Spandidos Publications style
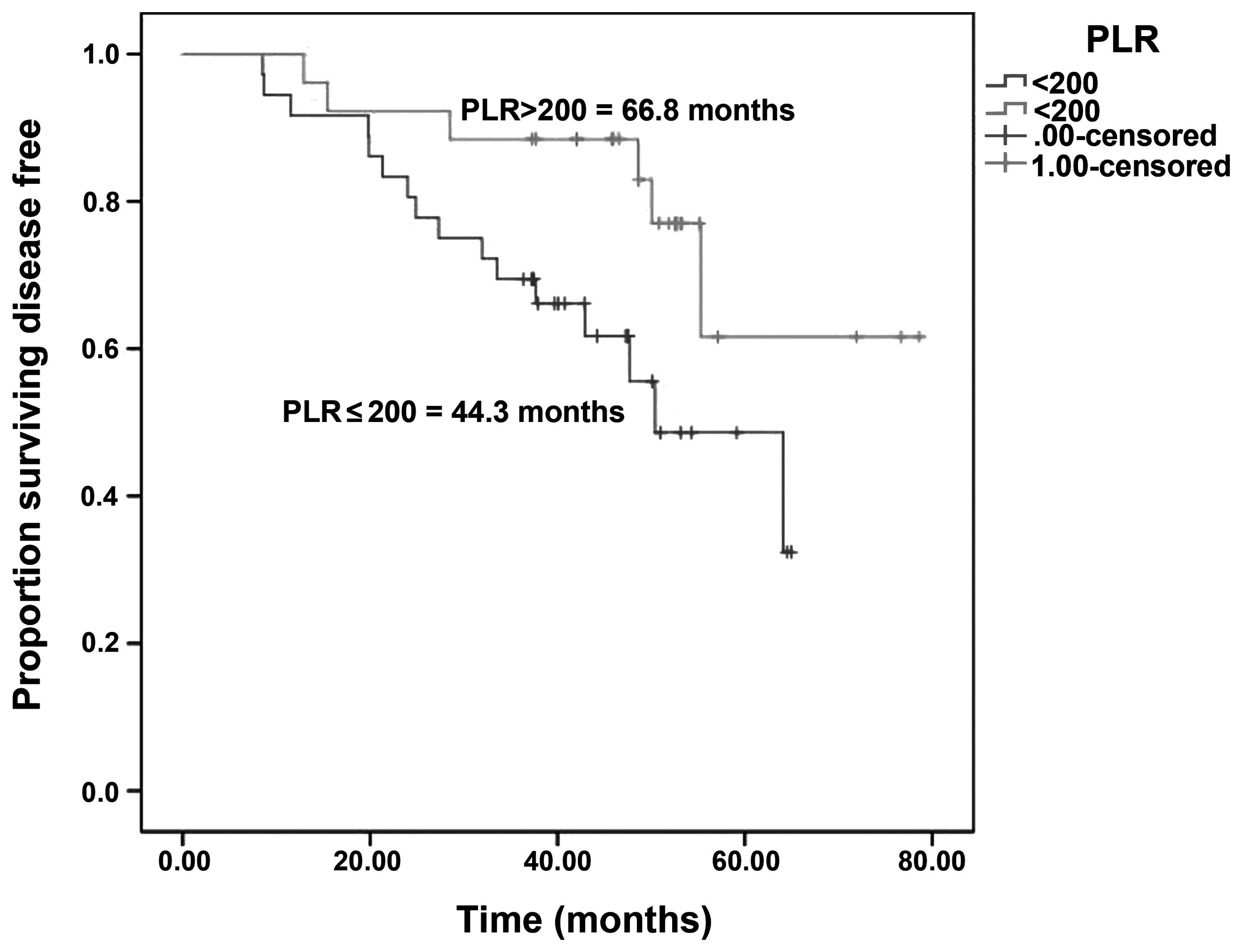
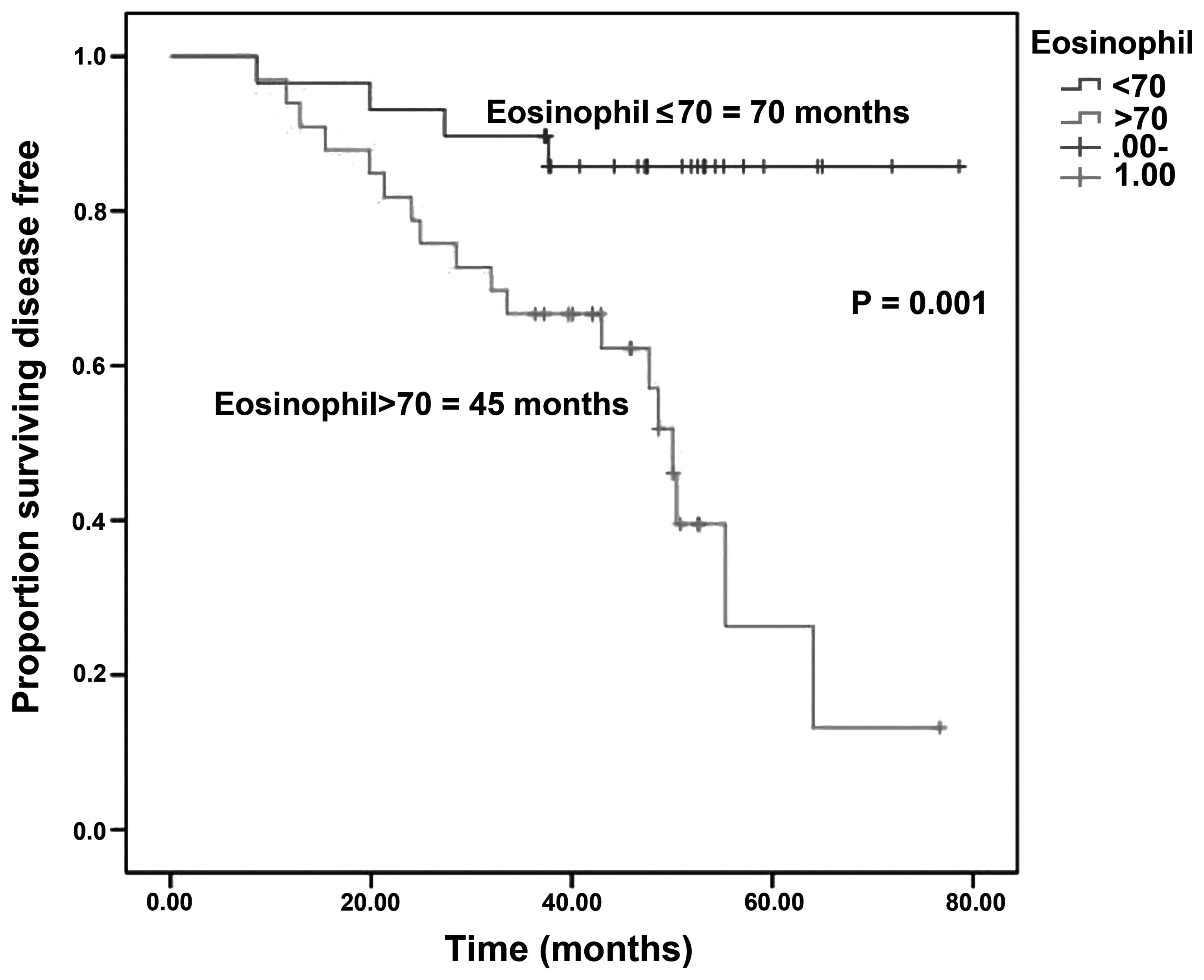
Gündüz S, Göksu SS, Arslan D, Tatli AM, Uysal M, Gündüz UR, Sevinç MM, Coşkun HS, Bozcuk H, Mutlu H, Mutlu H, et al: Factors affecting disease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab. Mol Clin Oncol 3: 1109-1112, 2015.
APA
Gündüz, S., Göksu, S.S., Arslan, D., Tatli, A.M., Uysal, M., Gündüz, U.R. ... Savas, B. (2015). Factors affecting disease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab. Molecular and Clinical Oncology, 3, 1109-1112. https://doi.org/10.3892/mco.2015.610
MLA
Gündüz, S., Göksu, S. S., Arslan, D., Tatli, A. M., Uysal, M., Gündüz, U. R., Sevinç, M. M., Coşkun, H. S., Bozcuk, H., Mutlu, H., Savas, B."Factors affecting disease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab". Molecular and Clinical Oncology 3.5 (2015): 1109-1112.
Chicago
Gündüz, S., Göksu, S. S., Arslan, D., Tatli, A. M., Uysal, M., Gündüz, U. R., Sevinç, M. M., Coşkun, H. S., Bozcuk, H., Mutlu, H., Savas, B."Factors affecting disease-free survival in patients with human epidermal growth factor receptor 2-positive breast cancer who receive adjuvant trastuzumab". Molecular and Clinical Oncology 3, no. 5 (2015): 1109-1112. https://doi.org/10.3892/mco.2015.610