Introduction
Several chemokine receptors have been implicated in
tumorigenesis and metastasis of various tumors (1). CXC chemokine ligand 12 (CXCL12), also
known as stromal cell-derived factor-1 (SDF-1), plays an important
role in progression and organ-specific metastasis of several
carcinomas (2), partly through
stimulation of the G protein-coupled receptor, CXCR4. However,
CXCR4 inhibition only partially inhibits tumor progression in
vivo, suggesting that other factors contribute to epithelial
cancer progression (3).
CXCR7, formerly known as orphan receptor RDC1, was
recently identified as a second CXCL12/SDF-1 receptor.
Specifically, high-affinity CXCR7-CXCL12/SDF-1 interactions
activate chemotaxis in CXCR7-expressing tumor cells and promote
tumor growth and metastasis in animal models (1,2). Moreover,
increased CXCR7 expression directly correlates with greater tumor
aggressiveness (3). Recent studies
support a mechanistic role for CXCR7 in several malignancies,
including lung cancer (1,4), prostate cancer (3), malignant pheochromocytomas (5), papillary thyroid carcinoma (6), ovarian cancer (7), hepatocellular carcinoma (8), breast cancer (9) and Kaposi's sarcoma (10). However, hardly any previous reports
have definitively linked CXCR7 upregulation with human colon cancer
and related lymph node metastasis and histopathology.
To the best of our knowledge, this study is the
first to investigate the association of CXCR7 mRNA and protein
upregulation with colon cancer using reverse transcription
quantitative polymerase chain reaction (RT-qPCR) and western blot
analysis, and determine CXCR7 expression in relation to lymph node
metastasis.
Patients and methods
Patients
Tissue samples from 34 human colon tumors and 18
normal colon tissue specimens were obtained from 34 patients with
primary colon cancer. The patient cohort comprised 19 male and 15
female patients, with a mean age of 52 years (range, 34–79 years).
None of the patients had received adjuvant radiotherapy or
chemotherapy prior to surgery. Patients with rectal primary tumors
were excluded.
The tumor specimens were dissected from resected
colon cancer tissues and the normal colon specimens were obtained
from the distal resected colon margin. All the resected primary
tumors and lymph nodes were histologically examined with
hematoxylin and eosin staining. Of the 34 colon cancer patients, 20
were diagnosed with lymph node metastasis. Histologically, 24
patients had well-differentiated adenocarcinomas and 10 had
moderately-to-poorly differentiated adenocarcinomas. The 18 normal
colon specimens were determined to be tumor-free by histological
examination. All the dissected samples were snap-frozen in liquid
nitrogen and preserved at −80°C until RNA or protein
extraction.
This study was approved by the Institutional Review
Board of the Third Xiangya Hospital of Central South University
(Hunan, China).
RT-qPCR analysis
Total RNA was extracted using TRIzol (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. RNA was treated with RQ1 RNase-Free
DNase (Promega Corporation, Madison, WI, USA). The RT reaction was
performed using the RevertAid™ RT kit (Fermentas Life Technologies,
Carlsbad, CA, USA) with 1 µl RNA template, 1 µl oligo(dT) primer
(0.5 µg/µl), 4 µl X5 reaction buffer, 1 µl RNase inhibitor, 2 µl
dNTPs (10 mM) and 1 µl reverse transcriptase. The reaction mixture
(20 µl) was incubated at 42°C for 60 min, then at 70°C for 10 min,
followed by cooling on ice. RT-qPCR was performed on the resulting
cDNA, using the manufacturer's protocol (Takara Bio Inc., Shiga,
Japan), in 25 µl reaction volume per capillary. The gene-specific
primer sequences (Invitrogen) were as follows: CXCR7 forward,
5′-CCGTTCCCTTCTCCATTATCGCTGTCTTCT-3′ and reverse,
5′-GTGAAGAGGGCGTGCTCCAGCCGGCAG GTGAA-3′; β-actin forward,
5′-GCGAGAAGATGACCC AGATC-3′ and reverse, 5′-CCAGTGGTACGGCCAGAGG-3′.
