Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related mortality and the sixth most common type of
cancer worldwide (1). Of the 12.7
million new cases of cancer diagnosed worldwide in 2008, HCC
accounted for 5.9% (748,000), while of the 7.6 million cancer
deaths worldwide in 2008, HCC accounted for 1,234,000 (9.7%)
(2). As the majority of HCC patients
are diagnosed at an advanced stage, the prognosis of HCC is
generally poor. Therefore, early and accurate diagnosis of HCC may
significantly improve the survival rate of the patients.
Squamous cell carcinoma antigen (SCCA) is a novel
tumor marker recently discovered to be of diagnostic value in
patients with HCC. SCCA is a serine protease inhibitor
physiologically found in the spinous and granular layers of normal
squamous epithelium, and typically expressed by neoplastic cells of
epithelial origin (3).
SCCA-immunoglobulin (Ig)M is the immunocomplex, the serpin SCCA
complexed with IgM. Increased levels of SCCA have been found in
epithelial cancers of the neck, cervix and lungs (4–6). Although
SCCA and SCCA-IgM reportedly exhibit low sensitivity (41.9 and
52.3%, respectively), they have a high specificity (82.6 and 75.7%,
respectively) for HCC (7). The aim of
the present study was to determine the diagnostic performance of
serum SCCA and SCCA-IgM for HCC diagnosis using a
meta-analysis.
Materials and methods
Search strategy and study
selection
Embase, Medline (using PubMed as the search engine),
Chinese Biomedical Literature Database (CBM), Weipu, Wanfang data
and CNKI databases were searched to identify relevant studies
without restrictions regarding year of publication, study design or
language. MeSH and keyword searches were used. A manual search was
also performed of the references listed in the original articles
and review articles retrieved. The keywords used for the literature
