Case report
A 23-year-old woman presented with enlarged right
inguinal lymph nodes during her seventh month of pregnancy in
December, 2005. The patient had no fever, chills, bleeding,
dizziness, facial pressure or hepatosplenomegaly. A review of the
remainder of the organ systems yielded unremarkable findings. The
past medical history was also unremarkable. A fine-needle
aspiration of the inguinal nodes was performed and abundant
lymphoid cells with a few scattered histiocytoid cells were found,
suggesting a lymphoproliferative disorder. The patient then
underwent an excisional biopsy of an enlarged right inguinal node.
The histological examination revealed effacement of the
architecture, with prominent expansion of interfollicular areas by
a neoplastic infiltrate composed of blasts cells with a high
nucleus:cytoplasm ratio, slightly irregular nuclei, fine chromatin
and small nucleoli. Numerous mitotic figures were present. The
immunohistopathological examination demonstrated that the
neoplastic cells were myeloblasts with aberrant T-cell antigen
expression: MPO+, CD5+, CD7+,
CD10+, CD34+, CD43+,
TdT+, Bcl-2+, CD1a−,
CD2−, CD3−, CD4−, CD8−,
CD20−, CD45RO−, CD56−,
CD57−, Bcl-1−, Bcl-6− and
Pax-5−. The pathological examination of the lymph nodes
revealed infiltration by myeloid sarcoma (MS). The peripheral blood
cell count and differentiation were within the normal range. A
subsequent bone marrow (BM) aspiration was performed, revealing a