The reaction mixture contained 12.5 µl SYBR® Premix Ex Taq™ II
(2×), 2 µl cDNA template, 1 µl primer pair mixture and
dH2O. RT-qPCR amplification consisted of an initial
denaturation step (95°C for 5 min), 40 cycles of denaturation (94°C
for 20 sec), annealing (53°C for 20 sec) and extension (72°C for 20
sec), followed by final incubation at 72°C for 5 min. All the
measurements were normalized to the expression of the 28S ribosomal
genes, considered as stable housekeeping genes (β-actin). Gene
expression was determined using the ΔΔCt method: 2−ΔΔCT
(ΔΔCt = [Ct (CXCR7) - Ct (β-actin)]target - [Ct
(CXCR7)-Ct (β-actin)]internal standard).
The CXCR7mRNA sequence was obtained from GenBank
(accession no. NM_020311).
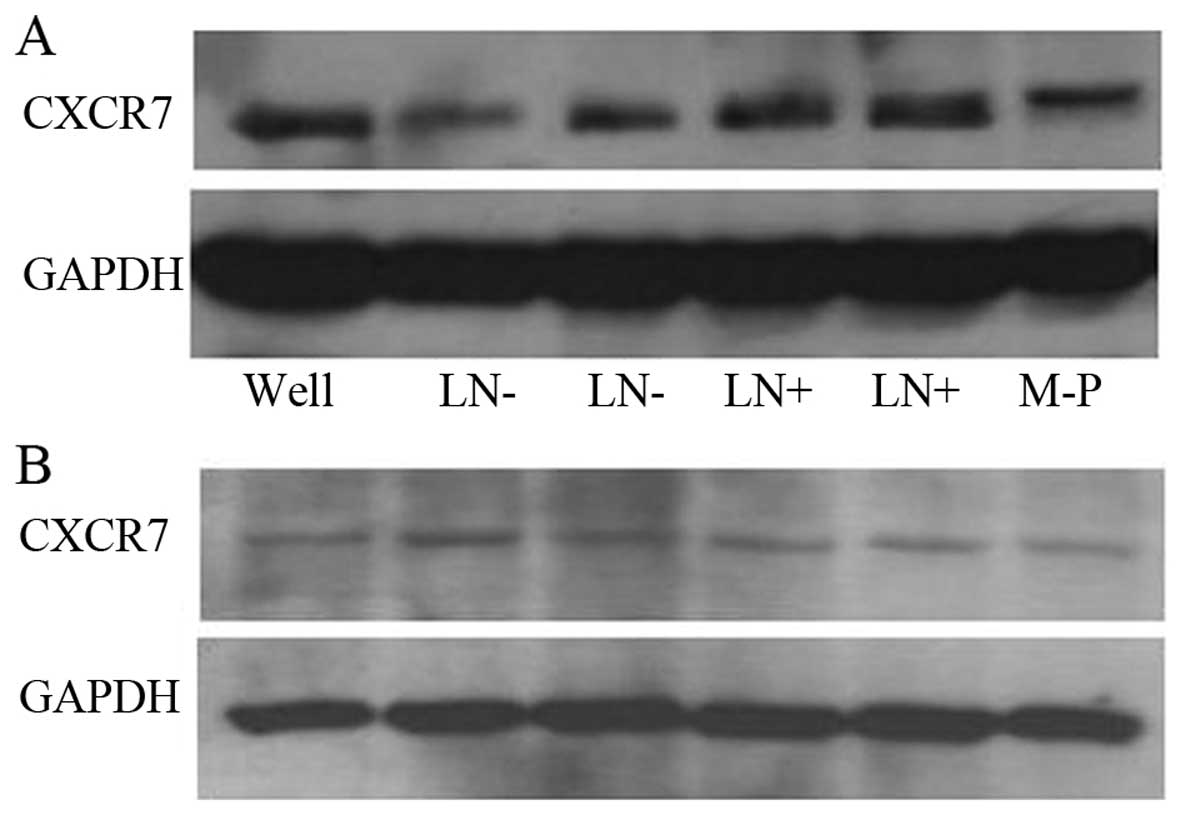
Western blot analysis
The tissue samples were lysed in ice-cold RIPA
buffer (Beyotime Institute of Biotechnology, Shanghai, China). The
protein concentrations were determined using bicinchoninic acid
(Pierce Biotechnology, Inc., Rockford, IL, USA) from lysates
clarified by centrifugation at 12,000 rpm for 20 min. Equivalent
protein quantities (30–50 µg) were separated by denaturing SDS-PAGE
electrophoresis, transferred to PVDF membranes and blocked in
Tris-buffered saline (TBS) containing 5% non-fat dried milk and
0.05% Tween-20. The membranes were incubated overnight with the