search were as follows: SCCA, squamous cell carcinoma antigen,
SCCA-IgM, HCC, liver cancer, liver tumor, liver neoplasm, hepatoma
and hepatic carcinoma.
The inclusion criteria were as follows: i) Studies
investigating the diagnostic performance of serum SCCA and SCCA-IgM
for HCC diagnosis; ii) sample size of HCC and non-HCC patients,
true-positive (TP), false-positive (FP), false-negative (FN) and
true-negative (TN) were reported or calculable; and iii) a minimal
sample size of 10 patients.
The exclusion criteria were as follows: i) Studies
conducted on animals; ii) duplicate reports; iii) studies with no
clearly reported outcomes of interest; iv) case reports and letters
to the editors; v) reviews or systematic reviews; vi) studies
investigating HCC recurrence following hepatectomy; and vii) the
assay type used was not ELISA.
Data extraction and quality
assessment
Two reviewers (Zhang and Zhou) independently
assessed the articles. The title and abstract of each article were
reviewed to identify eligible studies. Disagreements on study
eligibility were resolved through discussion. The information
extracted from the eligible studies included publication year,
country, characteristics of the participants, test methods,
reference standard and cut-off values.
Two reviewers (Zhang and Zhou) independently
assessed the quality of each study, according to the Quality
Assessment of Diagnostic Accuracy Studies (QUADAS-2) checklist
recommended by the Cochrane Collaboration (8). Each of the items in the QUADAS-2
checklist was scored as ‘yes’, ‘no’, or ‘unclear’.
Statistical analysis
In the present study, the recommended standard
methods for meta-analyses of diagnostic tests was used for
evaluation (9). The analyses were
performed using RevMan version 5.2 and MetaDisc version 1.4
software programs (10). A
random-effects model was used to pool sensitivity, specificity,
