Introduction
Ovarian cancer (OC) is the fifth most common cause
of cancer-related mortality in women. A high incidence of OC
correlates geographically with more economically developed
countries (1,2). The current treatments for OC are
cytoreductive surgery and platinum/paclitaxel (Taxol®)-based
chemotherapy (3). These therapies are
efficient in the treatment of 90% of patients diagnosed with OC,
but only when the disease is detected at an early stage (4). In addition, the treatments lack
specificity, further contributing to the high mortality rates of OC
(3,5,6). The
absence of an anatomical barrier around the ovary facilitates rapid
spreading of metastases in the peritoneal cavity and late diagnosis
is attributed to the minimal manifestations of early EOC (4,7). As a
consequence, the majority of OC cases are detected only when the
cancer has already metastasized to other anatomical structures
(8).
Epithelial OC (EOC) is the most common type of OC,
constituting 90% of diagnosed OC cases (9). One of the greatest challenges in EOC
research is to understand its cellular and molecular origin(s)
(10). Different in vivo and
in vitro systems have been used to model EOC. Drosophila
melanogaster (D. melanogaster) and Mus musculus
(M. musculus) EOC models have been helpful in elucidating
the biological characteristics of EOC, such as the molecular basis
of its metastatic mechanisms, which include alterations in cell
adhesion or migration, or expression of genes involved in EOC
development (11,12). Unlike humans, however, neither flies
nor mice spontaneously develop EOC; therefore, the translation of
outcomes to humans is limited. By contrast, in vitro EOC
models using human cells are a promising approach to testing
anticancer drugs, although the absence of the tumor cell
microenvironment is associated with certain limitations (13). Gallus gallus domesticus, the
domestic hen, is a model which appears to address some of these
shortcomings and, with the recent advances in laboratory tools for
chicken research, it is becoming a tractable system for the study
of EOC (14). The hen is the only
animal model that, like humans, develops the disease spontaneously
and exhibits similar pathology and disease progression; this
appears to be associated with prolific ovulation and ageing
(14).
The aim of the present review was: i) To provide an
overview of the current approaches and challenges in OC research,
with a focus on EOC; ii) to provide a comparative analysis of the
advantages and disadvantages of the different models used in EOC
research; and iii) to investigate Gallus gallus domesticus
as a model to answer fundamental questions regarding the origin of
EOC that remain unanswered and to advance modalities for treatment
and early diagnosis that may ultimately contribute to decreasing
the mortality rate of OC.
Pathology and origin of EOC in humans
Pathogenesis
More than 30 types of OC have been described, which
are all derived from only three major progenitor cell types, namely
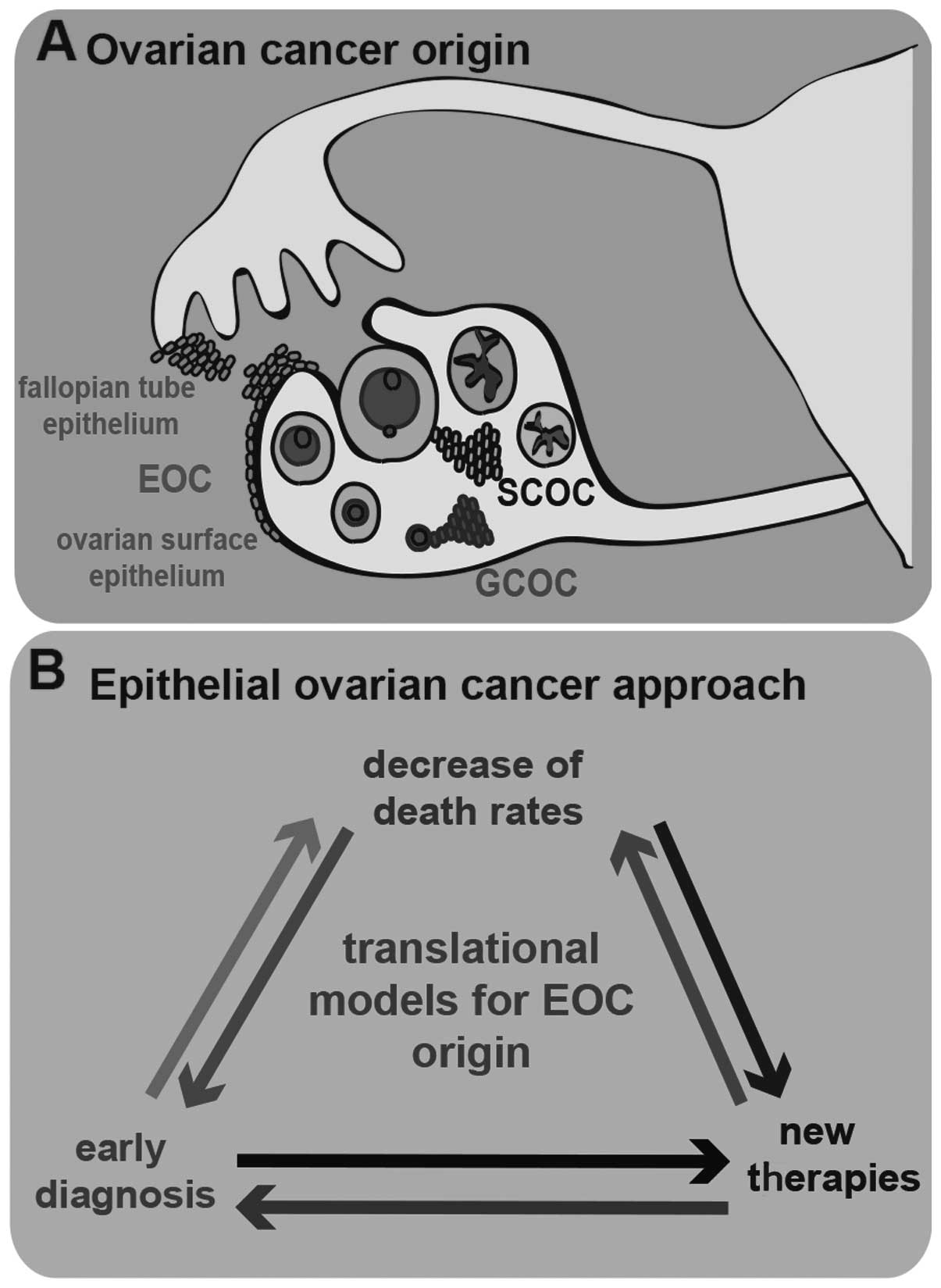
stromal cells, germ cells and surface epithelial cells (Fig. 1A). Stromal-cell OC (SCOC) results from
the transformation of stromal cells present in the ovary and has a
very low prevalence among OCs (7%); germ-cell OC (GCOC) results
from germ cell abnormalities that arise during development and is
the rarest histotypic origin of OC, with a prevalence of only 3%;
EOC is by far the most prevalent OC histotype origin, with a
prevalence of 90% (9). EOC results
from the abnormal development of epithelial cells and its origin is
discussed in detail below. The formation of malignant cysts from
malignant epithelial cells is currently considered to herald the
pathological development of EOC. Malignant epithelial ovarian cells
in the cysts undergo epithelial-to-mesenchymal transition (EMT),
becoming motile and capable of invading other tissues (15). The progress of the metastatic process
depends on the ability of these cells to survive and attach to
other structures (8,16).
