Introduction
Duodenal adenocarcinoma (DA) accounts for
approximately one-third of all small intestinal malignancies, with
the other major tumor types being neuroendocrine carcinoma, sarcoma
and lymphoma (1). Due to the relative
rarity of small bowel adenocarcinomas (SBAs), the prospective
trials limited to this disease are sparse and the optimal therapy
for advanced SBA as well as resected node-positive SBA has not been
determined (2). Patients with DA
exhibit poorer outcomes compared with patients with tumors at
others primary sites, such as the jejunum and ileum (1). This is the case report of a patient with
DA who underwent treatment with oxaliplatin and S-1 (SOX) and a
review of the relevant literature.
Case report
A 40-year-old man presented to the Shandong Cancer
Hospital and Institute on January, 2011 with a month-long history
of abdominal pain, repeated vomiting and weight loss. On January
18, 2011, abdominal magnetic resonance imaging was performed,
revealing a tumor of the descending duodenum, involving the
uncinate process of the pancreas. A deep ulcer (3.7×4.2 cm), with
irregular thickening of the wall of the descending duodenum was
confirmed by endoscopy. The histological examination revealed a
differentiated duodenal adenocarcinoma (DA). On January 28, 2011,
the patient underwent Whipple pancreatoduodenectomy, during which a
2-cm wide tumor was identified in the descending duodenum. The
histopathological findings confirmed a ‘differentiated
adenocarcinoma extending through the duodenal serosa and
infiltrating the pancreatic parenchyma without lymph node
metastases (0N+/18N)’. The postoperative period was uneventful,