diagnostic odds ratio (DOR) and their corresponding 95% confidence
intervals (CIs) and forest plots were used to depict the
heterogeneity of the eligible studies, as well as the sensitivity
and specificity of individual studies with the corresponding 95%
CIs. The summary receiver operating characteristic (SROC) curves
demonstrated the overall diagnostic performance of SCCA and
SCCA-IgM (11). The inconsistency
index (I2) reflected the degree of heterogeneity
(12). Spearmans rank correlation
coefficient was used to determine whether the heterogeneity could
be explained by a threshold effect and meta-regression was
performed to identify possible sources of heterogeneity caused by
non-threshold effect (9).
Results
Study eligibility
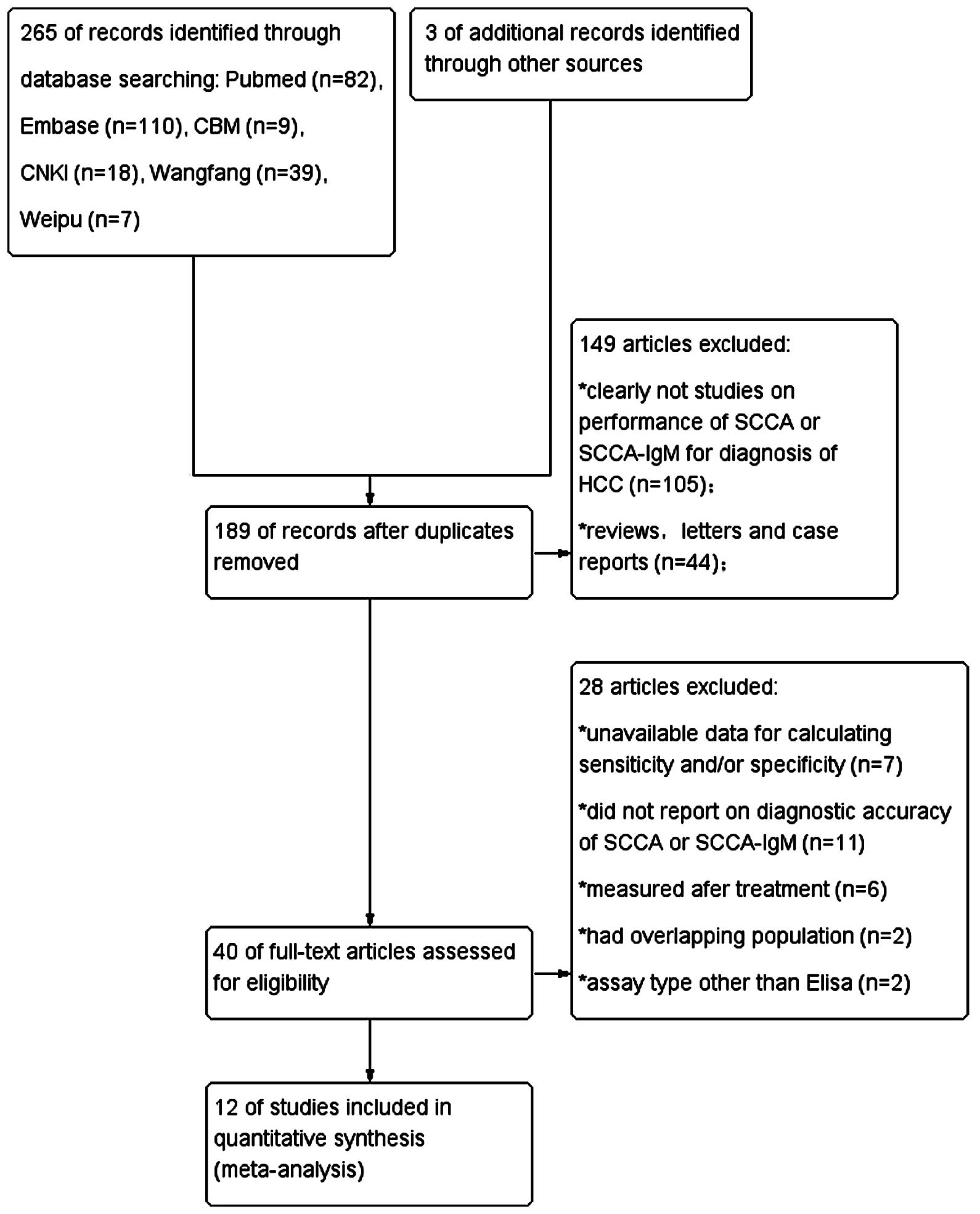
An independent search identified a total of 265
articles. Following exclusion of duplicate studies, a total of 189
articles remained. After reviewing the titles and abstracts, 40
articles were considered relevant. Following full-text review, 12
articles (7,13–23) were
finally included in our analysis, according to the strict inclusion
and exclusion criteria mentioned above. A flowchart of the study
selection process is shown in Fig. 1.
The 12 studies (8 studies on the diagnostic value of SCCA, 3
studies on SCCA-IgM and 1 study on both), included a total of 2,354
subjects (1,190 HCC and 1,164 non-HCC patients). The
characteristics of the included articles are summarized in Tables I and II. All the eligible studies were published
from 2005 onwards. The sample size ranged between 81 and 961. Five
studies were performed in Asian (14,20–23), 5 in
European (7,13,15,17,18)
and 2 in African populations (16,19).
 | Table I.Characteristics of included studies on
serum squamous cell carcinoma antigen. |
Table I.
Characteristics of included studies on
serum squamous cell carcinoma antigen.