Causality
There is currently no consensus regarding the origin
of EOC and it is considered to either derive from malignant
alterations of the ovarian surface epithelium (17), or from the abnormal development of the
Fallopian tube epithelium (18). The
complexity of EOC appears to indicate that the ovarian surface
epithelium as well as the Fallopian tube epithelium are involved in
the development of this disease (10). The ovarian surface epithelium as the
origin for EOC is the oldest hypothesis and has been associated
with the high frequency of ovulation in women (19–21).
During each ovulation, this epithelium is disrupted when the mature
oocyte is expelled from the ovary and inflammatory processes are
then required to repair it (17,22).
During the repair process, a proportion of the cells detach and
develop abnormalities, due to the DNA damage in response to
inflammatory molecules, resulting in EOC (23). A role for hormones in the damage of
the ovarian surface epithelium has also been suggested (24,25). The
observation that women who use progestin-estrogen oral
contraceptives have a 30–60% lower probability of developing EOC,
further strengthens the hypothesis that the ovarian surface
epithelium is the origin of EOC (26). However, female mice, which ovulate
approximately 4 times more than a woman during their lifespan, do
not develop this disease (27). It is
possible that structural differences in the ovarian surface
epithelium between mice and humans (27) allow mice to develop a form of
‘resistance’ against EOC development, despite their significantly
higher ovulation rates. Interestingly, it is estimated that the
number of ovulations of a 2-year-old hen is similar to the number
of ovulations of a woman at menopause (28). The fact that the hen is the only
animal model that develops spontaneous EOC suggests similarities in
the role of ovulation in the development of the disease between
hens and humans. On the other hand, EOC was recently associated
with abnormalities of the Fallopian tube epithelium. The Fallopian
tube epithelium has been proposed as an origin of EOC, since
several proteins normally expressed by the oviduct, such as paired
box 8 (PAX8) and cancer antigen (CA)-125, have been found to be
expressed in EOC biopsies (10,29).
Moreover, it has been suggested that a genetic predisposition in
Fallopian tube epithelial cells gives rise to EOC; this includes
mutations in DNA damage repair genes, such as BRCA1 and
BRCA2 and cell cycle regulators, such as P53 (30). However, since the ovarian surface
epithelium and the Fallopian tube epithelium are contiguous, have a
common early embryonic origin and are both affected by ovulation,
it is difficult to distinguish whether one or both tissues are the
origin of EOC (10).
Current EOC treatments
There are four main factors that impede early
detection of EOC. First, the location of the ovaries deep in the
pelvic cavity makes it difficult to detect the initial development
of EOC through pelvic probing and imaging. However, certain
technological advances in this field, such as ultrasound and
fluorodeoxyglucose-positron emission tomography/computed
tomography, allow for better imaging and earlier detection
(6,31). Second, EOC was until recently
considered to be an asymptomatic disease. Certain attempts have
been made to establish a symptom index for OC; the physical
symptoms may include gastrointestinal, genitourinary and
gynecological complaints. These symptoms are, however, variable
among patients, so this issue has not been resolved (4,7,32). Third, there are currently no early
tumor markers for EOC that allow early diagnosis, or population
screening and later management of the disease, or monitoring of
treatment effectiveness (33).
Finally, the spread of malignant carcinogenic cells in the pelvic
cavity is facilitated by the absence of a physical barrier around
the ovaries. This promotes the spread of EOC along other organs,
such as the contralateral ovary, the uterus and the peritoneum
(8).
Once EOC is diagnosed, the primary treatment is
surgical removal of the tumor. The surgery is normally followed by
platinum and Taxol chemotherapy, which impairs cancer cell
survival. Platinum-based treatments contain chemical compounds that
promote DNA crosslinking, inhibiting DNA repair and synthesis
(34), while Taxol promotes the
assembly of microtubules in an irreversible manner, preventing cell
division and promoting apoptosis of cancer cells (35). Regrettably, the chemotherapeutic
agents used against OC are very similar to those used in the 1970s,
when platinum-based therapies were first used in OC treatment
(3). Alternatives to platinum/Taxol
chemotherapy are currently under investigation (6). These include targeting tumor
angiogenesis using inhibitors of proangiogenic proteins, such as
vascular endothelial growth factor receptor (VEGFR),
platelet-derived growth factor receptor and angiopoietins; or
targeting key elements in cell growth, such as epidermal growth
factor receptor (EGFR), which is overexpressed in EOC cells, using
tyrosine kinase inhibitors and monoclonal antibodies against the
extracellular domain of EGFR (36–39). The
majority of these treatments are being developed in animal models
but, unfortunately, often fail in clinical trials (33), highlighting the shortcomings of the
animal models for human diseases (40). As a consequence, the OC post-diagnosis
survival rates at 1, 3 and 5 years have not changed significantly
over the last 20 years (1).
Accumulating knowledge on the origin of EOC is crucial to tackling
this disease in its early stages, through identifying predictive
EOC biomarkers for diagnosis and improvement of therapy (Fig. 1B). For this purpose, it is essential
to establish a reliable experimental model capable of capturing all
the characteristics of EOC pathology and origin.