appropriate primary antibodies (rabbit anti-human CXCR7 antibody
and rabbit anti-human GAPDH antibody; both from Abcam, Cambridge,
UK) at dilutions specified by the manufacturer, then washed 4 times
in TBS/Tween-20 and incubated with horseradish
peroxidase-conjugated secondary antibody (goat anti-rabbit IgG HRP
conjugate; Dako, Glostrup, Denmark). Blots were then developed
using an enhanced chemiluminescence kit (Beyotime). The resulting
values were reported as CXCR7/GAPDH densitometric arbitrary
units.
Statistical analysis
RT-qPCR data are reported as medians and evaluated
statistically using the non-parametric Mann-Whitney test. Western
blot analysis data are reported as means ± standard deviations and
statistically analyzed using t-tests. The differences were
considered statistically significant at P-values of <0.05.
Results
CXCR7 expression is increased in colon
cancer
CXCR7 expression was found to be upregulated in
human colon cancer and was associated with lymph node
metastasis.
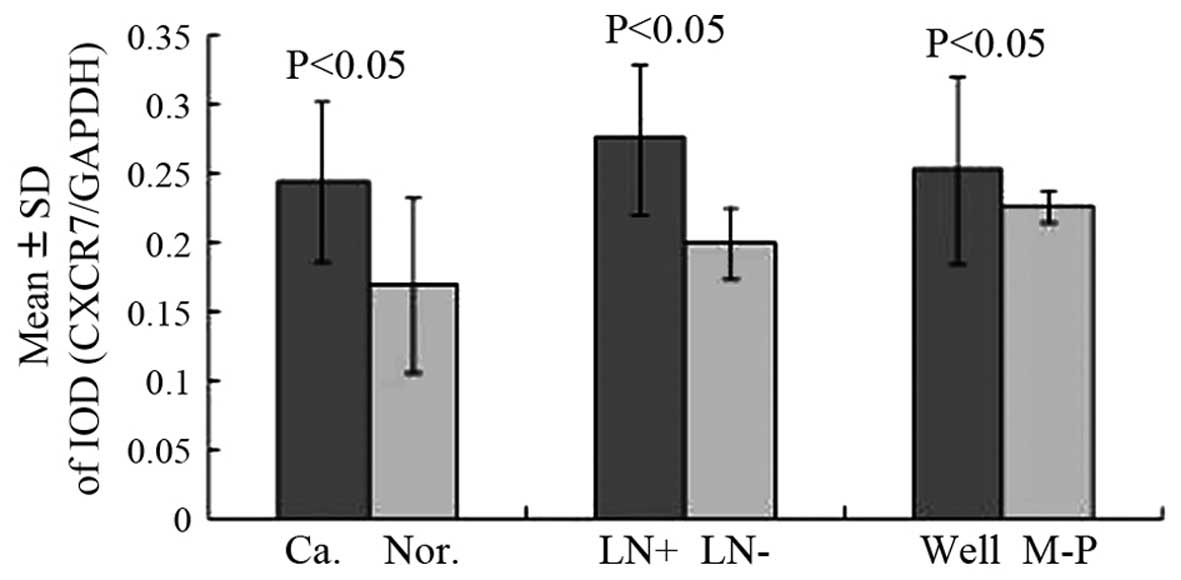
The normalized CXCR7 mRNA and protein levels were
significantly higher in colon cancer tissues, compared with those
in normal colon tissues (Table I,
Z=5.868, P<0.001; Figs. 1 and
2, t=4.25, P<0.001). These are the
first data demonstrating CXCR7 upregulation in human colon
cancer.