| Study (year) | Country | HCC/controls | Gender (M/F,
HCC) | Cut-off, ng/ml | TP | FP | TN | FN | AUC | Refs. |
|---|
| Trerotoli et
al (2009) | Italy | 55/27 | 44/11 | 1.1 | 40 | 0 | 27 | 15 | 0.897 | (15) |
| Giannelli et
al (2005) | Italy | 120/90 | 95/25 | 0.368 | 101 | 47 | 43 | 19 | 0.705 | (17) |
| Hussein et al
(2008) | Egypt | 49/45 | 39/10 | 1.5 | 38 | 7 | 38 | 11 | 0.869 | (16) |
| Soyemi et al
(2012) | Nigeria | 60/30 | 40/20 | 0.368 | 45 | 22 | 8 | 15 | 0.525 | (14) |
| Giannelli et
al (2007) | Italy | 499/462 | 404/95 | 3.8 | 209 | 80 | 382 | 290 | 0.656 | (7) |
| Salman et al
(2011) | Egypt | 30/60 | Unknown | 0.53 | 24 | 14 | 46 | 6 | Unknown | (19) |
| Zhai et al
(2009) | China | 50/50 | 41/9 | 0.12 | 40 | 22 | 28 | 10 | Unknown | (23) |
| Wu (2007) | China | 34/47 | 31/3 | 1.2 | 19 | 7 | 40 | 15 | 0.761 | (20) |
| Chen et al
(2010) | China | 105/30 | Unknown | 1.5 | 75 | 4 | 26 | 30 | 0.91 | (22) |
 | Table II.Characteristics of included studies
on serum squamous cell carcinoma antigen-immunoglobulin M. |
Table II.
Characteristics of included studies
on serum squamous cell carcinoma antigen-immunoglobulin M.
| Study (year) | Country | HCC/controls | Gender (M/F,
HCC) | Cut-off,
AUC/ml | TP | FP | TN | FN | AUC | Refs. |
|---|
| Beneduce et
al (2005) | Italy | 50/50 | Unknown | 120 | 35 | 13 | 37 | 15 | 0.741 | (18) |
| Pozzan et al
(2014) | Italy | 81/206 | 63/18 | 89 | 72 | 102 | 104 | 9 | 0.66 | (13) |
| Giannelli et
al (2007) | Italy | 499/462 | 404/95 | 104 | 261 | 112 | 350 | 238 | 0.675 | (7) |
| Zhai et al
(2014) | China | 57/67 | 41/16 | 110.5 | 42 |
8 | 59 | 15 | 0.853 | (21) |
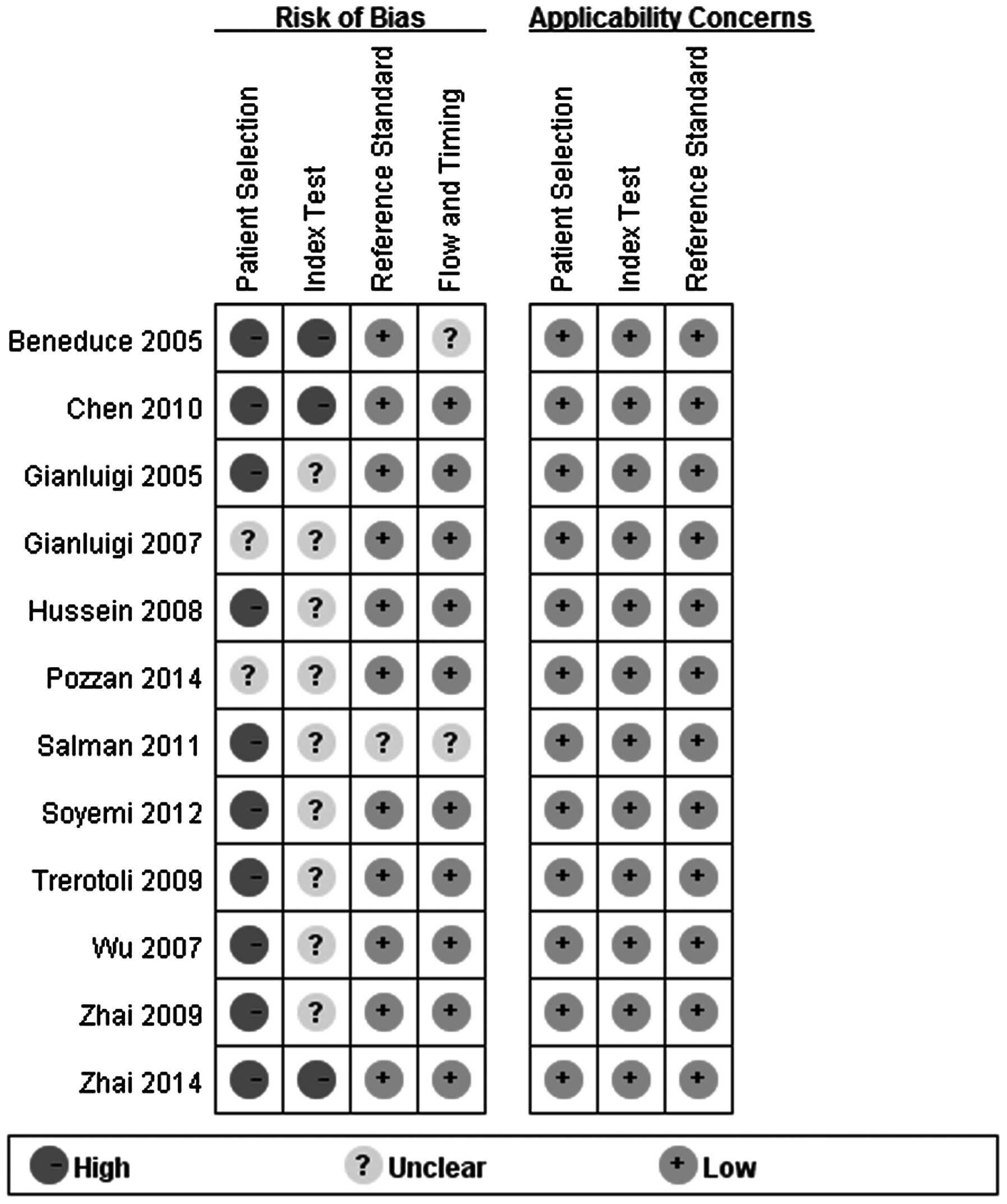
Quality of the studies
The quality assessment of the included studies using
the QUADAS-2 tool is shown in Fig. 2.
Certain design details could not be determined from the articles