Animal models in EOC research
D. melanogaster
The conserved mechanisms of molecular signalling
pathways between fruit flies and humans, in combination with the
ability to conduct large-scale genetic screens, makes D.
melanogaster an excellent model for understanding the basic
signalling mechanisms underlying the progression of EOC. Studies in
D. melanogaster have helped identify tumor suppressor genes
and oncogenes involved in OC development (16). Border cells present in the fly's
ovaries have been used as a model to study EMT, which is part of
the cancer metastatic process (41).
These studies have identified polarity markers in the epithelium,
such as E-cadherin and myosin IV, which play a role in the
deregulation of proliferation and cell invasion, similar to what
happens in human EOC (11). EGFR and
VEGFR are key regulators of border cell invasiveness and have been
studied in the fruit fly, since they are also involved in EOC
(11). The role of the Hippo
signalling pathway has also been investigated in the fruit fly as a
model for EOC. Interestingly, by overexpressing the Yes-associated
protein component of this pathway, which is also overexpressed in
human EOC, it has been possible to induce EOC in flies,
demonstrating its significance in EOC tumorigenesis and
conservation of the process in humans (42). Studying Hippo signalling in fruit
flies has revealed the role of this pathway in tissue growth
regulation, through programming cell death and cell fate, in flies
and humans (11). However, D.
melanogaster remains a less than ideal clinical translational
model, since it displays reduced metastatic potential and lacks the
complexity of the human physiology and human immune system
(41).
M. musculus
Mice are the most widely used animals for human
disease modelling. In addition to a number of conserved molecular
and physiological pathways, mice display a large repertoire of
genetic and laboratory tools, still unsurpassed by other laboratory
species (43). Mouse models in EOC
have been extensively used to investigate disease progression in
humans and to develop anti-OC drugs. Several mouse models of EOC
with different characteristics have been developed. In this review,
we aimed to focus on the comparison of advantages and disadvantages
of three major groups of mouse models in OC research, namely
xenograft, syngeneic and genetically engineered mice.
Xenograft mouse models, in which human OC cells are
introduced into host immunodeficient mice, enable the study of the
early disease stages, as well as invasion and spreading of the
cancer cells. These models have been used to evaluate therapeutic
approaches, since they constitute a good representation of the
disease and its heterogeneity (44,45). The
immune response, however, is completely absent in xenograft models,
since the procedures are performed in immunodeficient mouse strains
(43).
The development of syngeneic mouse models, in which
the cancer cells are derived from the same mouse strain and are
introduced into the immunocompetent host, overcome certain
limitations of xenografts (46),
although the EOC studied is mouse, rather than human. These models
enable the study of immune response, tumor-secreting factors,
epithelial-stromal interactions and tumor vascularization (43,47).
Since the development of EOC in mice is never
spontaneous and must always be induced (12), this is mostly achieved using
genetically engineered mice (43).
Mice have been engineered to overexpress genes associated with EOC
in humans. These genes include P53, AKT, Brca1 and
Brca2, which have been implicated in the progression and
regression of this disease (48–51).
However, the paucity of tissue-specific promoters for ovarian
surface epithelium or Fallopian tube epithelium is a major
limitation of this approach, since it is difficult to distinguish
tissue-specific malignancy from the more general oncogenic
properties of these genes (10).
Nevertheless, engineered or transgenic mice have enabled the study
of the effects of different mutations in EOC and the corresponding
immune interactions (12,43).
Taken together, these mouse models have overcome
certain limitations of D. melanogaster in EOC research.
However, they also present with their own biological limitations,
which compromise their extrapolation to humans. For example, the
heterogeneous origin of EOC requires its study in a heterogenetic
background, which is not provided by inbred laboratory mouse
strains. Moreover, EOC development in mice is not a spontaneous
process, but rather induced as mentioned above, which, by
definition, rules out the study of the origin and initial
development of this disease, limiting the success of therapeutic
response prediction in human patients. The development of new drugs
using animal models requires a major investment from pharmaceutical
companies, since only a limited number of these drugs continue to
clinical trials. Failure to translate is a major obstacle towards
finding cures for EOC (40).
In vitro models
In vitro systems, based particularly on human cell
lines, are in principle an attractive alternative in terms of
predictive power and also have the potential to be turned into
high-throughput formats for therapeutic target identification.
These in vitro systems may also capture patient genetic profiles,
an important step in personalized medicine (13). This promise of bench-to-clinical
translation has led to various attempts of developing reliable in
vitro models of EOC. The current challenges are determining the
best source of biomaterials and improving the culture conditions of
EOC in order to mimic biological environments (13,52).
Unfortunately, cells derived from untreated tumors exhibit a
tendency to develop drug resistance during primary culture using
the presently available methods, limiting their value (53). Immortalized normal ovarian surface
and/or Fallopian tube epithelia constitute promising alternatives,
since they may be genetically modified and cultured for long
periods, although they do not mimic the initial stages of the
disease (53).
With regard to culture conditions, cell-spreading
assays, where tumor cells spread on surfaces coated with
extracellular matrix (ECM) proteins, have been used to study the
migration of OC cells (54,55). However, although these ECM proteins
may also be present in the tumor, they do not mimic the tumor
microenvironment in vivo. For this reason, 3D culture
systems have been developed to provide a more appropriate
microenvironment for EOC cells (56).
3D culture systems also allow other factors, such as oxygen
tension, growth factor gradients and properties of the ECM, to be
tightly controlled in order to test their effects on EOC
development (57). However, despite
the sophistication of these 3D systems, several widely used OC cell
lines and immortalized ovarian surface or Fallopian tube epithelium
lines have not been able to capture the biology of the tumor
(13,58). This issue has been associated with
biomechanical and biophysical constraints and inappropriate ECM
and, thus far, has not been resolved (59). Several limitations, such as
establishment of a proper ECM environment, absence of functional
vasculature or cells that are able to mediate adaptive immune
responses, remain to be overcome in order to construct truly
representative EOC in vitro models (59). Improving in vitro models for
EOC may be costly, due to the need for specialized materials and
expertise, but is also dependent on a better understanding of the
tumor microenvironment in vivo, which the 3D cultures
attempt to mimic. This is presently considered to be ‘a work in
progress’.