without complications. Two cycles of adjuvant chemotherapy (oral
capecitabine monotherapy) were administered following surgery. The
clinical and instrumental follow-up revealed disease recurrence: On
February 20, 2012, computed tomography (CT) revealed a mass in the
right lobe of the liver, which was suspected to be a metastasis.
The patient underwent transcatheter arterial chemoembolization on
February 28 and radiofrequency ablation on March 5, 2012. At the
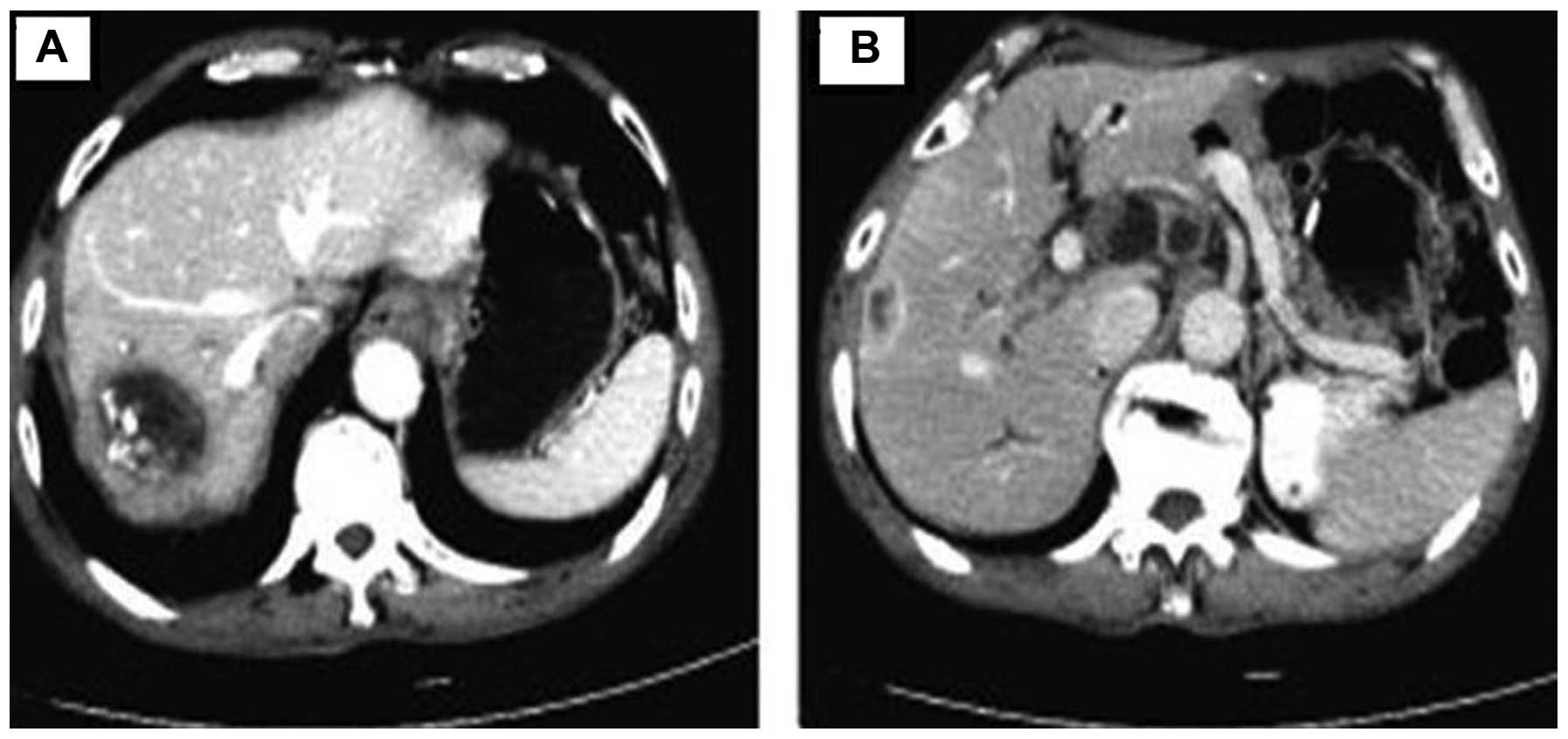
2-month follow-up, a CT examination revealed that the right lobe
mass had enlarged, with new multiple lymph nodes in the hilar
region (Fig. 1) and the disease was
considered as progressive. The Eastern Cooperative Oncology Group
performance status of the patient was 1. Between May and July,
2012, 4 cycles of palliative chemotherapy were performed,
comprising oxaliplatin (200 mg) on day 1 and S-1 (40
mg/m2 body surface area) administered orally, twice
daily on days 1–14. The cycle was repeated every 3 weeks. In August
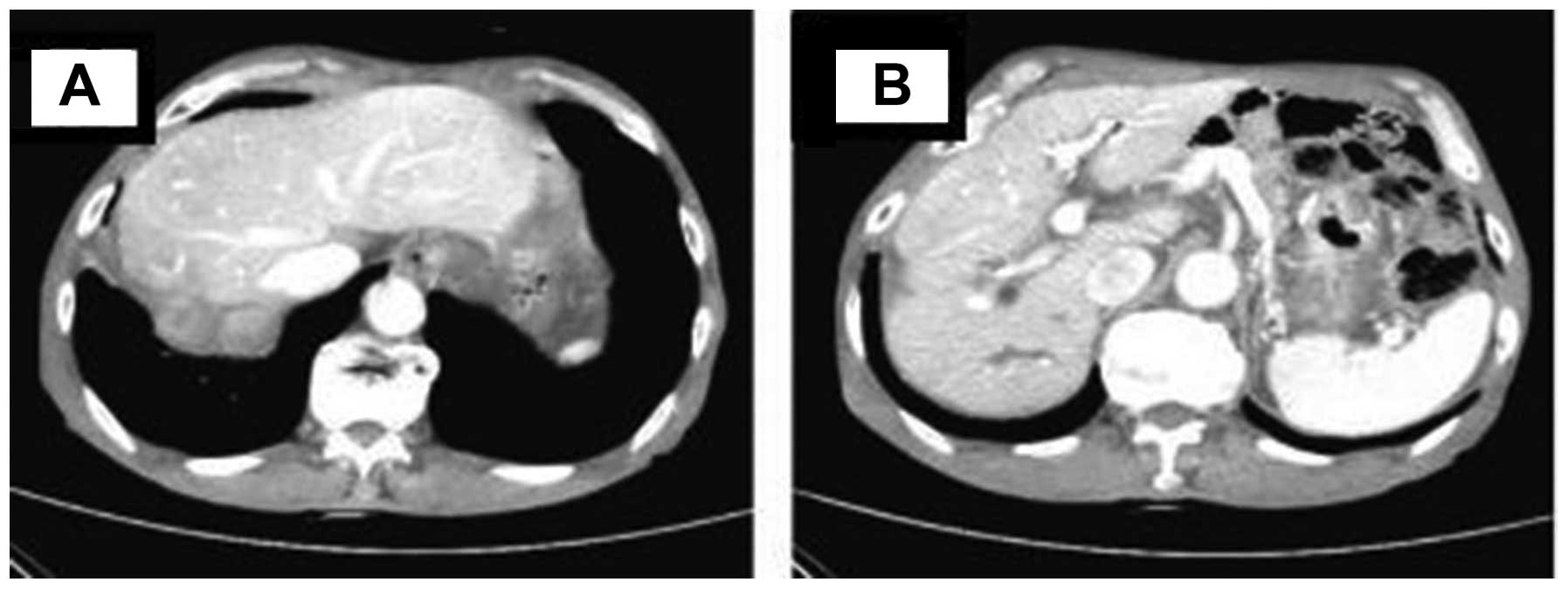
20, 2012, an abdominal CT revealed that the right lobe lesions had
shrunk and the hilar lymph nodes had disappeared. The patient was
classified as being in partial remission (Fig. 2). From August 21, 2012 onwards, 6
cycles of palliative chemotherapy were performed, comprising S-1
(40 mg/m2 body surface area) administered orally, twice
daily on days 1–28. The cycle was repeated every 6 weeks. In
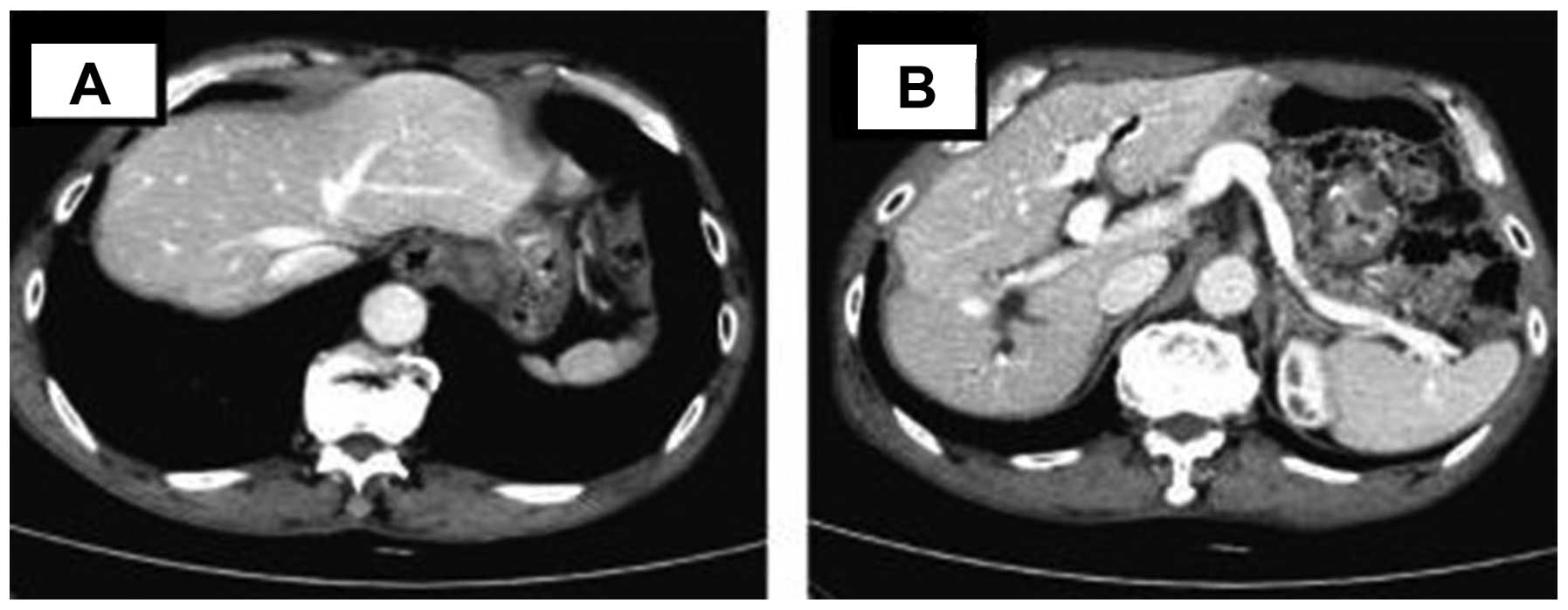
October 13, 2012, an abdominal CT revealed that the right lobe
lesions had disappeared and the patient was in complete remission
