Introduction
Colorectal cancer (CRC) is the third most common
type of cancer worldwide and one of the leading causes of
cancer-related mortality (1). Lack of
an effective method for screening early-stage colon cancer is an
important reason for its high mortality rate. Patients with
early-stage colon cancer have a favorable outcome with treatment,
with the 5-year survival rates exceeding 70% for stage I/II colon
cancer (2,3). Colonoscopy has been used for screening
early-stage colon cancer for several decades as a first choice;
however, due to its invasive character, it is associated with low
compliance in the clinical setting. Therefore, cost-effective and
non-invasive biomarkers with high sensitivity and specificity are
needed to enable early-detection of stage I/II colon cancer.
Recently, several studies have focused on the role
of various biomarkers in early-stage cancer progression and
diagnosis (4–6). Carcinoembryonic antigen (CEA), a
well-known tumor marker, is found at low levels in the serum of
heathy adults, but at higher levels in colon cancer patients
(4). However, CEA is not a reliable
independent marker for diagnosing cancer or for screening tests to
detect early-stage cancer (7), as its
levels are also increased in certain non-neoplastic diseases,
including ulcerative colitis, pancreatitis, cirrhosis and
hypothyroidism (5). Hence, there
remains a high demand for novel and reliable biomarkers with high
specificity.
An increasing number of recent studies have focused
on the potential of microRNAs (miRNAs) for assessing colon cancer
diagnosis or progression. miRNAs have been reported to play an
important role in a number of important cellular processes, and may
be involved in colon carcinogenesis through deregulation of
oncogenes or tumor suppressor genes (8). Several studies have recently identified
miRNAs expressed at different levels in the serum of cancer
patients compared with healthy controls (9–12). miRNAs
have been suggested as potential reliable biomarkers for tumor
screening (13,14) due to their extremely stable nature and
the fact that they may be easily measured by common laboratory
methods. In 2009, Ng et al (15) analyzed 95 miRNAs and identified miRNA
(miR)-17-3p as exhibiting the most significant overexpression in
the plasma and tumor tissues of colon cancer patients. However, a
later study found no significant differences between miR-17-3p
levels in the serum of CRC patients and heathy controls (16).
The aim of the present study was to investigate
whether CEA and miR-17-3p are elevated in the serum of stage I/II
colon cancer patients and assess their potential as novel
biomarkers for this type of cancer.
Subjects and methods
Subjects
Serum samples were obtained from 70 colon cancer
patients who were diagnosed at the First Affiliated Hospital of
Soochow University (Suzhou, China) and the First Affiliated
Hospital of Bengbu Medical College, (Bengbu, China) between 2011
and 2013. An additional 70 serum samples were obtained from healthy
control individuals with no previous history of any cancer, who
were recruited from the same hospitals. All the subjects were of
the same ethnicity (Chinese Han). The clinical and pathological
characteristics, including gender, age and clinical stage, were
recorded for colon cancer patients and are summarized in Table I. The patients were selected
retrospectively on the basis of clinical characteristics to include
only patients with stage I/II colon cancer. Informed consent was
obtained from each subject, and the study was approved by the
Ethics Committee of the First Affiliated Hospital of Soochow
University, Suzhou, China.
 | Table I.Summary of expression levels of CEA
and miR-17-3P detected in serum of colon cancer patients and
healthy donors expressed as median and interquartile range. |
Table I.
Summary of expression levels of CEA
and miR-17-3P detected in serum of colon cancer patients and
healthy donors expressed as median and interquartile range.
| Variables | Healthy donors
(n=70) | Colon cancer patients
(n=70) | P-valueb |
|---|
| Age (years) |
|
|
|
| Mean ±
SD | 47.34±10.13 | 49.58±12.62 |
|
| Gender, no. |
|
|
|
| Male | 40 | 48 |
|
|
Female | 30 | 22 |
|
| Stage, no. |
|
|
|
| I |
|
14 |
|
| II |
|
56 |
|
| CEA (ng/ml) |
|
|
|
| Mean ±
SD | 1.76±0.88 | 9.79±3.43 | 0.032 |
|
miR-17-3pa |
|
|
|
| Mean ±
SD | 0.61±0.48 | 3.25±2.69 | 0.01 |
Sample collection
Blood samples were obtained immediately following
diagnosis and prior to the patients receiving any oncological
treatment. Blood was processed for serum within 2 h and stored at
−80°C. The median time from storage to endpoint analysis was 12
months. Serum was obtained by centrifugation at 12,000 × g for 10
min at 4°C.