and, for these studies, the risk bias was labeled as ‘unclear’.
However, the quality was not considered to be satisfactory. All the
studies used a retrospective design and in only two studies were
the blood samples collected from consecutive patients. Five studies
recruited healthy individuals in the control group. All the studies
reported the diagnostic standard of HCC, but none of the 12 studies
interpreted serum SCCA and SCCA-IgM test levels with the
investigators blinded to the diagnosis. All 12 studies measured
SCCA and SCCA-IgM using ELISA.
Sensitivity and specificity of SCCA
and SCCA-IgM for HCC
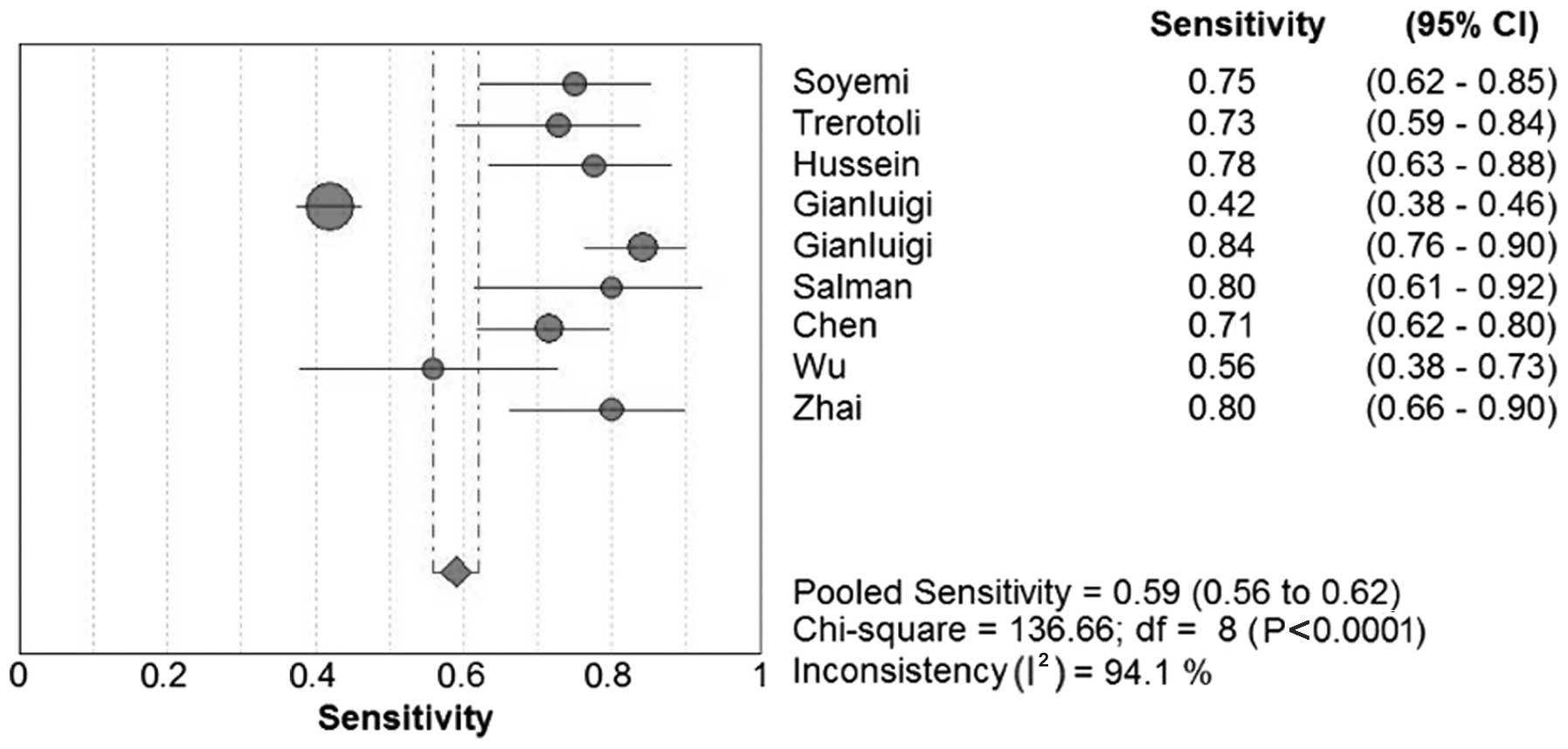
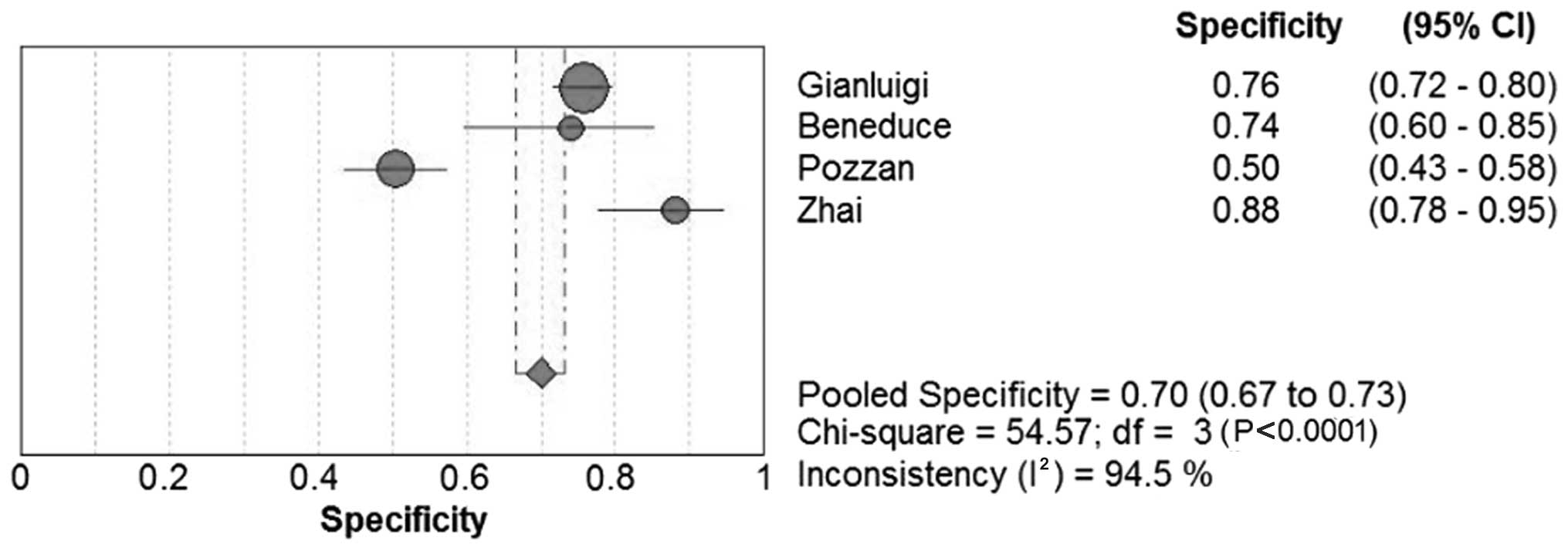
The sensitivity in the 12 studies ranged between
41.9 and 84.2% for SCCA and between 52.3 and 89.0% for SCCA-IgM;
the specificity range was 26.7–100.0% and 50.0–87.8%, respectively.
Forest plots for sensitivity, specificity and their respective 95%
CIs for SCCA and SCCA-IgM are shown in Figs. 3–6. The
results of the pooled sensitivity and specificity and were 59.0 and
76.0%, respectively, for SCCA and 60.0 and 70.0%, respectively, for
SCCA-IgM.
Threshold effect
The threshold effect is a significant source of
between-study heterogeneity in diagnostic meta-analyses. In our
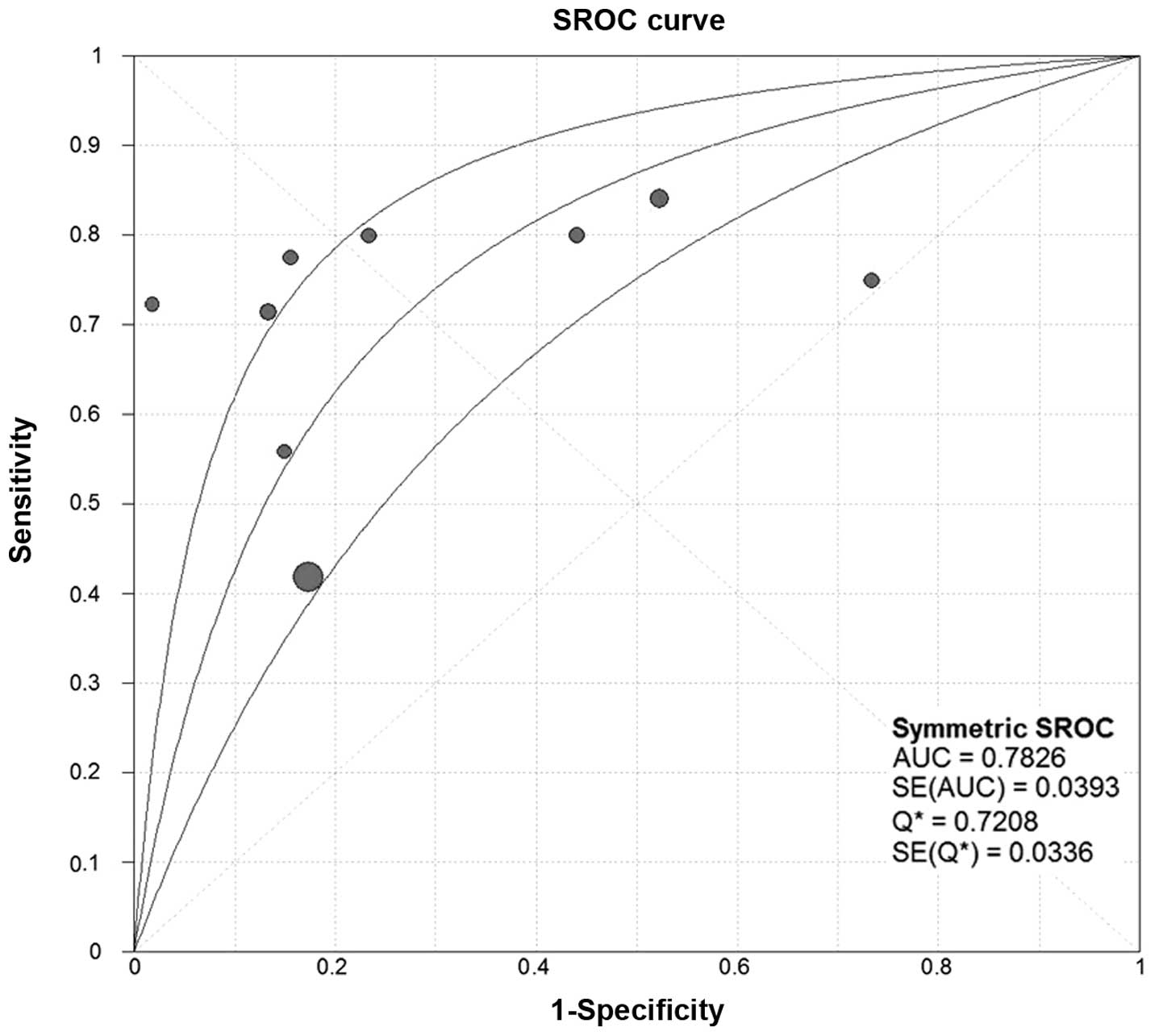
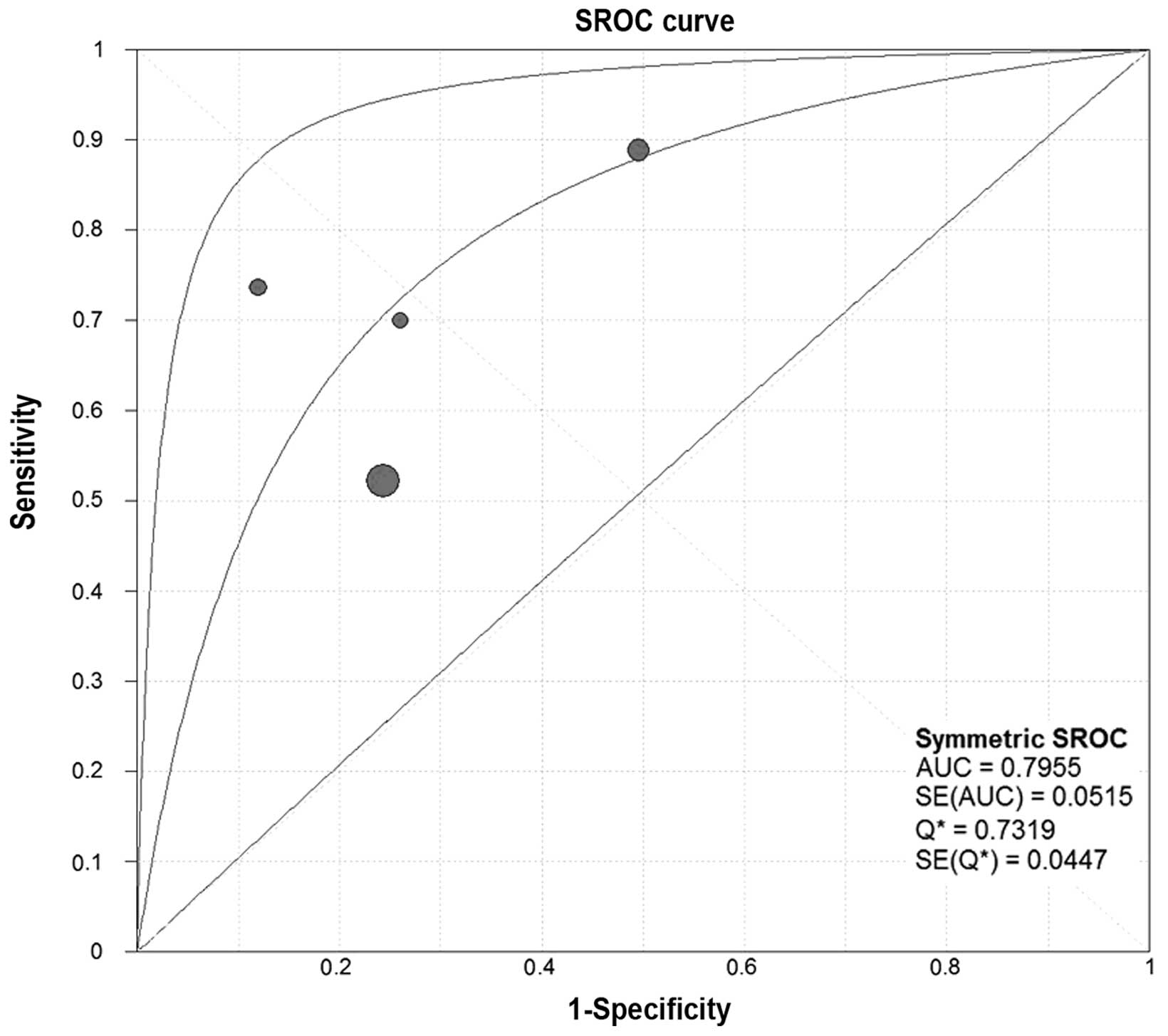
analysis, the SROC curves of SCCA and SCCA-IgM demonstrated that
the plane scatter plot did not exhibit the ‘shoulder-arm’ shape,
which is characteristic of the presence of the threshold effect
(Figs. 7 and 8). The Spearman's correlation coefficient
was 0.577 and 0.400 and the P-value was 0.104 and 0.600 for SCCA
and SCCA-IgM, respectively (Table
III). These results indicated that there was no heterogeneity
attributable to the threshold effect.
 | Table III.Results of analysis of diagnostic
threshold. |
Table III.
