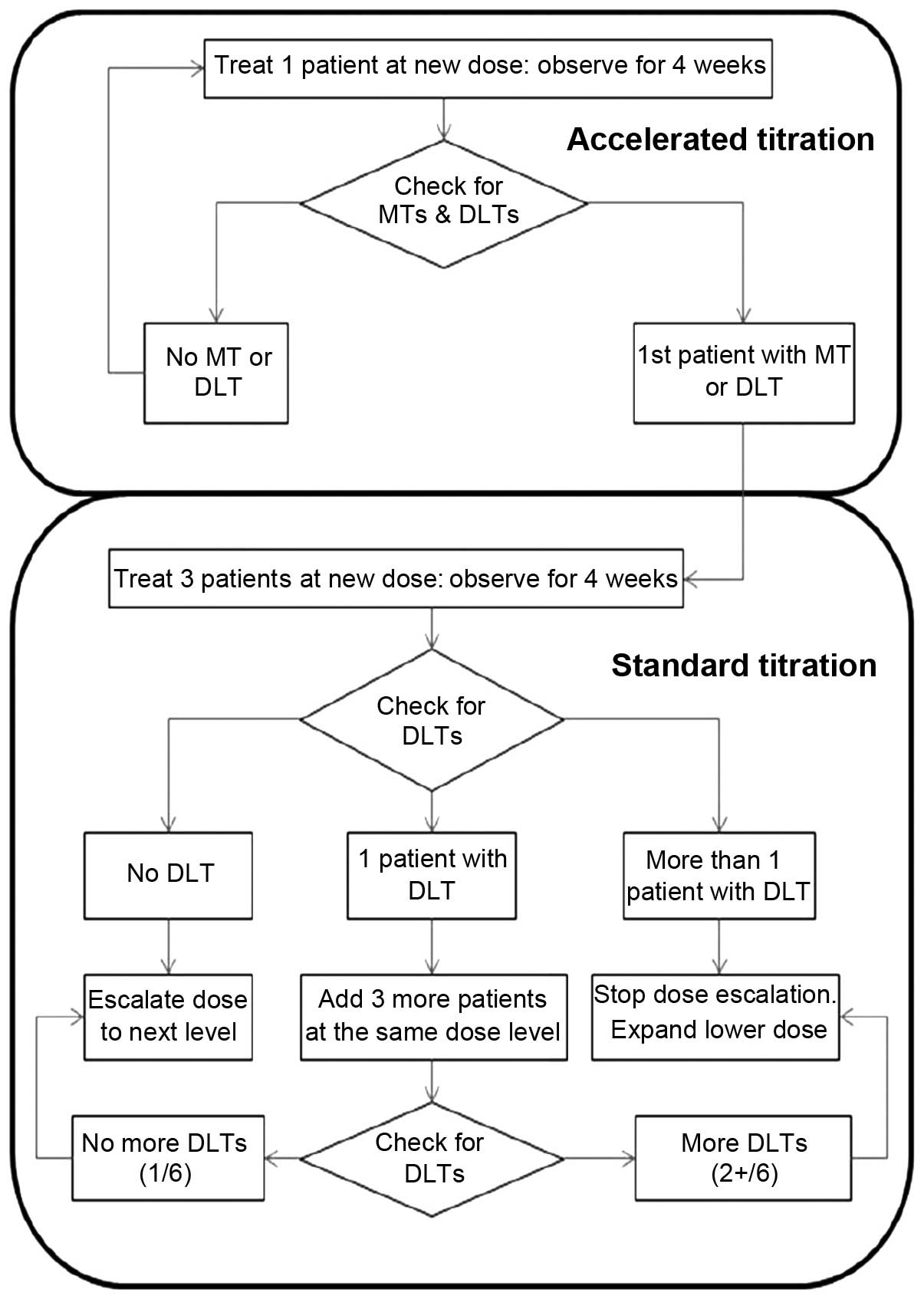
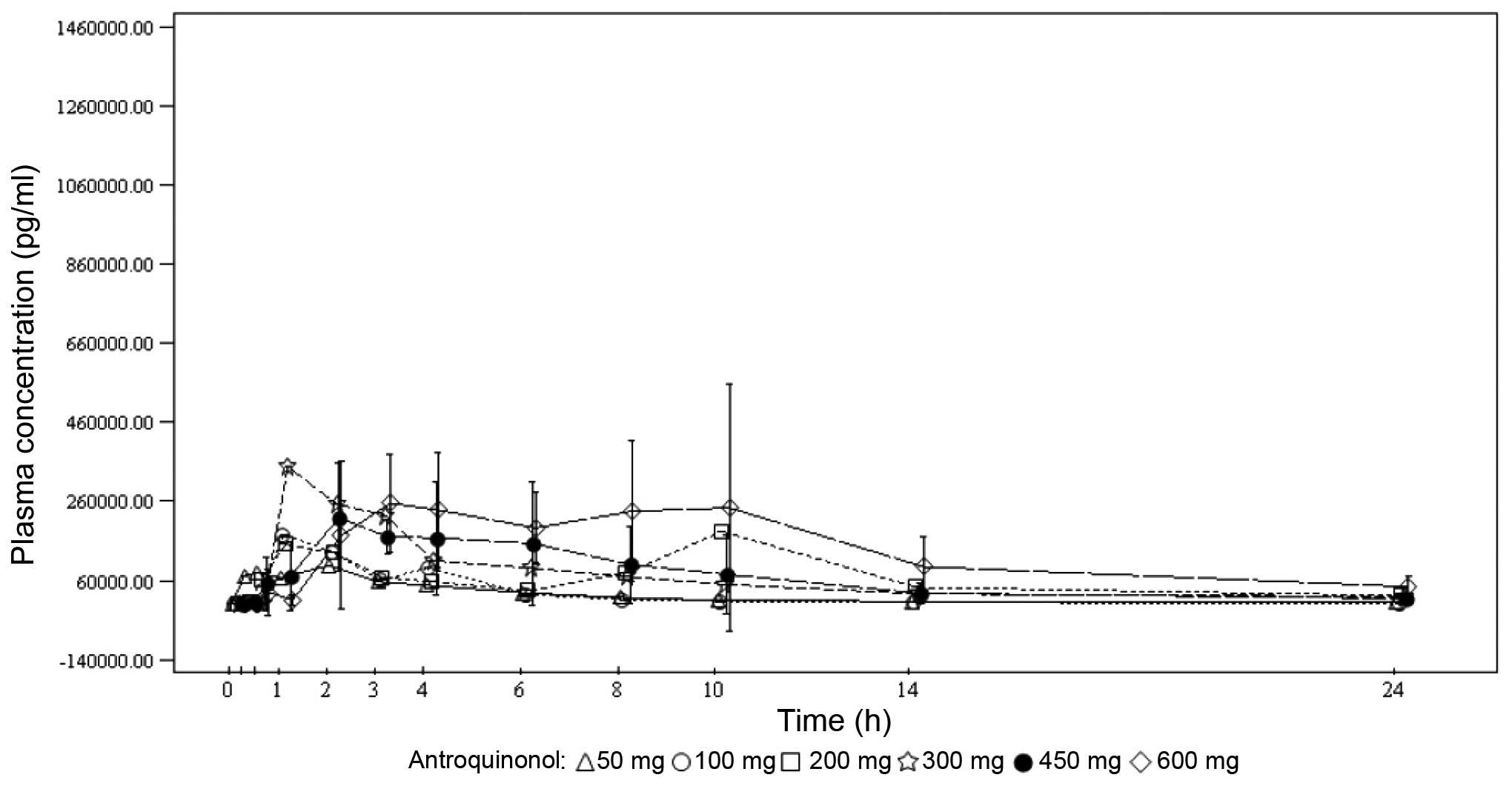
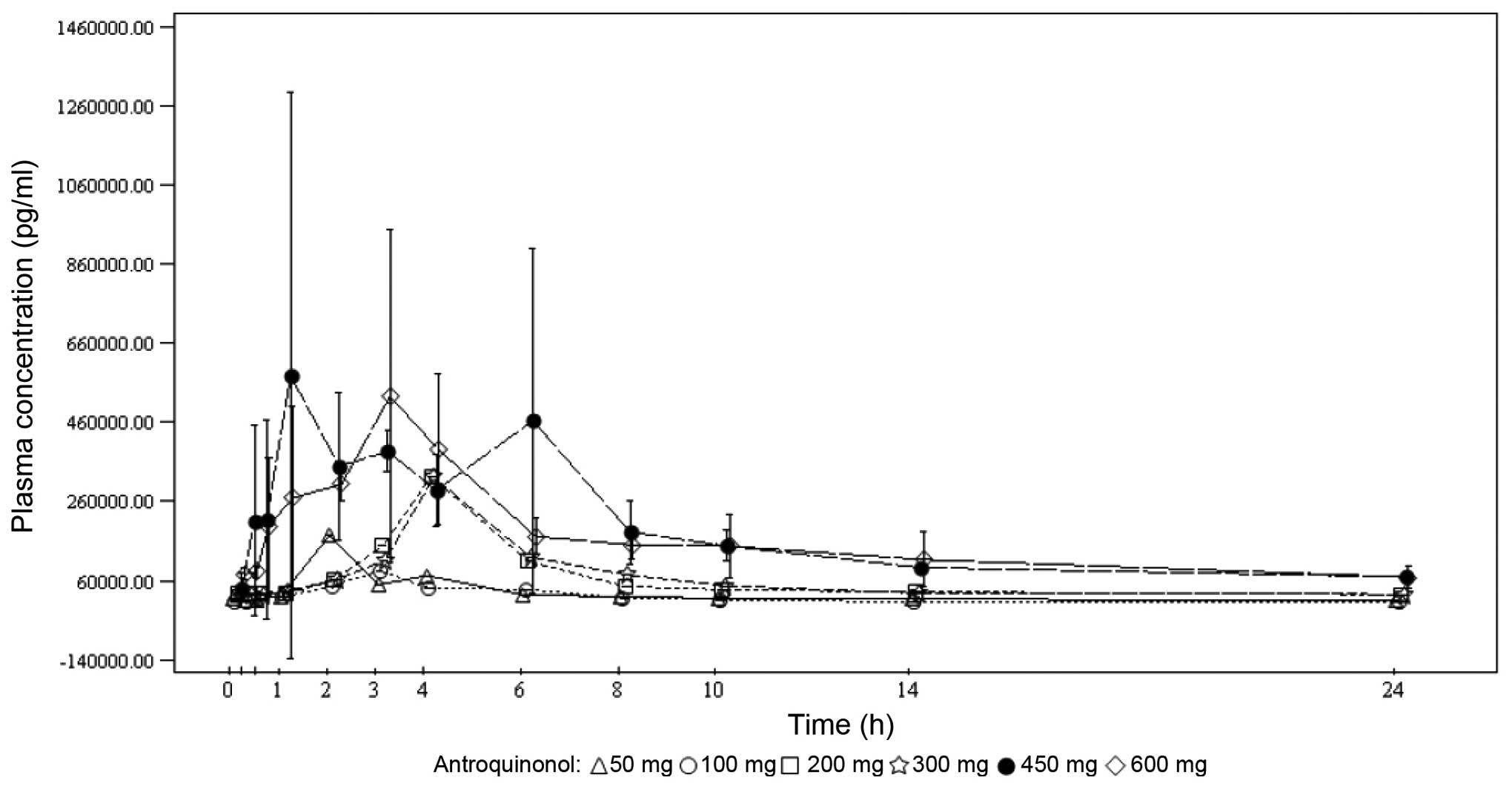
Spandidos Publications style
Lee Y, Ho C, Kao W and Chen Y: A phase I multicenter study of antroquinonol in patients with metastatic non-small-cell lung cancer who have received at least two prior systemic treatment regimens, including one platinum-based chemotherapy regimen. Mol Clin Oncol 3: 1375-1380, 2015.
APA
Lee, Y., Ho, C., Kao, W., & Chen, Y. (2015). A phase I multicenter study of antroquinonol in patients with metastatic non-small-cell lung cancer who have received at least two prior systemic treatment regimens, including one platinum-based chemotherapy regimen. Molecular and Clinical Oncology, 3, 1375-1380. https://doi.org/10.3892/mco.2015.642
MLA
Lee, Y., Ho, C., Kao, W., Chen, Y."A phase I multicenter study of antroquinonol in patients with metastatic non-small-cell lung cancer who have received at least two prior systemic treatment regimens, including one platinum-based chemotherapy regimen". Molecular and Clinical Oncology 3.6 (2015): 1375-1380.
Chicago
Lee, Y., Ho, C., Kao, W., Chen, Y."A phase I multicenter study of antroquinonol in patients with metastatic non-small-cell lung cancer who have received at least two prior systemic treatment regimens, including one platinum-based chemotherapy regimen". Molecular and Clinical Oncology 3, no. 6 (2015): 1375-1380. https://doi.org/10.3892/mco.2015.642