The domestic hen: A unique model to study
EOC
The female hen possesses a single functional ovary,
which undergoes ovulation at a high rate during its lifespan
(60). Despite anatomical
differences, the laying hen is the only experimental model that
develops spontaneous OC and, at the same time, offers the
possibility of easy manipulation of external factors, such as
nutrition or hormones and drug administration (61,62).
Moreover, the pathology and progression of the disease resembles
that in humans in several respects (63,64).
Specific characteristics of the hen also overcome several
limitations of the other models already discussed in the study of
OC.
Incessant ovulation hypothesis
Fathalla (17) was the
first to identify a possible association between the repeated
involvement of ovarian surface epithelium in the process of
ovulation and the frequency of the development of the common
ovarian neoplasms from this epithelium. In his ‘incessant ovulation
hypothesis’, Fathalla stresses the role of repeated repair of the
ruptured ovarian surface epithelium in the induction of genetic
aberrations in the tissue that culminate in the development of OC
(17,65). This hypothesis is in line with
observations on the domestic hen, which ovulates daily, on average,
for at least 2 years and exhibits an OC prevalence of 5–35% among
adult hens, depending on the genetic strain (66,67).
Moreover, the hypothesis relates EOC incidence in humans to the
fact that modern women are generally exposed to a continuous
ovulatory process from puberty to menopause. A continuous ovulatory
process without fertilization results from the decreased pregnancy
rates in modern society, also evidenced by the geographical
co-localization of high OC incidence in more economically developed
countries (17). There is strong
evidence supporting an association between low prevalence of EOC
and the use of oral contraceptives or/and pregnancy (65). While wild chickens may live for 20–30
years, the domestic hen has a relatively short lifespan and is
subject to intense and concentrated egg production during the first
2 years of its life, which makes it an interesting model to study
the role of ovulation in EOC. Indeed, Fathalla's theory laid the
foundation for different studies regarding the role of ovulation in
OC (17). The first study, using
medroxyprogesterone, demonstrated decreased egg production and a
15% reduction in the incidence of EOC in 3-year-old birds (68). More recently, using progestin as
contraceptive, a 90% decrease in OC incidence was achieved in
treated hens compared with the controls (69). A short generation time, the
possibility of controlling environmental factors and the
availability of different genetic strains make the domestic hen a
very useful model in chemoprevention experiments (61,68,69).
Biomolecular and metastatic
traits
The similarities between the hen and humans with
respect to EOC development are also observed in terms of pathology,
with several similar histopathological subtypes identified in both
species (68,70). Moreover, the sequencing of the chicken
genome 10 years ago enabled valuable molecular comparisons with
human cases (71). Different
biomarkers, such as CA-125, P53 and E-cadherin, were also expressed
in EOC in both species (28,72–74).
With respect to the EOC origin, the same
controversies apply to human and hens. In the hen, the expression
of proteins that are specifically expressed in the oviduct during
the later stages of the disease, such as ovoalbumin, ovostatin 2,
PAX2 protein or EGFR1, indicate involvement of the oviduct in
disease development (10,72). This finding supports the involvement
of the Fallopian tube epithelium in spontaneous EOC, as in humans.
This trait makes the hen a particularly useful model to better
understand the origin of the OC in humans, where the oviduct also
appears to play a role (63,67,75).
Since, as mentioned earlier, female mice do not develop spontaneous
EOC, the involvement of the Fallopian tube epithelium was only
recently demonstrated: In a transgenic mouse model, in which SV40
large T-antigen was expressed under control of a mouse
Müllerian-specific Ovgp-1 promoter, malignant progression of this
epithelium was observed (76).
With respect to the pathology of EOC in the hen,
this is a highly malignant cancer that metastasizes along the
abdominal cavity, spreading to different organs within a short
period of time (68).
Histopathological evaluation of OC metastasis reveals similar
characteristics between human and hen spontaneous adenocarcinomas
of the reproductive tract. Interestingly, the metastatic process of
EOC, in terms of the position and location of the ascites during
the later stages of hen EOC, also resembles that in humans
(18).
Despite significant evidence supporting the presence
of similar molecular patterns in the origin and development of EOC,
the lack of commercially available antibodies for
immunohistochemistry and western blot analysis remains a major
limitation in the use of hen models in EOC (14,67). In
order to increase the translational power of the laying hen as a
model in OC research, it is crucial to develop further chicken
laboratory tools in the fields of genomics, proteomics and
metabolomics. These tools will likely be useful for the study of
OC, as well as that of other pathologies (77).
Anatomy and heterogenetic
background
Different EOC types display remarkable diversity at
the cellular and molecular levels (10,78). There
is currently a scarcity of evidence regarding the role of specific
genes in the development of EOC in humans, which appears to have
heterogenic causes (78). The
evidence indicating a heterogeneous background to EOC suggests that
it is of paramount importance to establish an experimental model
with a heterogenetic background to study this disease, rather than
using inbred species (67). The
domestic hen has been extensively bred for agricultural purposes,
but its genome maintains the genetic diversity of the wild chicken
(71). Studies regarding the role of
ageing in the development of EOC in hens have demonstrated
differences in EOC prevalence rates among different strains.
Different strains appear to develop OC in parallel with ageing;
however, the incidence rate of the disease differs among strains
(66).
Development of the reproductive
system
The fact that the hen develops in ovo,
provides a significant advantage for in vivo manipulation and
imaging of embryonic processes (79).