Results of analysis of diagnostic
threshold.
|
| Spearman's
correlation |
| No. of |
|---|
| Markers | coefficient | P-value | studies |
|---|
| SCCA | 0.577 | 0.104 | 9 |
| SCCA-IgM | 0.400 | 0.600 | 4 |
Meta-regression analysis for
heterogeneity
We attempted to explain this heterogeneity as
induced by factors other than the threshold effect, by
investigating the study characteristics using meta-regression
analysis. We examined race, sample size and the number of controls
as possible sources of heterogeneity. Due to the small number of
studies, we only tested meta-regression of the effects of
methodological characteristics in the SCCA group. The P-value
reflected the various test factors affecting the SCCA diagnostic
efficiency (Table IV) and the
differences were not found to be statistically significant.
 | Table IV.Results of various factors in
meta-regression. |
Table IV.
Results of various factors in
meta-regression.
| Variables | Coeff. | SE | P-value | RDOR | 95% CI |
|---|
| Race | 1.071 | 0.8790 | 0.2898 | 2.92 | 0.25–33.51 |
| Sample size | −0.646 | 1.0383 | 0.5674 | 0.52 | 0.03–9.36 |
| Controls | −0.778 | 1.1952 | 0.5505 | 0.46 | 0.02–12.68 |
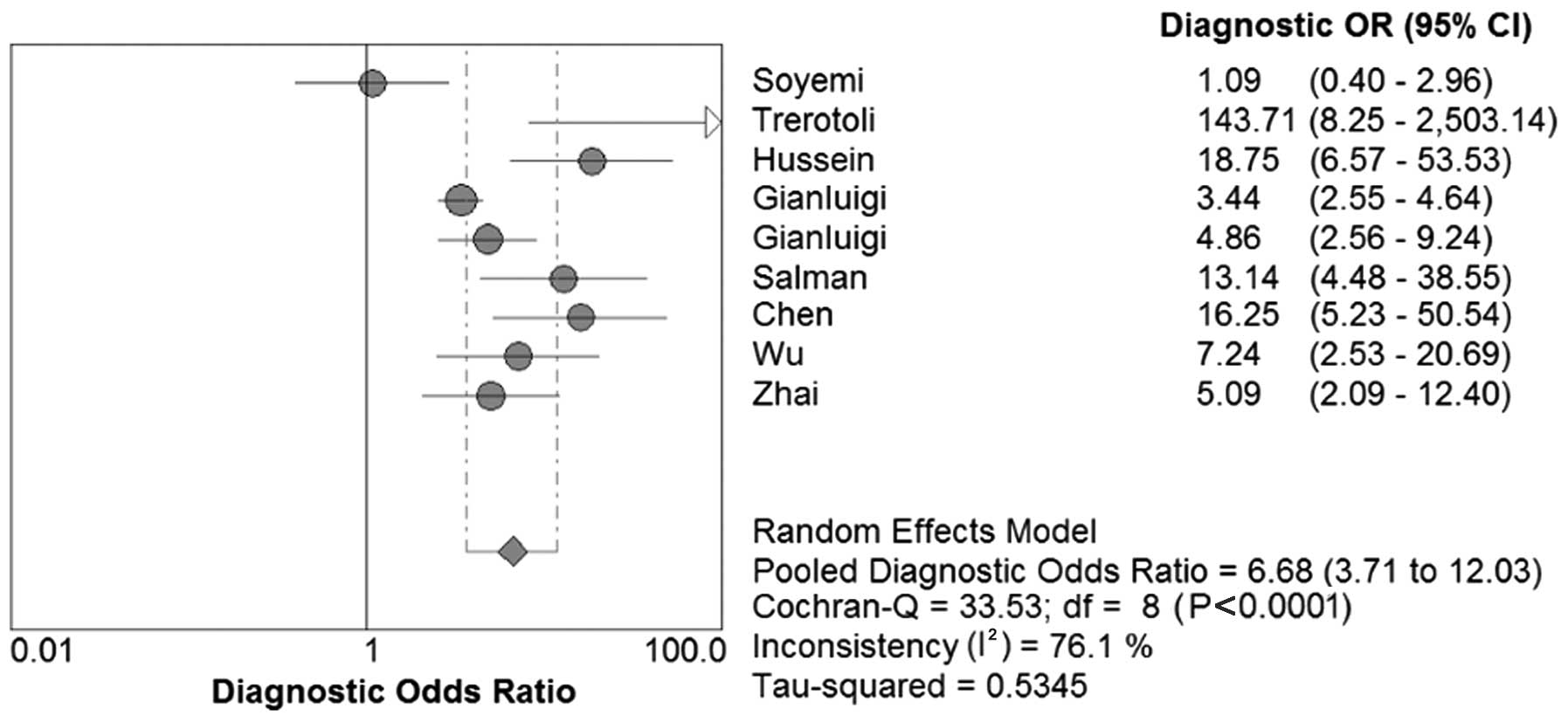
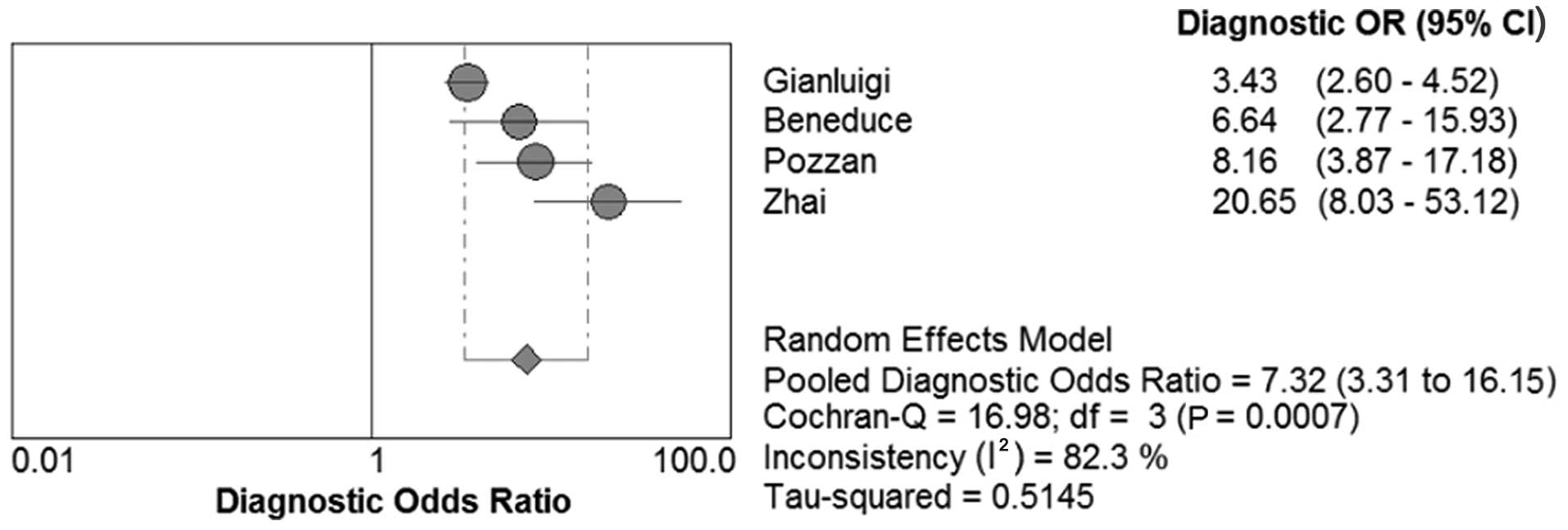
DOR, SROC and AUC of SCCA and SCCA-IgM
for HCC
We constructed the SROC curves and calculated the
AUC for SCCA and SCCA-IgM (Figs. 7
and 8); the DOR was found to be 6.68
(95% CI: 3.71–12.03) for SCCA and 7.32 (95% CI: 3.31–16.15) for
SCCA-IgM (Figs. 9 and 10).