(CR) (Fig. 3). The primary lesion was
not identified at the last follow-up in May, 2013.
According the National Cancer Institute Common
Toxicity Criteria, version 3.0 toxicity scale, the most common
adverse reactions, including skin pigmentation, leukopenia and
diarrhea, subsided 3–4 weeks after treatment completion,
spontaneously or with symptomatic treatment. The toxicity was
considered tolerable by the patient (3,4).
Discussion
Although the duodenum is the most common location of
small intestinal adenocarcinoma, DA is a rare malignancy,
comprising 1% of all gastrointestinal cancers (5,6). DA is
usually diagnosed at an advanced stage and the resectability is
low. Even with optimal resection, however, survival is poor and
recurrence is common, with a median duration of survival for
metastatic disease of <8 months (7,8). Ryder
et al (9) reported on their
40-year experience with DA at the UCLA Medical Center and
demonstrated that DAs are characterized by large size, moderate to
poor differentiation and invasion of the surrounding fat or
mesentery, which are associated with decreased survival. Metastatic
lymph node involvement and the location of the tumor within the
duodenum were not found to be associated with survival.
Due to the relative rarity of DA, the prospective
trials limited to advanced DA treated with chemotherapy are sparse
(2). Retrospective studies indicate
that chemotherapy may improve the survival of patients with
metastatic SBA compared to no treatment (9). Czaykowski and Hui (10) published a study on patients receiving
palliative chemotherapy and reported an increase in median survival