 | Table I.Analysis of clinicopathological
factors and CXCR7 mRNA expression (RT-qPCR). |
Table I.
Analysis of clinicopathological
factors and CXCR7 mRNA expression (RT-qPCR).
| Clinicopathological
factors | No. | M | IQR | Z-value | P-value |
|---|
| Normal colon
tissues | 18 | 0.22 | 0.216 | 5.868 | <0.001 |
| Colon cancer
tissue | 34 | 1.14 | 0.85 |
|
|
| Lymph node metastatic
status |
|
|
|
|
|
|
Positive | 20 | 1.455 | 1.553 | 3.92 | <0.001 |
|
Negative | 14 | 0.74 | 0.585 |
|
|
| Histological
classification |
|
|
|
|
|
|
Wella | 24 | 1.415 | 1.356 | 1.096 | 0.273 |
|
M-Pb | 10 | 1.065 | 0.233 |
|
|
Our 34 colon cancer patients were divided into lymph
node metastatic (20 patients, positive) and non-metastatic (14
patients, negative) groups. Additionally, the patients were
classified histopathologically into 24 cases with
well-differentiated and 10 cases with moderately-to-poorly
differentiated adenocarcinomas. The CXCR7 mRNA levels were
significantly different between the positive and negative lymph
node groups (Table I, Z=3.92,
P<0.001). Moreover, CXCR7 mRNA upregulation was positively
correlated with lymph node metastasis, namely colon tumors with
metastatic lymph nodes exhibited higher CXCR7 levels, whereas
non-metastatic tumors exhibited lower CXCR7 expression. No
significant difference was found based on histopathological
criteria (Table I, Z=1.096, P=0.273).
CXCR7 protein expression was consistent with our CXCR7 mRNA
results. The CXCR7 protein level was significantly increased in
colon tumors with lymph node metastases compared with the
non-metastatic group (Figs. 1 and
2, t=5.36, P<0.001), whereas no
significant difference was observed between histopathologically
distinct tumor types (Figs. 1 and
2, t=1.768, P=0.089). Therefore, our
results suggest that CXCR7 mRNA and protein upregulation is
correlated with lymph node metastasis, but is not associated with
the histopathological type.
Discussion
Chemokines are a group of low-molecular-weight
proteins that regulate cell trafficking by binding to specific
G-protein-coupled seven-span transmembrane receptors on target
cells. Chemokine receptors are classified by the type of chemokines
they bind (CXC, CC, XC, CX3C), followed by R for receptor and a
number indicating the order of discovery (2). A number of chemokine receptors have
>1 known ligand and several chemokines are able to activate
>1 receptor. Thus, there is significant promiscuity in
chemokine/receptor signaling.
The chemokine CXCL12/SDF-1 regulates a number of
essential biological processes, including cardiac and neuronal
development, stem cell motility, neovascularization, angiogenesis
and apoptosis. In particular, CXCL12/SDF-1 promotes primary tumor
growth and progression to metastasis. These processes are mediated
by SDF-1 via its canonical receptor, known as CXCR4 (4).