hypercellular BM (~70% cellularity), without histomorphological
evidence of lymphoma or leukemia, although trilineage hematopoiesis
with myeloid hyperplasia was observed. The BM smear results were as
follows: blasts 2%, progranulocytes 0%, myelocytes 21%,
metamyelocytes 19%, granulocytes 20%, eosinophils 2%, basophils 0%,
lymphocytes 11%, plasma cells 1%, monocytes 2% and normoblasts 22%.
BM flow cytometry detected no significant increase in blasts or
abnormal cells. The T-, B- and NK-cell populations were normal in
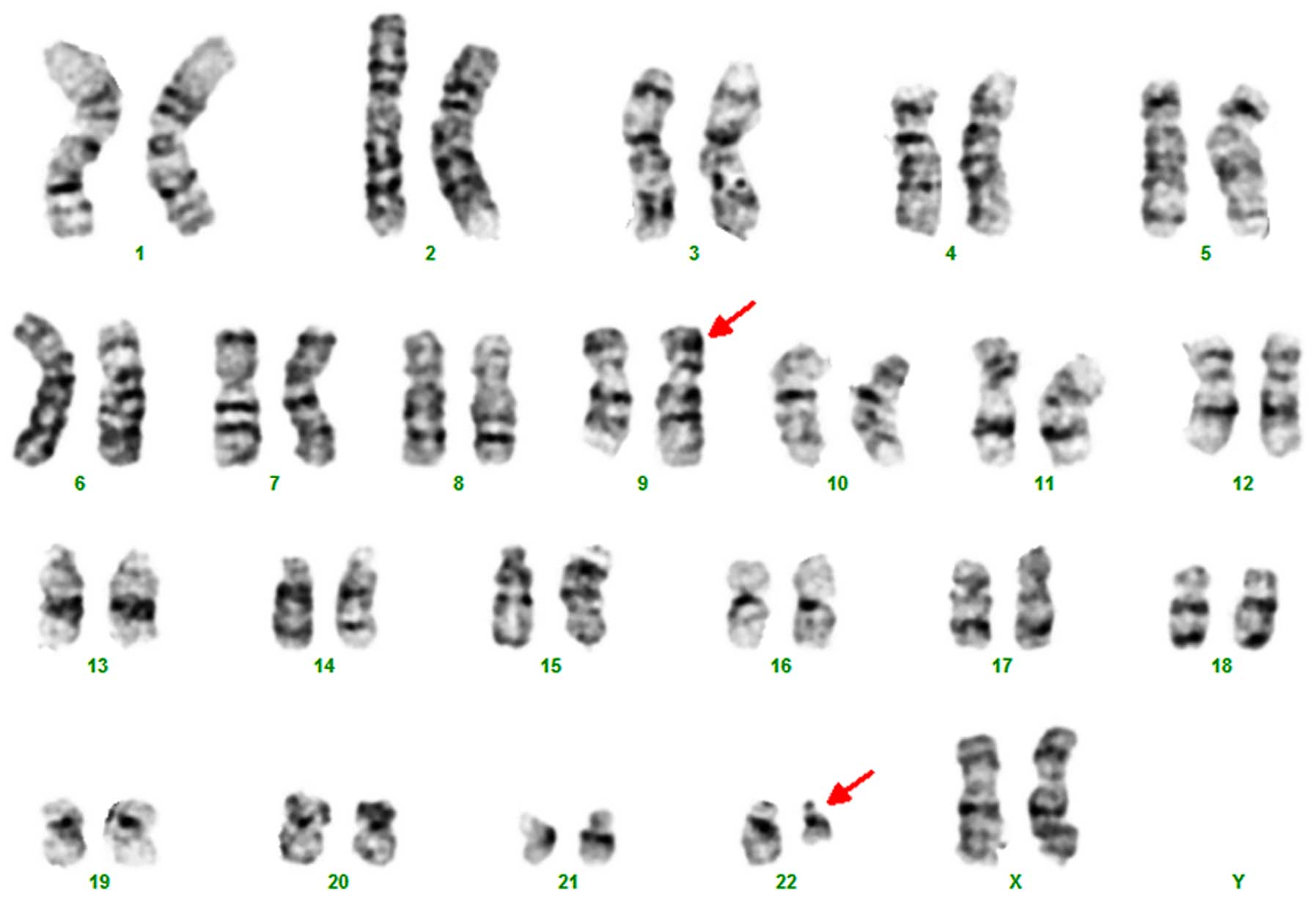
number. Interestingly, however, the karyotype analysis of BM
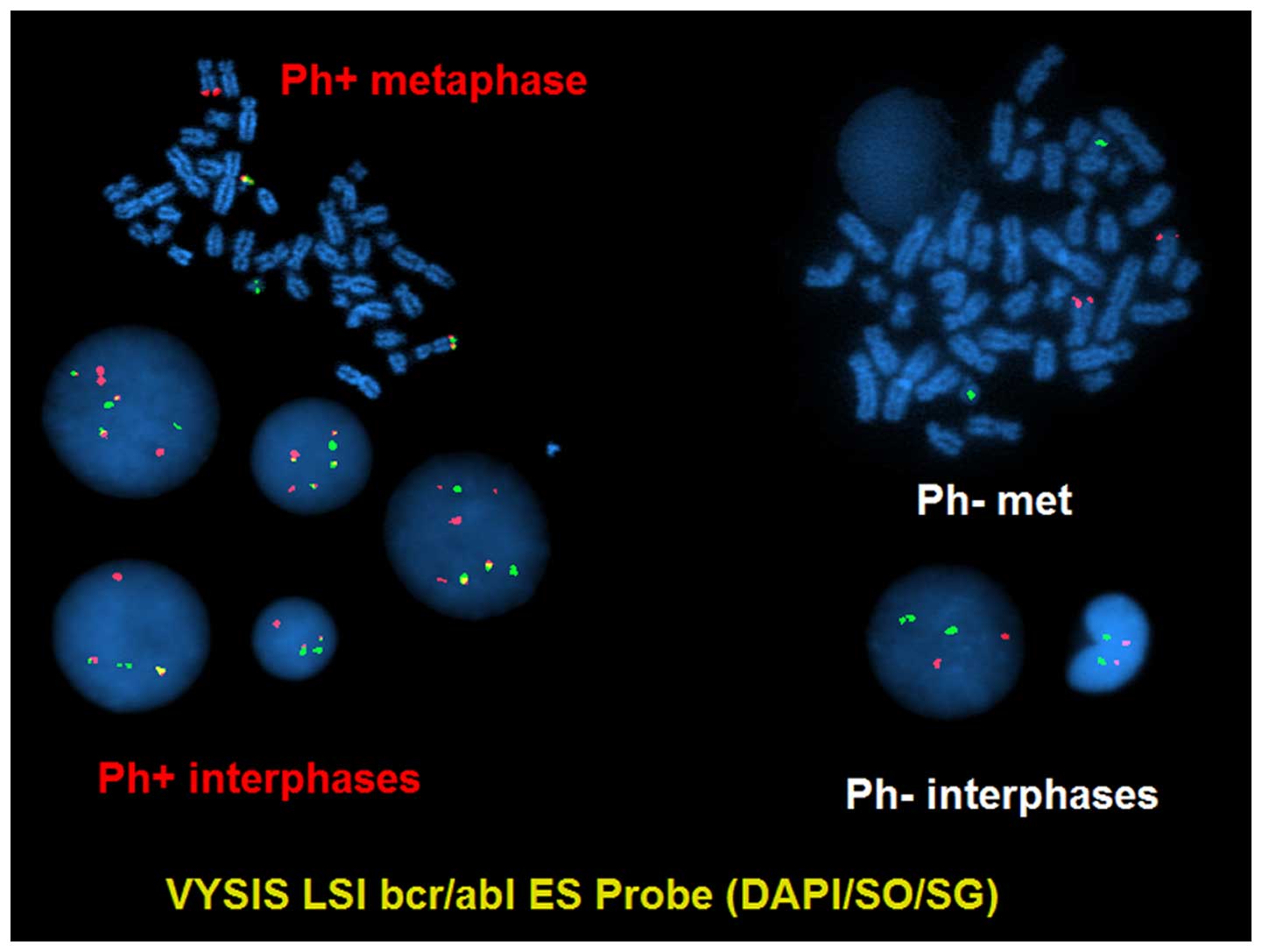
detected 46,XX,t(9;22)(q34;q11.2)[15]/46,XX[5] (Fig. 1) and the fluorescence in situ
hybridization (FISH) test result was positive for BCR-ABL1
rearrangement (116/200) (Fig. 2). The
peripheral blood counts remained normal.
In March 2006, after the delivery of the fetus, BM
aspiration was repeated. The BM biopsy revealed 70% cellularity,
whereas the morphology of megakaryocytes was unremarkable. The BM
smear revealed 2% blasts and slightly left-shifted granulocytes.
The karyotype analysis of BM detected
46,XX,t(9;22)(q34;q11.2)[13]/46,XX[7] and FISH confirmed
BCR-ABL1 rearrangement (129/200). Quantitative polymerase
chain reaction (PCR) analysis of the peripheral blood identified
the e1a2 BCR-ABL1 fusion transcript. The percentage of
BCR-ABL1 to ABL1 transcripts was 66.78. The physical