The use of chicken embryos, which are amniotes, in cell interaction
studies, cell fate tracing or mechanisms of embryonic patterning,
has allowed investigation of several processes that have analogies
in humans (79). The development of
the urogenital system is a case in point, particularly with regard
to understanding the signalling pathways underlying the development
of the testes and ovaries (80). The
development of the gonads in chickens displays one particularly
striking characteristic: During gonadogenesis, the development of
the gonads is asymmetric, resulting in two functional testes in
males, but only one functional ovary on the left side in females
(81). This asymmetric development of
the chicken gonads affects gonadal morphology and the development
of germ cells, as exemplified by the asymmetric expression of
meiotic markers (unpublished data). In mammals, asymmetry between
the two gonads is also established during development; this does
not affect their functionality, as a pair of functional testes or
ovaries form. However, this asymmetry becomes evident in the
development of certain sexual differentiation disorders, such as
hermaphroditism (82,83). With respect to OC, it is interesting
to note that there appears to be a higher prevalence of GCOC in the
right gonad compared to that in the left gonad. This asymmetric
prevalence of GCOC suggests an association between this asymmetry
and germ cell development (84). The
chicken provides a model for asymmetric ovarian development, a
mechanism that appears to play a role in germ cell development,
which is affected in GCOC. Therefore, the higher prevalence of GCOC
in the right ovary may be further elucidated by understanding the
asymmetrical development of the gonads in the chicken. Regarding
EOC, there is no evidence supporting a role for gonadal asymmetry
in the prevalence of the disease in the right or left ovary
(84); interestingly, however,
paired-like homeodomain transcription factor 2 (PITX2), which is
overexpressed in EOC, is also a key player in the asymmetric
development of chicken female gonads (85,86). The
expression of PITX2 in the left gonad promotes proliferation of the
left cortex, leading to the asymmetric development of the gonads
(85,87). Moreover, when induced in the left
gonad, PITX2 promotes the formation of the right cortex (87). Interestingly, PITX2 plays an important
role during development, but is normally silenced in the adult; its
role in cancer was recently demonstrated in several tumor types,
such as metastatic prostate cancer and breast cancer (88–91). The
chicken embryo offers a unique experimental model to understand the
role of PITX2 in gonadal development and the effects of the
inhibition or overexpression of this transcription factor during
development, which may provide insight into its role in the
signalling pathways involved in the development of EOC.
Genetic tools: Boosting avian models in
EOC
Since Aristotle, the first to study the avian model,
the laying hen has been used extensively in experimental
embryology, disease modelling and evolutionary studies (77). The hen has contributed to our
understanding of numerous processes relevant to humans, including
(abnormal) cardiac development and somitogenesis, through which
much of the skeletal musculature is formed (77). The differences between birds and
humans, that may complicate EOC modelling, stem from the endocrine
system and relate to the sexual hormone cycle (14,69).
However, the drawbacks of avian models in studying the origin of
EOC are mostly associated with the lack of technology that provides
appropriate laboratory tools (92).
In contrast to flies and mice, there are few commercial sources of
antibodies for immunohistochemistry, FACS or western blot analysis
and transgenic approaches are only now becoming available in the
chicken laboratories (92).
Transgenesis in chicken is progressing slowly, despite the
publication of the chicken genome sequence (71). Nevertheless, small interfering RNA and
morpholino oligonucleotides have already been tested successfully
in the avian model, allowing gain- and loss-of-function gene
studies that are controlled in space and time (93). The development of isolation and
culture methods of chicken embryonic stem cells has opened new
doors in exploring chicken cell biology (94); however, as the available protocols are
far from producing the first avian knockouts, it is currently
necessary to rely on data from other models. New genetic tools,
associated with its extensive history as an experimental model and
low costs of acquisition and maintenance compared to other models,
predict remarkable advances in the use of the hen for disease
modelling (77,92). For EOC, long-term studies using the
appropriate tools with regard to gene and protein expression will
soon become more accessible. Together with the possibility of
controlling gene expression and culturing chicken cells, these will
allow researchers to investigate the spontaneous origin of EOC in a
heterogenetic background and overcome certain of the limitations of
other models.
The avian model as a key player in EOC
research
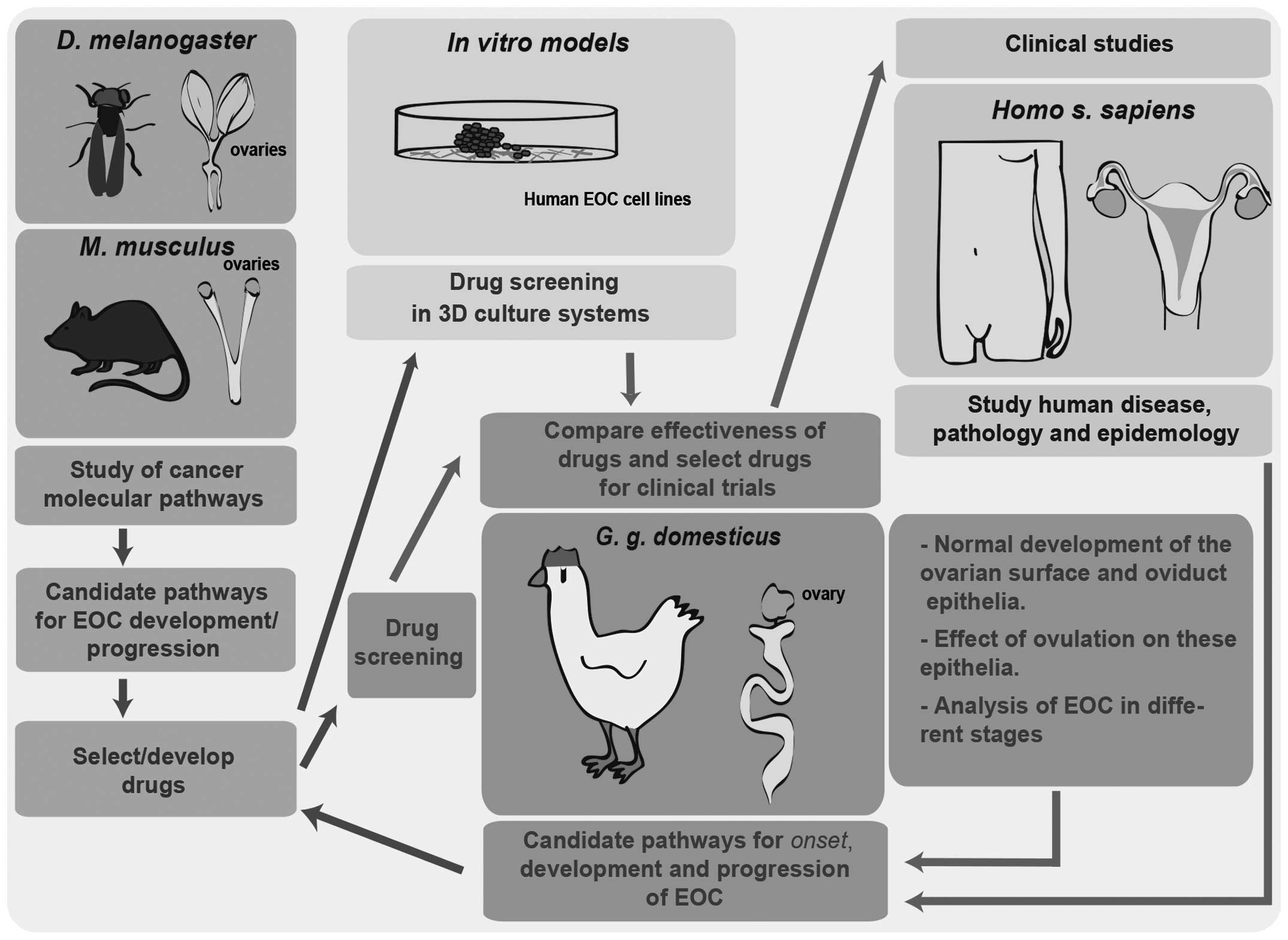
Highlighting the advantages of the hen in studying
the origin of EOC does not minimize the importance of improving
other EOC models in parallel, but rather warrants the development
of an integrative approach using different models, in vivo
and in vitro, that may complement the discoveries made in
the avian model (Fig. 2). Fruit fly
and mouse models in EOC research will continue to unravel the basic
mechanisms in EOC development and allow the development/selection
of drugs that may be screened in 3D culture systems of human EOC
cells. Subsequently, the hen offers the possibility of large-scale
drug screenings in heterogeneous populations, enabling the
comparison of drug efficiency in a robust model in order to better
select drugs for clinical trials.