Serum CEA determination
CEA was assayed with a immunoradiometric assay
(Beijing Dongya Biotechnology; Beijing, China) and the threshold
for a positive result was 5 ng/ml. The assay was performed
according to the manufacturer's instructions.
RNA isolation, reverse transcription
(RT) and quantitative-polymerase chain reaction (qPCR)
Total RNA enriched for small RNAs was isolated from
250 µl aliquots of serum using Qiagen miRNeasy Mini kits (Qiagen
GmbH, Hilden, Germany) according to the manufacturer's modified
protocol. For each sample, 1.25 µl of 0.8 µg/µl MS2 RNA carrier was
added to 800 µl of QIAzol solution. The extracted RNA was eluted in
30 µl of preheated elution solution, and the concentration and
purity of the RNA were estimated spectrophotometrically
(A260/280>2.0; A260/230>1.8) using a NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA).
The RNA samples were either further processed immediately or stored
at −80°C.
Complementary DNA was synthesized from total RNA
using gene-specific primers according to the TaqMan MicroRNA Assay
protocol (Applied Biosystems, Foster City, CA, USA). For RT
reactions, 10 ng of RNA sample, 50 nM of stem-loop RT primer, 1X RT
buffer, 0.25 mM each of dNTPs, 3.33 U/µl MultiScribe RT and 0.25
U/µl RNase inhibitor were used. The RT mixtures (15 µl) were
incubated for 30 min at 16°C, 30 min at 42°C and 5 min at 85°C, and
then maintained at 4°C (PTC-200 Thermal Cycler; MJ Research, Inc.,
Waltham, MA, USA). qPCR was performed using the 7500 Real-Time PCR
system (Applied Biosystems). The 20-µl PCR reaction mixture
included 1.33 µl of RT product, 1X TaqMan Universal PCR master mix,
and 1 µl of primer and probe mix from the TaqMan MicroRNA Assay kit
(Applied Biosystems). The qPCR reactions were incubated in a
96-well optical plate at 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and at 60°C for 10 min.
Data normalization and statistical
analysis
The cycle threshold (Ct) values were calculated
using SDS 2.0.1 software (Applied Biosystems) using the default
threshold settings (automatic baseline, threshold 0.2). All the
qPCR reactions were run as triplicates and the mean Ct and standard
deviation values were calculated. The average expression levels of
miR-17-3p were normalized using miR-16 (Applied Biosystems) as a
reference gene and subsequently the 2−ΔΔCT method was
applied. The Mann-Whitney U test was used to compare the serum CEA
and miRNA expression between the different groups, as it is a
non-parametric test of the null hypothesis that two samples come
from the same population against an alternative hypothesis,
particularly that one population tends to exhibit larger values
compared with the other, and may be applied on unknown
distributions. Receiver operating characteristic (ROC) curves and
the area under the ROC curve (AUC) assessed the feasibility of
using serum miRNA as a diagnostic tool for colon cancer. The Youden
index determined the threshold for the plasma miRNA concentrations.
All the tests were two-sided and P<0.05 was considered to
indicate statistically significant differences. The statistical
analysis was conducted using SPSS 16.0 software (SPSS Ltd., Woking,
Surrey, UK) and the graphs were generated with GraphPad Prism 5.0
(GraphPad Software Inc., San Diego, CA, USA).
Results
Serum sample examination
To evaluate the diagnostic value of miR-17-3p and
CEA that were identified in previous studies (16,17), serum
samples of 70 stage I/II colon cancer patients and 70 healthy
controls were examined by radioimmunoassay and RT-qPCR,
respectively. miR-16 was used as the endogenous control, as it
displayed high stability, high abundance and low variability in
analyzed serum samples.
CEA and miR-17-3p serum levels
Using the Mann-Whitney U test, the CEA levels were
found to be significantly elevated in the serum of stage I/II colon
cancer patients compared with healthy controls (P<0.01).