However, the metastatic behavior of carcinomas was
only partially blocked by CXCR4 inhibition in vivo,
suggesting that other genes may be involved in
CXCL12/SDF-1-mediated tumorigenesis (3). An alternate receptor, the
G-protein-coupled receptor CXCR7 (formerly known as RDC1) was
recently shown to bind with high affinity to CXCL12/SDF-1 and
CXCL11/I-TAC. Membrane-associated CXCR7 is expressed on a number of
tumor cell lines, activated endothelial cells and fetal liver
cells, but is absent on other cell types (4). A receptor exhibited a four-fold higher
expression in malignant pheochromocytomas compared with benign ones
(5). Ectopic CXCR7 expression in NIH
3T3 cells significantly increased cell proliferation and formed
tumors when xenografted in nude mice (10). CXCR7 overexpression provided tumor
cells with increased adhesive and invasive properties, in addition
to a growth and survival advantage in vivo. By contrast,
CXCR7 downregulation using RNA interference exerted opposite
effects. Specifically, RNAi-mediated CXCR7 downregulation inhibited
tumor cell adhesion and invasion, thus conferring growth and
survival disadvantages in vivo (1,3). Xue et
al (8) demonstrated that CXCR7
enhances the risk of extrahepatic metastasis in relatively
well-differentiated hepatocellular carcinomas, potentially
functioned by upregulation of osteopontin.
Several studies reported that CXCR7 regulates tumor
development in vivo. Specifically, CXCR7 was shown to
enhance tumor formation from breast, lung and prostate cancer cells
(1,3)
and promoted experimental lung metastases from breast and
osteosarcoma in mouse models (1,11).
Furthermore, several human malignancies, including breast, lung,
cervical, prostate, renal and esophageal tumors, pancreatic
adenocarcinoma, Kaposi's sarcoma and cutaneous squamous cell
carcinoma, were found to express elevated CXCR7 levels compared
with normal tissues (1,3,10,12,13).
Additionally, in the majority of human cancers, the CXCR7
expression levels increased as the tumors became more
aggressive.
Despite this evidence, a CXCR7 pathogenic mechanism
has not been clearly determined. CD44 and cadherin-11 are among the
potential downstream targets of CXCR7, which likely contribute to
tumor cell invasiveness. CXCR7 also regulates the expression of the
pro-angiogenic factors interleukin-8 or vascular endothelial growth
factor (3). It was recently suggested
that the biological effect of proliferation was attenuated and cell
cycle arrest was caused by CXCR7 silencing in MCF-7 human breast
cancer cells. Furthermore, CXCR7 knockdown attenuated the levels of
phosphorylated epithelial growth factor receptor (EGFR) at tyrosine
1110 following EGF-stimulation and also decreased the
phosphorylation of extracellular signal-regulated protein kinases 1
and 2 (ERK1/2) in MCF-7 cells (9).
Liu et al (14) observed that
CXCR7 knockdown by siRNA decreased the phosphorylation of ERK1/2
and attenuated cell proliferation, invasion and migration in
multiple glioblastoma multiforme cell lines. Therefore, we
hypothesize that CXCR7 is involved in colon cancer development and
lymph node metastasis.
The aim of the present study was to demonstrate that
upregulated CXCR7 was correlated with human colon cancer. Our data
indicated that the CXCR7 transcript level was significantly
elevated in colon cancer compared with normal colon tissue using a
combination of mRNA and protein detection, and suggest that
elevated CXCR7 expression promotes colon carcinogenesis.
Additionally, as CXCR7 was found to be expressed, although at lower
levels, in normal colon tissues, it is likely involved in
non-malignant processes within the human colon.
Our results unequivocally demonstrated that CXCR7
was significantly upregulated in colon tumors with lymph node
metastasis compared with non-metastatic colon tumors. These results
are consistent with previous findings in prostate cancer (3). Lymph node status was a significant risk
factor predicting cancer recurrence (15). Our study suggests that CXCR7 may be an
important predictor of lymph node metastasis in colon cancer, but
does not account for histopathological differences among colon
cancer types. The sample size of the present study may not be
optimal, but should be sufficient to draw a conclusion that may
guide clinical decision-making.