of ~8 months compared with patients with advanced disease receiving
no chemotherapy. Overman et al (11) conducted a phase II study of
capecitabine and oxaliplatin for advanced adenocarcinoma of the
small bowel and the ampulla of Vater and reported that the
confirmed overall response rate was 50%, the median time to
progression was 11.3 months and the median overall survival was
20.4 months.
S-1 is an orally active derivative of 5-fluorouracil
(5-FU), which is a fourth-generation oral fluoropyrimidine
(12) and has been used instead of
5-FU in certain clinical trials (13,14). S-1
was first approved for the treatment of advanced or metastatic
gastric cancer, and there are currently no reports on the treatment
of DA. Clinical studies have reported that S-1 in combination with
oxaliplatin may achieve a high response rate, ranging between 53
and 59%, with an excellent toxicity profile in the treatment of
advanced gastric cancer (15). In
this study, the DA patient underwent 4 cycles of the SOX regimen
(S-1 40 mg/m2/day administered orally, twice daily, with
a schedule of 14 days on and 7 days off, and oxaliplatin 130
mg/m2 administered on day 1), followed by 6 cycles of
single-agent S-1 maintenance chemotherapy, with disappearance of
the lesions on imaging and complete remission. The progression-free
survival was 14 months. This treatment was associated with high
efficacy and an acceptable toxicity profile. In conclusion, the
study demonstrated that S-1 may exhibit a favorable efficacy and
safety profile in patients with advanced DA, although it requires
further validation in clinical trials.