On the other hand, the laying domestic hen
represents a unique system that mimics the disease in humans with
regard to origin, development, metastatic processes and association
with the ageing oviduct epithelium; in addition, the
characterization of EOC in different progression stages in the
adult hen may elucidate the mechanisms underlying the origin of EOC
in humans. Therefore, the hen constitutes a fundamental model for
the identification of candidate pathways associated with the onset,
development and progression of EOC and the selection of drugs that
target cancer pathways. Those drugs may be screened in other
models, such as fruit fly, mouse and in vitro systems, but
also in the hen itself. The complementary study of the different
models may help us elucidate the pathology and epidemiology of this
disease.
In conclusion, only an integrative research effort,
where the avian model plays a crucial role, will enable the
identification of new markers, thereby allowing the development of
novel diagnostics and therapies for OC, which remains the most
common cause of gynecological cancer-related mortlaity in
humans.
Acknowledgements
This study was supported by the Fundação para a
Ciência e a Tecnologia SFRH/BD/94387/2013 to A.M.B.
References
|
1
|
Lowe KA, Chia VM, Taylor A, et al: An
international assessment of ovarian cancer incidence and mortality.
Gynecol Oncol. 130:107–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma JM, Zou ZH and Jemal A:
Cancer Statistics, 2014. CA Cancer J Clin. 64:9–29. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaughan S, Coward JI, Bast RC, et al:
Rethinking ovarian cancer: recommendations for improving outcomes.
Nat Rev Cancer. 11:719–725. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bharwani N, Reznek RH and Rockall AG:
Ovarian cancer management: the role of imaging and diagnostic
challenges. Eur J Radiol. 78:41–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luvero D, Milani A and Ledermann JA:
Treatment options in recurrent ovarian cancer: latest evidence and
clinical potential. Ther Adv Med Oncol. 6:229–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Menon U, Griffin M and Gentry-Maharaj A:
Ovarian cancer screening - current status, future directions.
Gynecol Oncol. 132:490–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goff BA, Mandel LS, Drescher CW, et al:
Development of an ovarian cancer symptom index: possibilities for
earlier detection. Cancer. 109:221–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bast RC, Hennessy B and Mills GB: The
biology of ovarian cancer: new opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero I and Bast RC Jr: Minireview: human
ovarian cancer: biology, current management, and paths to
personalizing therapy. Endocrinology. 153:1593–1602. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
King SM and Burdette JE: Evaluating the
progenitor cells of ovarian cancer: analysis of current animal
models. BMB Rep. 44:435–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
RosalesNieves AE and Gonzalez-Reyes A:
Genetics and mechanisms of ovarian cancer: parallels between
Drosophila and humans. Semin Cell Dev Biol. 28:104–109.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fong MY and Kakar SS: Ovarian cancer mouse
models: a summary of current models and their limitations. J
Ovarian Res. 2:122009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacob F, Nixdorf S, Hacker NF and
Heinzelmann-Schwarz VA: Reliable in vitro studies require
appropriate ovarian cancer cell lines. J Ovarian Res. 7:602014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson PA and Giles JR: The hen as a
model of ovarian cancer. Nat Rev Cancer. 13:432–436. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vergara D, Merlot B, Lucot JP, et al:
Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett.
291:59–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naora H and Montell DJ: Ovarian cancer
metastasis: integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fathalla MF: Incessant ovulation - a
factor in ovarian neoplasia? Lancet. 2:1631971. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Erickson BK, Conner MG and Landen CN Jr:
The role of the Fallopian tube in the origin of ovarian cancer. Am
J Obstet Gynecol. 209:409–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fleming JS, Beaugie CR, Haviv I,
ChenevixTrench G and Tan OL: Incessant ovulation, inflammation and
epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol
Cell Endocrinol. 247:4–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murdoch WJ and McDonnel AC: Roles of the
ovarian surface epithelium in ovulation and carcinogenesis.
Reproduction. 123:743–750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murdoch WJ: Ovarian surface epithelium,
ovulation and carcinogenesis. Biol Rev Camb Philos Soc. 71:529–543.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Auersperg N, Wong AS, Choi KC, Kang SK and
Leung PC: Ovarian surface epithelium: biology, endocrinology, and
pathology. Endocr Rev. 22:255–288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maccio A and Madeddu C: Inflammation and
ovarian cancer. Cytokine. 58:133–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salehi F, Dunfield L, Phillips KP, Krewski
D and Vanderhyden BC: Risk factors for ovarian cancer: an overview
with emphasis on hormonal factors. J Toxicol Environ Health B Crit
Rev. 11:301–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Sousa Damião R, Fujiyama Oshima CT,
Stávale JN and Gonçalves WJ: Analysis of the expression of estrogen
receptor, progesterone receptor and chicken ovalbumin upstream
promoter-transcription factor I in ovarian epithelial cancers and
normal ovaries. Oncol Rep. 18:25–32. 2007.PubMed/NCBI
|
|
26
|
Moorman PG, Havrilesky LJ, Gierisch JM, et
al: Oral contraceptives and risk of ovarian cancer and breast
cancer among high-risk women: a systematic review and
meta-analysis. J Clin Oncol. 31:4188–4198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Auersperg N: Specific keynote:
experimental models of epithelial ovarian carcinogenesis. Gynecol
Oncol. 88:S47–51; discussion S52-55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hakim AH, Turbov J and Rodriguez G:
Ovarian adenocarcinomas in the laying hen and women share similar
alterations in p53, ras, and HER-2/neu. Cancer Prev Res (Phila).