Moreover, miR-17-3p also exhibited significantly different
expression levels (P<0.001) between colon cancer patients and
controls (Fig. 1). Additionally, the
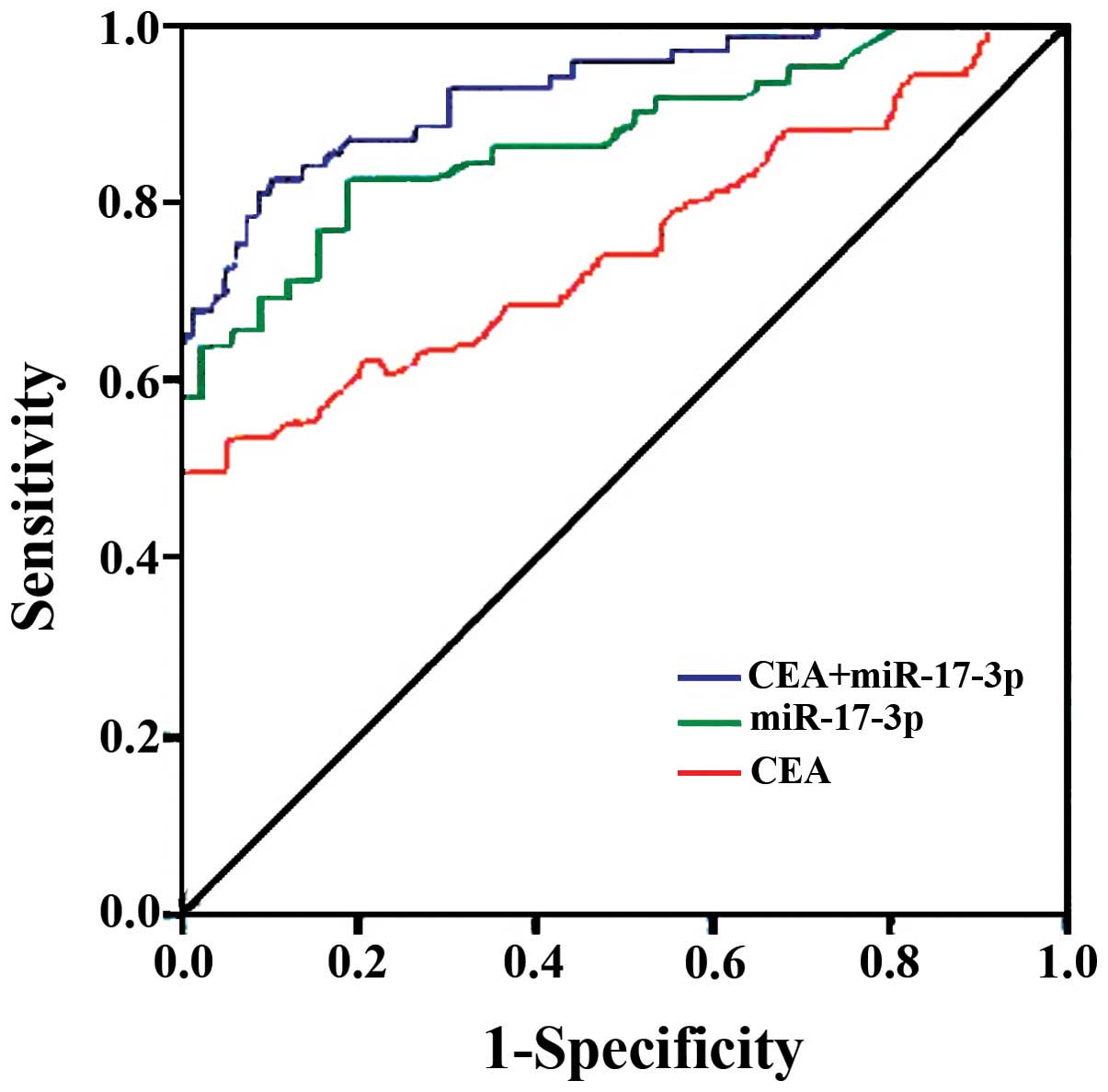
ROC curve analysis indicated their potential diagnostic values: The
AUCs for CEA and miR-17-3p were 0.719 (95% CI: 0.658–0.843) and
0.807 (95% CI: 0.748–0.906), respectively. At a threshold of 9.6
ng/ml for CEA, the optimal sensitivity and specificity were 74.6
and 84.3% in separating colon cancer patients from normal controls.
At a threshold of 2.98 for miR-17-3p, the sensitivity and the
specificity were 83.6 and 72.9%, respectively. When the test
results for CEA and miR-17-3p were considered jointly, the ROC
analysis revealed an AUC of 0.929 (95% CI: 0.834–0.978) with a
sensitivity of 96.4% and specificity 95.7% in discriminating colon
cancer patients from healthy controls.