examination identified a right inguinal node measuring 3×4 cm,
whereas no cervical or axillary nodes were palpable. The patient
was administered idarubicin, cytarabine, vincristine, dexamethasone
and imatinib (600 mg/day) as initial treatment. After two courses
of treatment, the patient achieved complete resolution of
lymphadenopathy and complete cryptogenic response in the BM. The
percentage of BCR-ABL1 to ABL1 transcripts decreased
from 66.78 to 5.14. However, after 3 more courses of treatment, the
percentage of BCR-ABL1 to ABL1 transcripts exhibited
no further decline. Therefore, chemotherapy was discontinued and
the patient underwent related allogeneic hematopoietic stem cell
transplant (Allo-HSCT) from her HLA-identical brother in September,
2006. Following engraftment (100% donor), the patient achieved a
major, but not complete, molecular remission (the percentage of
BCR-ABL1 to ABL1 transcripts was 0.03–0.04). Four
months after Allo-HSCT, the BCR-ABL1 level increased to 0.7.
Therefore, imatinib 400 mg/day was administered. In June, 2007, the
BM exhibited diploid female karyotypes and molecular studies
revealed 90% of the cells to be donor. Blast cells (3%) were found
in the BM, and FISH on BM examination detected no BCR-ABL1
rearrangement. The patient underwent a series of donor lymphocyte
infusions (DLIs) in August, 2007, February and June, 2008 and
January, 2009. The imatinib dose was escalated to 800 mg/day.
However, the BCR-ABL1 level remained between 0.28 and 0.65.