2:114–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolwijck E, Span PN, Thomas CM, et al:
Prognostic value of CA 125 in ovarian cyst fluid of patients with
epithelial ovarian cancer. Oncol Rep. 23:579–584. 2010.PubMed/NCBI
|
|
30
|
Walsh T, Casadei S, Lee MK, et al:
Mutations in 12 genes for inherited ovarian, Fallopian tube, and
peritoneal carcinoma identified by massively parallel sequencing.
Proc Natl Acad Sci USA. 108:18032–18037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grant P, Sakellis C and Jacene HA:
Gynecologic oncologic imaging with PET/CT. Semin Nucl Med.
44:461–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chene G, PenaultLlorca F, Robin N, et al:
Early detection of ovarian cancer: Tomorrow? A review. J Gynecol
Obstet Biol Reprod (Paris). 42:5–11. 2013.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lokshin AE: The quest for ovarian cancer
screening biomarkers: Are we on the right road? Int J Gynecol
Cancer. 22 Suppl 1:S35–S40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muggia F: Platinum compounds 30 years
after the introduction of cisplatin: implications for the treatment
of ovarian cancer. Gynecol Oncol. 112:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kumar S, Mahdi H, Bryant C, et al:
Clinical trials and progress with paclitaxel in ovarian cancer. Int
J Womens Health. 2:411–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang RY, Chung VY and Thiery JP:
Targeting pathways contributing to epithelial-mesenchymal
transition (EMT) in epithelial ovarian cancer. Curr Drug Targets.
13:1649–1653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kalachand R, Hennessy BT and Markman M:
Molecular targeted therapy in ovarian cancer: what is on the
horizon? Drugs. 71:947–967. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Syrios J, Banerjee S and Kaye SB: Advanced
epithelial ovarian cancer: from standard chemotherapy to promising
molecular pathway targets - where are we now? Anticancer Res.
34:2069–2077. 2014.PubMed/NCBI
|
|
39
|
Itamochi H and Kigawa J: Clinical trials
and future potential of targeted therapy for ovarian cancer. Int J
Clin Oncol. 17:430–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Caponigro G and Sellers WR: Advances in
the preclinical testing of cancer therapeutic hypotheses. Nat Rev
Drug Discov. 10:179–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brumby AM and Richardson HE: Using
Drosophila melanogaster to map human cancer pathways. Nat
Rev Cancer. 5:626–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hall CA, Wang R, Miao J, et al: Hippo
pathway effector Yap is an ovarian cancer oncogene. Cancer Res.
70:8517–8525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
House CD, Hernandez L and Annunziata CM:
Recent technological advances in using mouse models to study
ovarian cancer. Front Oncol. 4:262014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shaw TJ, Senterman MK, Dawson K, Crane CA
and Vanderhyden BC: Characterization of intraperitoneal,
orthotopic, and metastatic xenograft models of human ovarian
cancer. Mol Ther. 10:1032–1042. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fu X and Hoffman RM: Human ovarian
carcinoma metastatic models constructed in nude mice by orthotopic
transplantation of histologically-intact patient specimens.
Anticancer Res. 13:283–286. 1993.PubMed/NCBI
|
|
46
|
Roby KF, Taylor CC, Sweetwood JP, et al:
Development of a syngeneic mouse model for events related to
ovarian cancer. Carcinogenesis. 21:585–591. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Urzua U, Roby KF, Gangi LM, et al:
Transcriptomic analysis of an in vitro murine model of
ovarian carcinoma: functional similarity to the human disease and
identification of prospective tumoral markers and targets. J Cell
Physiol. 206:594–602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Orsulic S, Li Y, Soslow RA, et al:
Induction of ovarian cancer by defined multiple genetic changes in
a mouse model system. Cancer Cell. 1:53–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xing D and Orsulic S: A mouse model for
the molecular characterization of brca1-associated ovarian
carcinoma. Cancer Res. 66:8949–8953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
FleskenNikitin A, Choi KC, Eng JP, Shmidt
EN and Nikitin AY: Induction of carcinogenesis by concurrent
inactivation of p53 and Rb1 in the mouse ovarian surface
epithelium. Cancer Res. 63:3459–3463. 2003.PubMed/NCBI
|
|
51
|
Wu R, HendrixLucas N, Kuick R, et al:
Mouse model of human ovarian endometrioid adenocarcinoma based on
somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling
pathways. Cancer Cell. 11:321–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fuller ES and Howell VM: Culture models to
define key mediators of cancer matrix remodeling. Front Oncol.