Discussion
Several studies recently reported that CEA and
miR-17-3p individually may be used as potential markers for the
diagnosis of CRC (16) and pancreatic
cancer (17) by its differentiated
expression level in the plasma or serum. In this study, we analyzed
the levels of CEA and miR-17-3p in the serum of stage I/II colon
cancer patients and healthy controls. Compared with healthy
controls, both CEA and miR-17-3p were overexpressed in the serum of
colon cancer patients. Moreover, the results of the ROC analysis
using a combination of CEA and miR-17-3p revealed an AUC of 0.927
with a sensitivity of 93% and a specificity of 94.7% in
discriminating colon cancer patients from healthy controls.
CEA is one of the most widely used tumor markers,
and has been found to be elevated in the plasma or serum of
patients with various types of cancer, including CRC, pancreatic,
lung and breast cancer (17,18). Based on the high expression of CEA in
colon cancer, the diagnosis of colon cancer was improved via CEA
level detection (19,20). However, CEA expression has also been
reported to be increased in certain non-cancerous conditions, such
as ulcerative colitis, pancreatitis, cirrhosis and hypothyroidism
(5,21). Therefore, CEA alone may not offer a
reliable screening test for early cancer detection (7). Our results confirmed that CEA is
elevated in the serum of patients with stage I/II colon cancer
compared with healthy controls, and demonstrated that the combined
ROC analysis using both CEA and miR-17-3p may significantly
increase the sensitivity and specificity of CEA; thus, CEA may be a
useful component in combined biomarker detection due to its high
sensitivity.
miRNAs play an important role in carcinogenesis,
they have been assessed as diagnostic biomarkers (13,22) and
prognostic factors (23) and have
been used in the therapeutic strategies for various cancers
(13). Various miRNAs have been found
to be present at reduced or elevated levels in the serum or plasma
of colon cancer patients (12,22,24).
Considering miR-17-3p levels in the serum of colon cancer patients,
previous investigations have yielded contradictory results. In
2009, Ng et al identified an elevation of miR-17-3p levels
in the plasma of CRC patients (15).
Our results support this finding, as we found elevated miR-17-3p
levels in the serum of early-stage colon cancer patients. However,
in 2013, Faltejskova et al reported that there was no
significant difference in the serum levels of miR-17-3p between CRC
patients and healthy controls (16).
As we analyzed miR-17-3p expression using the same protocol as that
used in the Faltejskova et al study, we also used miR-16 as
control to evaluate the expression. A potential explanation for
these contradictory results may be that the study of Ng et
al and our study were performed on Asian populations, whereas
the study of Faltejskova et al included a Caucasian
population.
Combining detection of different biomarkers has been
reported to be a useful strategy in several studies (12,13,17,22),
as it increases the sensitivity and specificity of each biomarker.
In the present study, CEA exhibited high specificity and low
sensitivity, while miR-17-3p exhibited the opposite properties. In
a combined ROC analysis using the two markers, both sensitivity and
specificity reached a relatively high value in discriminating
early-stage colon cancer patients from healthy controls. To date,
no reliable independent biomarker has been established for
early-stage colon cancer screening. Our results suggest that the
combination of CEA and miR-17-3p serum assays may offer a useful
tool for the detection of early-stage colon cancer detection.
In conclusion, the combined detection of CEA and
miR-17-3p may prove to be of clinical value for the early screening
of colon cancer. Combined CEA and miR-17-3p detection was found to
significantly increase sensitivity and specificity in early-stage
colon cancer diagnosis. Therefore, the combined detection of serum
CEA and miR-17-3p may have the potential to become a new laboratory
method for the clinical diagnosis of colon cancer.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolpin BM, Meyerhardt JA, Mamon HJ and
Mayer RJ: Adjuvant treatment of colorectal cancer. CA Cancer J
Clin. 57:168–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Storli KE, Søndenaa K, Bukholm IR, Nesvik
I, Bru T, Furnes B, Hjelmeland B, Iversen KB and Eide GE: Overall
survival after resection for colon cancer in a national cohort
study was adversely affected by TNM stage, lymph node ratio,
gender, and old age. Int J Colorectal Dis. 26:1299–1307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stanford Cancer Center. Cancer diagnosis,
. Information about cancer. https://stanfordhealthcare.org/medical-clinics/cancer-center.htmlAccessed.