In June, 2010, a fifth DLI was performed, following which the
patient developed a mild continued skin graft-versus-host disease
(GVHD). In September, 2010, imatinib was switched to dasatinib.
From March, 2011 onwards, the patient switched to 100% donor cells
and the BM showed no Philadelphia chromosome. The peripheral blood
BCR-ABL1 PCR was negative. Since then, the patient has
remained in complete molecular remission and has been leukemia-free
for >48 months.
Discussion
In >95% chronic myeloid leukemia (CML) patients,
the two major BCR-ABL1 transcripts are b2a2 and b3a2, which
encode the P210 oncoprotein. Very rarely, CML patients have the
e1a2 transcript, which encodes the P190 oncoprotein. In a large
series study of 1,292 CML patients from the MD Anderson Cancer
Center (1), only 14 patients
expressed the e1a2 transcript alone [9 in chronic phase (CP), 4 in
accelerated phase (AP) and 1 in blast crisis (BC)]. Several studies
from other countries (Korean and Mexican) also reported a similar
incidence of the e1a2 transcript, which was ~1% in CML (2,3). Thus,
e1a2 is a rare BCR-ABL1 transcript in CML. Although an
extramedullary BC may be observed in CML, mainly in CML-AP or BC,
its presence in CML-CP is uncommon. Our CML patient with the e1a2
BCR-ABL1 transcript exhibited unusual characteristics at
initial presentation of CML, namely manifesting an extramedullary
BC. To the best of our knowledge, this is the first case report of
CML with both the e1a2 BCR-ABL1 transcript and
extramedullary BC as initial presentation.
In addition to harboring the uncommon e1a2
transcript, this case had the notable characteristic of CML being
atypical or occult. The peripheral blood count was within the
normal range and the patient had no hepatosplenomegaly. In
addition, the morphological examination of BM smear and biopsy
revealed no abnormalities. However, the BM cytogenetics and FISH on
BM confirmed the diagnosis of CML. It was reported that P190
BCR-ABL1 CML often exhibits monocytosis, with cytomorphological
characteristics intermediate between CML and chronic myelomonocytic
leukemia (4). However, our patient
did not have monocytosis. The BM and blood tests indicated that CML
was still in the early or occult stage, but the incidence of
extramedullary BC demonstrated the aggressive characteristic of
P190-positive CML. According to the World Health Organization
recommendations, despite BM and blood status, extramedullary BC is
a sign of CML BC (5).
This case also indicated that a complete and
comprehensive BM examination is required in extramedullary MS
cases. Indeed, MS is always accompanied by BM clonal disease. Even
in certain ‘isolated’ MS cases, leukemic clones are already present
in the BM. Although several groups have reported certain ‘isolated’
or ‘aleukemic’ MS cases without overt evidence of acute myeloid
leukemia (AML), AML clones may be detected in the BM by
cytogenetics and/or PCR-based methods (6,7).
Therefore, if extramedullary MS has been diagnosed, it is necessary
to perform the abovementioned tests to definitively exclude
leukemia associated with MS, even for those cases with ‘normal’
morphological characteristics.
The other characteristic of this case is the
difficulty in management. Following treatment by a combination of
traditional chemotherapy and imatinib, the extramedullary BC
disappeared and patient achieved hematological and cytogenetic
remission, along with minor molecular remission. Later on, after a
series of treatments, including Allo-HSCT, DLI and
second-generation tyrosine kinase inhibitor (TKI), a complete
molecular remission was achieved after 7 years of continuous
treatment. The optimal treatment for CML with the e1a2 transcript
has not been formulated, due to the rarity of this disease. TKIs
have been proven to improve the outcome of CML patients, although
the limited clinical data have shown that e1a2 BCR-ABL CML exhibits
a poorer response to first-line TKIs (1,8). In a
study by Verma et al (1),
among 14 CML patients with only the e1a2 transcript who received
therapy containing imatinib, nilotinib or dasatinib, only 2
patients achieved a major molecular remission and only 6 patients
(5 in CP and 1 in AP) remained alive at a median of 39 months after
diagnosis. That clinical study demonstrated that the efficacy of
TKIs for P190 BCR-ABL1 CML is significantly lower compared with
classic P210 BCR-ABL1 CML, whereas the clinical efficacy of
new-generation TKIs, such as ponatinib, in e1a2 BCR-ABL1
CML, is not yet certain. Jain et al reported two CML-CP
patients with the e1a2 transcript receiving ponatinib therapy
(9). One patient achieved complete
hematological remission, but never achieved a cytogenetic response,
whereas the other patient achieved a minor cytogenetic response
after 3 months of ponatinib therapy. Therefore, Allo-HSCT appears
to be the best treatment option for CML at present. Our patient did
not achieve a complete molecular remission following Allo-HSCT.