References
|
1
|
Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY,
Bennett CL and Talamonti MS: Small bowel cancer in the United
States: Changes in epidemiology, treatment, and survival over the
last 20 years. Ann Surg. 249:63–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halfdanarson TR, McWilliams RR, Donohue JH
and Quevedo JF: A single-institution experience with 491 cases of
small bowel adenocarcinoma. Am J Surg. 199:797–803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Overman MJ, Hu CY, Wolff RA and Chang GJ:
Prognostic value of lymph node evaluation in small bowel
adenocarcinoma: Analysis of the surveillance, epidemiology, and end
results database. Cancer. 116:5374–5382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choo R, Pearse M, Danjoux C, et al:
Analysis of gastrointestinal and genitourinary morbidity of
postoperative radiotherapy for pathologic T3 disease or positive
surgical margins after radical prostatectomy using national cancer
institute expanded common toxicity criteria. Int J Radiat Oncol
Biol Phys. 72:989–995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kautio AL, Haanpää M, Kautiainen H, et al:
Oxaliplatin scale and National Cancer Institute-Common Toxicity
Criteria in the assessment of chemotherapy-induced peripheral
neuropathy. Anticancer Res. 31:3493–3496. 2011.PubMed/NCBI
|
|
6
|
Overman MJ, Hu C-Y, Kopetz S, Abbruzzese
JL, Wolff RA and Chang GJ: A population-based comparison of
adenocarcinoma of the large and small intestine: Insights into a
rare disease. Ann Surg Oncol. 19:1439–1445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gibson MK, Holcroft CA, Kvols LK and
Haller D: Phase II study of 5-fluorouracil, doxorubicin, and
mitomycin C for metastatic small bowel adenocarcinoma. Oncologist.
10:132–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Fröbom R and Lagergren J: Incidence
patterns of small bowel cancer in a population-based study in
Sweden: Increase in duodenal adenocarcinoma. Cancer Epidemiol.
36:e158–e163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryder NM, Ko CY, Hines OJ, Gloor B and
Reber HA: Primary duodenal adenocarcinoma: A 40-year experience.
Arch Surg. 135:1070–1075. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Czaykowski P and Hui D: Chemotherapy in
small bowel adenocarcinoma: 10-year experience of the British
Columbia Cancer Agency. Clin Oncol (R Coll Radiol). 19:143–149.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Overman MJ, Varadhachary GR, Kopetz S,
Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL and Wolff RA:
Phase II study of capecitabine and oxaliplatin for advanced
adenocarcinoma of the small bowel and ampulla of Vater. J Clin
Oncol. 27:2598–2603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Wang ML, Zhou LY, Lu XY, Yang JF
and Yu HG: Randomized phase II study comparing paclitaxel with S-1
vs. S-1 as first-line treatment in patients with advanced gastric
cancer. Clin Transl Oncol. 15:836–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ajani JA, Rodriguez W, Bodoky G,
Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A,
Lang I and Falcon S: Multicenter phase III comparison of
cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced
gastric or gastroesophageal adenocarcinoma study: The FLAGS trial.
J Clin Oncol. 28:1547–1553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosati G, Ferrara D and Manzione L: New
perspectives in the treatment of advanced or metastatic gastric
cancer. World J Gastroenterol. 15:2689–2692. 2009. View Article : Google Scholar : PubMed/NCBI
|