October 15–2008.
|
|
5
|
Schimanski CC, Frerichs K, Rahman F,
Berger M, Lang H, Galle PR, Moehler M and Gockel I: High miR-196a
levels promote the oncogenic phenotype of colorectal cancer cells.
World J Gastroenterol. 15:2089–2096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo X, Stock C, Burwinkel B and Brenner H:
Identification and evaluation of plasma microRNAs for early
detection of colorectal cancer. PLoS One. 8:e628802013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duffy MJ, van Dalen A, Haglund C, Hansson
L, Klapdor R, Lamerz R, Nilsson O, Sturgeon C and Topolcan O:
Clinical utility of biochemical markers in colorectal cancer:
European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer.
39:718–727. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bandrés E, Cubedo E, Agirre X, Malumbres
R, Zárate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzó M, et
al: Identification by real-time PCR of 13 mature microRNAs
differentially expressed in colorectal cancer and non-tumoral
tissues. Mol Cancer. 5:292006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamichi N, Shimomura R, Inada K, Sakurai
K, Haraguchi T, Ozaki Y, Fujita S, Mizutani T, Furukawa C,
Fujishiro M, et al: Locked nucleic acid in situ hybridization
analysis of miR-21 expression during colorectal cancer development.
Clin Cancer Res. 15:4009–4016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lanza G, Ferracin M, Gafà R, Veronese A,
Spizzo R, Pichiorri F, Liu CG, Calin GA, Croce CM and Negrini M:
mRNA/microRNA gene expression profile in microsatellite unstable
colorectal cancer. Mol Cancer. 6:542007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schepeler T, Reinert JT, Ostenfeld MS,
Christensen LL, Silahtaroglu AN, Dyrskjøt L, Wiuf C, Sørensen FJ,
Kruhøffer M, Laurberg S, et al: Diagnostic and prognostic microRNAs
in stage II colon cancer. Cancer Res. 68:6416–6424. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Etheridge A, Lee I, Hood L, Galas D and
Wang K: Extracellular microRNA: A new source of biomarkers. Mutat
Res. 717:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ng EK, Chong WW, Jin H, Lam EK, Shin VY,
Yu J, Poon TC, Ng SS and Sung JJ: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Faltejskova P, Bocanek O, Sachlova M,
Svoboda M, Kiss I, Vyzula R and Slaby O: Circulating miR-17-3p,
miR-29a, miR-92a and miR-135b in serum: Evidence against their
usage as biomarkers in colorectal cancer. Cancer Biomark.
12:199–204. 2012.PubMed/NCBI
|
|
17
|
Chen Y, Gao SG, Chen JM, Wang GP, Wang ZF,
Zhou B, Jin CH, Yang YT and Feng XS: Serum CA242, CA199, CA125,
CEA, and TSGF are biomarkers for the efficacy and prognosis of
cryoablation in pancreatic cancer patients. Cell Biochem Biophys.
2014.
|
|
18
|
Hammarström S: The carcinoembryonic
antigen (CEA) family: Structures, suggested functions and
expression in normal and malignant tissues. Semin Cancer Biol.
9:67–81. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Monzo M, Navarro A, Bandres E, Artells R,
Moreno I, Gel B, Ibeas R, Moreno J, Martinez F, Diaz T, et al:
Overlapping expression of microRNAs in human embryonic colon and
colorectal cancer. Cell Res. 18:823–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi B, SeppLorenzino L, Prisco M, Linsley
P, deAngelis T and Baserga R: Micro RNA 145 targets the insulin
receptor substrate-1 and inhibits the growth of colon cancer cells.
J Biol Chem. 282:32582–32590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maestranzi S, Przemioslo R, Mitchell H and
Sherwood RA: The effect of benign and malignant liver disease on
the tumour markers CA19-9 and CEA. Ann Clin Biochem. 35:99–103.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Q, Huang Z, Ni S, Xiao X, Xu Q, Wang
L, Huang D, Tan C, Sheng W and Du X: Plasma miR-601 and miR-760 are
novel biomarkers for the early detection of colorectal cancer. PLoS
One. 7:e443982012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng H, Zhang L, Cogdell DE, Zheng H,
Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR and Zhang W:
Circulating plasma miR-141 is a novel biomarker for metastatic
colon cancer and predicts poor prognosis. PLoS One. 6:e177452011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|