However, after a series of DLIs, particularly after the last DLI,
mild skin GVHD occurred, after which the patient switched to 100%
donor cells and finally achieved complete molecular remission,
which may be attributed, at least partially, to dasatinib.
According to our limited experience with this
disease type, CML patients with the P190 oncoprotein always exhibit
an aggressive disease course, poor response to TKI treatment and a
high risk of transforming to AP and BC. The clinical
characteristics and difficulties during the entire treatment course
in this case are consistent with the abovementioned
characteristics.
We consider this case to be very interesting and
meaningful for hematologists and hematopathologists. First, to the
best of our knowledge, this is the first report of an e1a2-P190 CML
with extramedullary BC as initial presentation; and second, this
case highlights the higher agressiveness of CML when P190 is
present, and its refractoriness to treatment.
References
|
1
|
Verma D, Kantarjian HM, Jones D, Luthra R,
Borthakur G, Verstovsek S, Rios MB and Cortes J: Chronic myeloid
leukemia (CML) with P190 BCR-ABL: Analysis of characteristics,
outcomes, and prognostic significance. Blood. 114:2232–2235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goh HG, Hwang JY, Kim SH, Lee YH, Kim YL
and Kim DW: Comprehensive analysis of BCR-ABL transcript types in
Korean CML patients using a newly developed multiplex RT-PCR.
Transl Res. 148:249–256. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arana-Trejo RM, Sánchez Ruíz E,
Ignacio-Ibarra G, de la Fuente Báez E, Garces O, Morales Gómez E,
Granados Castro M, Martínez Ovilla R, Rubio-Borja ME, Anaya Solís
L, et al: BCR/ABL p210, p190 and p230 fusion genes in 250 Mexican
patients with chronic myeloid leukaemia (CML). Clin Lab Haematol.
24:145–150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Melo JV, Myint H, Galton DA and Goldman
JM: P190BCR-ABL chronic myeloid leukaemia: The missing link with
chronic myelomonocytic leukaemia? Leukemia. 8:208–211.
1994.PubMed/NCBI
|
|
5
|
Pileri SA, Orayi A and Falini B: Myeloid
sarcoma. WHO Classification of Tumours of Haematopoietic Lymphoid
Tissues. Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein
H, Thiele J and Vardiman JW: 2:(4th). (Lyon). IARC Press. 32–34.
2008.
|
|
6
|
Billio A, Pianezze G, Amato B and Fabris
P: ‘Isolated’ peritoneal granulocytic sarcoma with molecular and
chromosomal bone marrow involvement. Haematologica.
87:EIM012002.PubMed/NCBI
|
|
7
|
Huang B, You P, Zhu P, DU Z, Wu B, Xu X
and Chen Z: Isolated duodenal myeloid sarcoma associated with the
CBFβ/MYH11 fusion gene followed by acute myeloid leukemia
progression: A case report and literature review. Oncol Lett.
8:1261–1264. 2014.PubMed/NCBI
|
|
8
|
Pardanani A, Tefferi A, Litzow MR, Zent C,
Hogan WJ, McClure RF and Viswanatha D: Chronic myeloid leukemia
with p190BCR-ABL: Prevalence, morphology, tyrosine kinase inhibitor
response, and kinase domain mutation analysis. Blood.
114:3502–3503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jain P, Romo CG, Khoury HJ, Kantarjian H
and Cortes J: Clinical activity of ponatinib in patients with
chronic myeloid leukemia in chronic phase with e1a2 transcripts.
Haematologica. 98:e141–e142. 2013. View Article : Google Scholar : PubMed/NCBI
|