Discussion
According to the present meta-analysis, serum SCCA
and SCCA-IgM may be useful diagnostic biomarkers for HCC; however,
the included studies had certain limitations due to their design
and future well-designed studies are required to rigorously
evaluate the diagnostic accuracy of SCCA and SCCA-IgM.
Serum biomarkers are crucial in HCC diagnosis and
several biomarkers have been identified, including α-fetoprotein
(AFP), AFP-L3, glycoprotein 73, SCCA, glypican-3, transforming
growth factor-β and des-γ-carboxy prothrombin (24–31). Among
these serum biomarkers, AFP is the most commonly clinically applied
in the early diagnosis of HCC. However, the clinical value of AFP
has been challenged over the last few years, due to its low
sensitivity and specificity (1,32–34). The latest guidelines on the management
of HCC by the American Association for the Study of Liver Diseases
in 2010 did not recommend AFP as a tumor marker for HCC screening
(35).
In our study, we performed a meta-analysis of 12
articles investigating the diagnostic accuracy of serum SCCA and
SCCA-IgM in HCC. The results indicated that the sensitivity and
specificity were 59 and 76%, respectively, for SCCA, and 60 and
70%, respectively, for SCCA-IgM; this means that 59 and 76% of the
HCC patients had elevated levels, and 60 and 70% of non-HCC
patients had decreased levels of serum SCCA and SCCA-IgM,
respectively. The DOR is the ratio of the odds of positive test
results in patients with or without disease and a single indicator
of test accuracy that incorporates sensitivity and specificity into
a single index (36). In the present
meta-analysis, the mean DOR was 6.68 and 7.32 for SCCA and
SCCA-IgM, respectively, indicating that the odds for positivity
among subjects with HCC were 6.68 and 7.32 times higher compared
with the odds for positivity among non-HCC subjects. In addition,
the area under the SROC curve (AUC) for SCCA was 0.7826 and for
SCCA-IgM 0.7955, indicating a moderate diagnostic accuracy for
HCC.
Heterogeneity was significant and could not be
explained by the threshold effect. We hypothesized that the
heterogeneity was due to differences in race, sample size and
controls. As the number of studies was limited and certain
information was unavailable, we were unable to determine the
reasons for the existing heterogeneity by meta-regression.
Of note, one study reported that the SCCA levels
were inversely correlated with tumor size and the AUC of smaller
HCCs (<3 cm) was 0.7 (95% CI: 0.66–0.74), with a cut-off value
of 3.2 ng/ml, a sensitivity of 56.1% and a specificity of 74.9%
(7), suggesting that SCCA may be
helpful in detecting HCC at an early stage. The Cox multivariate
analysis of another study demonstrated that SCCA-IgM levels
(P=0.004) was an independent predictor of survival and, combining
SCCA-IgM with AFP, the sensitivity reached 94% (13). Another study also reported that the
combination of AFP and SCCA yielded a correct serological diagnosis
in 90.83% of HCC patients, indicating that combining the two
markers may achieve a higher sensitivity (17).
There were certain limitations to the present
meta-analysis. First, there were no randomized clinical trials and
the number of studies included in the present study was limited.
Therefore, more well-designed and large-sample sized studies are
required. Second, it was not feasible to include studies with
completely identical standards, particularly since the tumor and
liver function characteristics were different among different
patients. Third, significant heterogeneity was observed among
eligible studies and the heterogeneity could not be explained by
meta-regression. We used the more conservative random-effects model
to address this issue. Finally, hepatitis C or B virus-infected and
cirrhotic patients were at high risk of developing HCC, which
represented a target population, as it was considered inappropriate
to use healthy individuals as controls.
In conclusion, the present meta-analysis indicated
that SCCA and SCCA-IgM exhibit moderate diagnostic accuracy as
novel tumor makers of HCC, although the value of the combination of
SCCA/SCCA-IgM and AFP requires further investigation. Considering
the significant bias on this topic, the results of published
studies and present meta-analysis should be interpreted with
caution. Further studies should be undertaken to investigate the
value of the SCCA and SCCA-IgM for the diagnosis of HCC. In
addition, a well-designed prospective and large-sample size study
is required to rigorously evaluate the diagnostic accuracy of SCCA
and SCCA-IgM and confirm whether they provide an additional
diagnostic benefit when replacing or combined with other widely
used biomarkers.
Acknowledgements
The authors would like to thank Dr Chuxiao Shao for
his help with the linguistics and writing of this manuscript.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pirastu R, Biggeri A and Comba P:
International Agency for Research on Cancer Monographs (IARC). G
Ital Med Lav Ergon. 30:83–84; author reply 86–87. 2008.(In
Italian). PubMed/NCBI
|
|
3
|
Pontisso P, Calabrese F, Benvegnù L, et
al: Overexpression of squamous cell carcinoma antigen variants in
hepatocellular carcinoma. Br J Cancer. 90:833–837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Catanzaro JM, Guerriero JL, Liu J, et al:
Elevated expression of squamous cell carcinoma antigen (SCCA) is
associated with human breast carcinoma. PLoS One. 6:e190962011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YT, Yoon BS, Kim JW, et al:
Pretreatment levels of serum squamous cell carcinoma antigen and
urine polyamines in women with squamous cell carcinoma of the
cervix. Int J Gynaecol Obstet. 91:47–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stenman J, Hedström J, Grénman R, et al:
Relative levels of SCCA2 and SCCA1 mRNA in primary tumors predicts
recurrent disease in squamous cell cancer of the head and neck. Int
J Cancer. 95:39–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giannelli G, Fransvea E, Trerotoli P, et
al: Clinical validation of combined serological biomarkers for
improved hepatocellular carcinoma diagnosis in 961 patients. Clin
Chim Acta. 383:147–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM: QUADAS-2 Group: QUADAS-2: A revised tool for the quality
assessment of diagnostic accuracy studies. Ann Intern Med.