4:572014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gillet JP, Calcagno AM, Varma S, et al:
Redefining the relevance of established cancer cell lines to the
study of mechanisms of clinical anti-cancer drug resistance. Proc
Natl Acad Sci USA. 108:18708–18713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yagi H, Yotsumoto F and Miyamoto S:
Heparin-binding epidermal growth factor-like growth factor promotes
transcoelomic metastasis in ovarian cancer through
epithelial-mesenchymal transition. Mol Cancer Ther. 7:3441–3451.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sodek KL, Brown TJ and Ringuette MJ:
Collagen I but not Matrigel matrices provide an MMP-dependent
barrier to ovarian cancer cell penetration. BMC Cancer. 8:2232008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kenny HA, Krausz T, Yamada SD and Lengyel
E: Use of a novel 3D culture model to elucidate the role of
mesothelial cells, fibroblasts and extra-cellular matrices on
adhesion and invasion of ovarian cancer cells to the omentum. Int J
Cancer. 121:1463–1472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yamada KM and Cukierman E: Modeling tissue
morphogenesis and cancer in 3D. Cell. 130:601–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee JM, MhawechFauceglia P, Lee N, et al:
A three-dimensional microenvironment alters protein expression and
chemosensitivity of epithelial ovarian cancer cells in vitro. Lab
Invest. 93:528–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
White EA, Kenny HA and Lengyel E:
Three-dimensional modeling of ovarian cancer. Adv Drug Deliv Rev
79–80. 184–192. 2014. View Article : Google Scholar
|
|
60
|
Pollock CG and Orosz SE: Avian
reproductive anatomy, physiology and endocrinology. Vet Clin North
Am Exot Anim Pract. 5:441–474. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Barnes MN, Berry WD, Straughn JM, et al: A
pilot study of ovarian cancer chemoprevention using
medroxyprogesterone acetate in an avian model of spontaneous
ovarian carcinogenesis. Gynecol Oncol. 87:57–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Carver DK, Barnes HJ, Anderson KE, et al:
Reduction of ovarian and oviductal cancers in calorie-restricted
laying chickens. Cancer Prev Res (Phila). 4:562–567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Giles JR, Elkin RG, Trevino LS, et al: The
restricted ovulator chicken: a unique animal model for
investigating the etiology of ovarian cancer. Int J Gynecol Cancer.
20:738–744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Seo HW, Rengaraj D, Choi JW, et al: The
expression profile of apoptosis-related genes in the chicken as a
human epithelial ovarian cancer model. Oncol Rep. 25:49–56.
2011.PubMed/NCBI
|
|
65
|
Havrilesky LJ, Gierisch JM, Moorman PG, et
al: Oral contraceptive use for the primary prevention of ovarian
cancer. Evid Rep Technol Assess (Full Rep). 212:1–514.
2013.PubMed/NCBI
|
|
66
|
Johnson PA and Giles JR: Use of genetic
strains of chickens in studies of ovarian cancer. Poult Sci.
85:246–250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hawkridge AM: The chicken model of
spontaneous ovarian cancer. Proteomics Clin Appl. 8:689–699. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fredrickson TN: Ovarian tumors of the hen.
Environ Health Perspect. 73:35–51. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Trevino LS, Buckles EL and Johnson PA:
Oral contraceptives decrease the prevalence of ovarian cancer in
the hen. Cancer Prev Res (Phila). 5:343–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Barua A, Bitterman P, Abramowicz JS, et
al: Histopathology of ovarian tumors in laying hens: a preclinical
model of human ovarian cancer. Int J Gynecol Cancer. 19:531–539.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
International Chicken Genome Sequencing
Consortium, . Sequence and comparative analysis of the chicken
genome provide unique perspectives on vertebrate evolution. Nature.
432:695–716. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Trevino LS, Giles JR, Wang W, Urick ME and
Johnson PA: Gene expression profiling reveals differentially
expressed genes in ovarian cancer of the hen: support for oviductal
origin? Horm Cancer. 1:177–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ansenberger K, Zhuge Y, Lagman JA, et al:
E-cadherin expression in ovarian cancer in the laying hen,
Gallus domesticus, compared to human ovarian cancer. Gynecol
Oncol. 113:362–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jackson E, Anderson K, Ashwell C, Petitte
J and Mozdziak PE: CA 125 expression in spontaneous ovarian
adenocarcinomas from laying hens. Gynecol Oncol. 104:192–198. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lim CH, Lim W, Jeong W, et al: Avian WNT4
in the female reproductive tracts: potential role of oviduct
development and ovarian carcinogenesis. PLoS One. 8:e659352013.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kim J, Coffey DM, Creighton CJ, et al:
High-grade serous ovarian cancer arises from Fallopian tube in a
mouse model. Proc Natl Acad Sci USA. 109:3921–3926. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kain KH, Miller JW, JonesParis CR, et al:
The chick embryo as an expanding experimental model for cancer and
cardiovascular research. Dev Dyn. 243:216–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer: a proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Davey MG and Tickle C: The chicken as a
model for embryonic development. Cytogenet Genome Res. 117:231–239.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ayers KL, Sinclair AH and Smith CA: The
molecular genetics of ovarian differentiation in the avian model.
Sex Dev. 7:80–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Smith CA and Sinclair AH: Sex
determination: insights from the chicken. Bioessays. 26:120–132.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mittwoch U: Phenotypic manifestations
during the development of the dominant and default gonads in
mammals and birds. J Exp Zool. 281:466–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mittwoch U: Sex determination. EMBO Rep.
14:588–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Roychoudhuri R, Putcha V and Moller H:
Cancer and laterality: a study of the five major paired organs
(UK). Cancer Causes Control. 17:655–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
RodriguezLeon J, Rodriguez Esteban C,
Marti M, et al: PITX2 regulates gonad morphogenesis. Proc Natl Acad
Sci USA. 105:11242–11247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fung FK, Chan DW, Liu VW, et al: Increased
expression of PITX2 transcription factor contributes to ovarian
cancer progression. PLoS One. 7:e370762012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Guioli S and Lovell-Badge R: PITX2
controls asymmetric gonadal development in both sexes of the chick
and can rescue the degeneration of the right ovary. Development.
134:4199–4208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Vela I, Morrissey C, Zhang X, et al: PITX2
and non-canonical Wnt pathway interaction in metastatic prostate
cancer. Clin Exp Metastasis. 31:199–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gobel G, Auer D, Gaugg I, et al:
Prognostic significance of methylated RASSF1A and PITX2 genes in
blood- and bone marrow plasma of breast cancer patients. Breast
Cancer Res Treat. 130:109–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wilting J and Hagedorn M: Left-right
asymmetry in embryonic development and breast cancer: common
molecular determinants? Curr Med Chem. 18:5519–5527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Vandenberg LN and Levin M: A unified model
for left-right asymmetry? Comparison and synthesis of molecular
models of embryonic laterality. Dev Biol. 379:1–15. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Stern CD: The chick embryo - past, present
and future as a model system in developmental biology. Mech Dev.
121:1011–1013. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
SaukaSpengler T and Barembaum M: Gain- and
loss-of-function approaches in the chick embryo. Methods Cell Biol.
87:237–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Intarapat S and Stern CD: Chick stem
cells: current progress and future prospects. Stem Cell Res.
11:1378–1392. 2013. View Article : Google Scholar : PubMed/NCBI
|