Acknowledgements
We would like to thank Professor Xiaowei Xing and Dr
Hongwei Lv (Centralab of Xiangya Third Hospital of Central South
University) for technical assistance and proofreading of the
manuscript. We would also like to thank Professor Huiqing Mao
(Medical College of Qinghai University) for helping with the data
analysis.
References
|
1
|
Miao Z, Luker KE, Summers BC, et al: CXCR7
(RDC1) promotes breast and lung tumor growth in vivo and is
expressed on tumor-associated vasculature. Proc Natl Acad Sci USA.
104:15735–15740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghanem I, Riveiro ME, Paradis V, et al:
Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma
carcinogenesis. Am J Transl Res. 6:340–352. 2014.PubMed/NCBI
|
|
3
|
Wang J, Shiozawa Y, Wang J, et al: The
role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in
prostate cancer. J Biol Chem. 283:4283–4294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burns JM, Summers BC, Wang Y, et al: A
novel chemokine receptor for SDF-1 and I-TAC involved in cell
survival, cell adhesion, and tumor development. J Exp Med.
203:2201–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thouënnon E, Pierre A, Tanguy Y, et al:
Expression of trophic amidated peptides and their receptors in
benign and malignant pheochromocytomas: high expression of
adrenomedullin RDC1 receptor and implication in tumoral cell
survival. Endocr Relat Cancer. 17:637–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Yang L, Teng X, et al: The
involvement of CXCR7 in modulating the progression of papillary
thyroid carcinoma. J Surg Res. 91:379–388. 2014. View Article : Google Scholar
|
|
7
|
Yu Y, Li H, Xue B, et al: SDF-1/CXCR7 axis
enhances ovarian cancer cell invasion by MMP-9 expression through
p38 MAPK pathway. DNA Cell Biol. 33:543–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xue TC, Chen RX, Ren ZG, et al:
Transmembrane receptor CXCR7 increases the risk of extrahepatic
metastasis of relatively well-differentiated hepatocellular
carcinoma through upregulation of osteopontin. Oncol Rep.
30:105–110. 2013.PubMed/NCBI
|
|
9
|
Salazar N, Muñoz D, Kallifatidis G, et al:
The chemokine receptor CXCR7 interacts with EGFR to promote breast
cancer cell proliferation. Mol Cancer. 13:1982014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raggo C, Ruhl R, McAllister S, et al:
Novel cellular genes essential for transformation of endothelial
cells by Kaposi's sarcoma-associated herpesvirus. Cancer Res.
65:5084–5095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goguet-Surmenian E, Richard-Fiardo P,
Guillemot E, et al: CXCR7-mediated progression of osteosarcoma in
the lungs. Br J Cancer. 109:1579–1585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Teng XY, Meng XP and Wang BS:
Expression of stromal cell-derived factor 1 and CXCR7 ligand
receptor system in pancreatic adenocarcinoma. World J Surg Oncol.
12:3482014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu SC, Yu HS, Yen FL, et al: CXCR7
expression correlates with tumor depth in cutaneous squamous cell
carcinoma skin lesions and promotes tumor cell survival through ERK
activation. Exp Dermatol. 23:902–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Carson-Walter E and Walter KA:
Targeting chemokine receptor CXCR7 inhibits glioma cell
proliferation and mobility. Anticancer Res. 35:53–64.
2015.PubMed/NCBI
|
|
15
|
Gill S, Haince JF, Shi Q, et al:
Prognostic value of molecular detection of lymph node metastases
after curative resection of stage II colon cancer: A systematic
pooled data analysis. Clin Colorectal Cancer. Dec 24–2014.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|