155:529–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Devillé WL, Buntinx F, Bouter LM, et al:
Conducting systematic reviews of diagnostic studies: Didactic
guidelines. BMC Med Res Methodol. 2:92002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zamora J, Abraira V, Muriel A, Khan K and
Coomarasamy A: Meta-DiSc: a software for meta-analysis of test
accuracy data. BMC Med Res Methodol. 6:312006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walter SD: Properties of the summary
receiver operating characteristic (SROC) curve for diagnostic test
data. Stat Med. 21:1237–1256. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pozzan C, Cardin R, Piciocchi M, et al:
Diagnostic and prognostic role of SCCA-IgM serum levels in
hepatocellular carcinoma (HCC). J Gastroenterol Hepatol.
29:1637–1644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soyemi OM, Otegbayo JA, Ola SO, Akere A
and Soyemi T: Comparative diagnostic efficacy of serum squamous
cell carcinoma antigen in hepatocellular carcinoma. BMC Res Notes.
5:4032012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trerotoli P, Fransvea E, Angelotti U, et
al: Tissue expression of squamous cellular carcinoma antigen (SCCA)
is inversely correlated to tumor size in HCC. Mol Cancer. 8:292009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hussein MM, Ibrahim AA, Abdella HM,
Montasser IF and Hassan MI: Evaluation of serum squamous cell
carcinoma antigen as a novel biomarker for diagnosis of
hepatocellular carcinoma in Egyptian patients. Indian J Cancer.
45:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giannelli G, Marinosci F, Trerotoli P, et
al: SCCA antigen combined with alpha-fetoprotein as serologic
markers of HCC. Int J Cancer. 117:506–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beneduce L, Castaldi F, Marino M, et al:
Squamous cell carcinoma antigen-immunoglobulin M complexes as novel
biomarkers for hepatocellular carcinoma. Cancer. 103:2558–2565.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salman T, Raouf AA, Saleh SM, Salama M and
Mohammed AAE: Comparative study between serum alpha-fetoprotein,
VEGF and SCCA in enhancing detection of hepatocellular carcinoma in
Egyptian patients. Hepatol Int. 5:392011.
|
|
20
|
Wu X: Clinical application value of serum
squamous cell carcinoma antigen in hepatocellular carcinoma.
Wenzhou Medical University. 2007.(In Chinese).
|
|
21
|
Zhai L, Li J, Yang X, et al: Combine serum
AFP, GP73 and SCCA IgM IC to detect early hepatocellular carcinoma
of HBV related. Shandong Medical Journal. 34–37. 2014.(In
Chinese).
|
|
22
|
Chen X, Sun P and Yao X: Squamous cell
carcinoma antigen detection in the diagnosis of primary liver
cancer. Chin J Pract Med. 37:69–70. 2010.(In Chinese).
|
|
23
|
Zhai Q: Tumor markers in the diagnosis of
hepatocellular carcinoma. Chin J Prim Med Pharm. 1614–1615.
2009.(In Chinese).
|
|
24
|
Zhu J, Jiang F, Ni HB, et al: Combined
analysis of serum γ-glutamyl transferase isoenzyme II,
α-L-fucosidase and α-fetoprotein detected using a commercial kit in
the diagnosis of hepatocellular carcinoma. Exp Ther Med. 5:89–94.
2013.PubMed/NCBI
|
|
25
|
Witjes CD, van Aalten SM, Steyerberg EW,
et al: Recently introduced biomarkers for screening of
hepatocellular carcinoma: A systematic review and meta-analysis.
Hepatol Int. 7:59–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi JY, Jung SW, Kim HY, et al:
Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma
according to total-AFP. World J Gastroenterol. 19:339–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Yin X, Ying J and Zhang B: Golgi
protein 73 versus alpha-fetoprotein as a biomarker for
hepatocellular carcinoma: A diagnostic meta-analysis. BMC Cancer.
12:172012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marrero JA and El-Serag HB:
Alpha-fetoprotein should be included in the hepatocellular
carcinoma surveillance guidelines of the American Association for
the Study of Liver Diseases. Hepatology. 53:1060–1061; author reply
1061–1062. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu JS, Wu DW, Liang S and Miao XY: GP73, a
resident Golgi glycoprotein, is sensibility and specificity for
hepatocellular carcinoma of diagnosis in a hepatitis B-endemic
Asian population. Med Oncol. 27:339–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akutsu N, Yamamoto H, Sasaki S, et al:
Association of glypican-3 expression with growth signaling
molecules in hepatocellular carcinoma. World J Gastroenterol.
16:3521–3528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shirakawa H, Kuronuma T, Nishimura Y, et
al: Glypican-3 is a useful diagnostic marker for a component of
hepatocellular carcinoma in human liver cancer. Int J Oncol.
34:649–656. 2009.PubMed/NCBI
|
|
32
|
Zoli M, Magalotti D, Bianchi G, et al:
Efficacy of a surveillance program for early detection of
hepatocellular carcinoma. Cancer. 78:977–985. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trevisani F, Dintino PE, Morselli-Labate
AM, et al: Serum alpha-fetoprotein for diagnosis of hepatocellular
carcinoma in patients with chronic liver disease: Influence of
HBsAg and anti-HCV status. J Hepatol. 34:570–575. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gambarin-Gelwan M, Wolf DC, Shapiro R,
Schwartz ME and Min AD: Sensitivity of commonly available screening
tests in detecting hepatocellular carcinoma in cirrhotic patients
undergoing liver transplantation. Am J Gastroenterol. 95:1535–1538.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glas AS, Lijmer JG, Prins MH, Bonsel GJ
and Bossuyt PM: The diagnostic odds ratio: A single indicator of
test performance. J Clin Epidemiol. 56:1129–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|