for determining potential extensive pulmonary
resection.
Acknowledgements
This study was supported in part by a Grant-in-aid
for Scientific Research (C) 24592098 and 26462140 from the Japanese
Ministry of Education, Culture, Sports, Science and Technology.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small-cell lung cancer
|
|
FDG-PET
|
2-deoxy-2-(18F)fluoro-D-glucose-positron emission
tomography
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
SUV
|
standardized uptake value
|
|
IASLC
|
International Association for the
Study of Lung Cancer
|
|
OS
|
overall survival
|
References
|
1
|
Mountain CF: Revisions in the
international system for staging lung cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rusch VW, Crowley J, Giroux DJ, Goldstraw
P, Im JG, Tsuboi M, Tsuchiya R and Vansteenkiste JInternational
Staging Committee; Cancer Research and Biostatistics; Observers to
the Committee; Participating Institutions: The IASLC Lung Cancer
Staging Project: proposals for the revision of the N descriptors in
the forthcoming seventh edition of the TNM classification for lung
cancer. J Thorac Oncol. 2:603–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watanabe S, Asamura H, Suzuki K and
Tsuchiya R: Problems in diagnosis and surgical management of
clinical N1 non-small cell lung cancer. Ann Thorac Surg.
79:1682–1685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hishida T, Yoshida J, Nishimura M,
Nishiwaki Y and Nagai K: Problems in the current diagnostic
standards of clinical N1 non-small cell lung cancer. Thorax.
63:526–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyasaka Y, Suzuki K, Takamochi K,
Matsunaga T and Oh S: The maximum standardized uptake value of
fluorodeoxyglucose positron emission tomography of the primary
tumour is a good predictor of pathological nodal involvement in
clinical N0 non-small-cell lung cancer. Eur J Cardiothorac Surg.
44:83–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinert HC, Hauser M, Allemann F, Engel
H, Berthold T, von Schulthess GK and Weder W: Non-small cell lung
cancer: Nodal staging with FDG PET versus CT with correlative lymph
node mapping and sampling. Radiology. 202:441–446. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vansteenkiste JF, Stroobants SG, De Leyn
PR, Dupont PJ, Verschakelen JA, Nackaerts KL and Mortelmans
LALeuven Lung Cancer Group: Mediastinal lymph node staging with
FDG-PET scan in patients with potentially operable non-small cell
lung cancer: A prospective analysis of 50 cases. Chest.
112:1480–1486. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vansteenkiste JF, Stroobants SG, Dupont
PJ, De Leyn PR, De Wever WF, Verbeken EK, Nuyts JL, Maes FP and
Bogaert JG: FDG-PET scan in potentially operable non-small cell
lung cancer: Do anatometabolic PET-CT fusion images improve the
localisation of regional lymph node metastases? The Leuven Lung
Cancer Group. Eur J Nucl Med. 25:1495–1501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hellwig D, Graeter TP, Ukena D, Groeschel
A, Sybrecht GW, Schaefers HJ and Kirsch CM: 18F-FDG PET
for mediastinal staging of lung cancer: Which SUV threshold makes
sense? J Nucl Med. 48:1761–1766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carrillo SA, Daniel VC, Hall N, Hitchcock
CL, Ross P Jr and Kassis ES: Fusion positron emission/computed
tomography underestimates the presence of hilar nodal metastases in
patients with resected non-small cell lung cancer. Ann Thorac Surg.
93:1621–1624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rusch VW, Asamura H, Watanabe H, Giroux
DJ, Rami-Porta R and Goldstraw PMembers of IASLC Staging Committee:
The IASLC Lung Cancer Staging Project: a proposal for a new
international lymph node map in the forthcoming seventh edition of
the TNM classification for lung cancer. J Thorac Oncol. 4:568–577.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vansteenkiste JF, Stroobants SG, Dupont
PJ, De Leyn PR, Verbeken EK, Deneffe GJ, Mortelmans LA and Demedts
MGLeuven Lung Cancer Group: Prognostic importance of the
standardized uptake value on
18F-fluoro-2-deoxy-glucose-positron emission tomography
scan in non-small-cell lung cancer: An analysis of 125 cases. J
Clin Oncol. 17:3201–3206. 1999.PubMed/NCBI
|
|
14
|
Fischer B, Lassen U, Mortensen J, Larsen
S, Loft A, Bertelsen A, Ravn J, Clementsen P, Høgholm A, Larsen K,
et al: Preoperative staging of lung cancer with combined PET-CT. N
Engl J Med. 361:32–39. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Silvestri GA, Gould MK, Margolis ML,
Tanoue LT, McCrory D, Toloza E and Detterbeck FAmerican College of
Chest Physicians: Noninvasive staging of non-small cell lung
cancer: ACCP evidenced-based clinical practice guidelines (2nd
edition). Chest. 132 (Suppl 3):178S–201S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ernst A, Eberhardt R, Krasnik M and Herth
FJ: Efficacy of endobronchial ultrasound-guided transbronchial
needle aspiration of hilar lymph nodes for diagnosing and staging
cancer. J Thorac Oncol. 4:947–950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin S, Kim HK, Choi YS, Kim K, Kim J and
Shim YM: Prognosis of unexpected and expected pathologic N1
non-small cell lung cancer. Ann Thorac Surg. 96:969–975;
discussion. 975–976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moreno AC, Morgensztern D, Boffa DJ,
Decker RH, Yu JB, Detterbeck FC, Wang Z, Rose MG and Kim AW:
Treating locally advanced disease: An analysis of very large, hilar
lymph node positive non-small cell lung cancer using the National
Cancer Data Base. Ann Thorac Surg. 97:1149–1155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe S, Asamura H, Suzuki K and
Tsuchiya R: Recent results of postoperative mortality for surgical
resections in lung cancer. Ann Thorac Surg. 78:999–1002;
discussion. 1002–1003. 2004. View Article : Google Scholar : PubMed/NCBI
|