Introduction
Osteosarcomas are the most common primary
musculoskeletal malignant tumors in children, accounting for ~5% of
all pediatric tumors (1).
Conventionally, chemotherapy has been used to improve patient
survival; however, this therapeutic approach has reached a plateau
in the last two decades. Over the last decade, targeted therapy was
introduced and has achieved great success in various malignancies,
such as leukemia, melanoma, and lung and breast cancer. However,
patients with osteosarcoma have not yet benefited from targeted
therapy, due to the fact that the genetic etiology of osteosarcomas
is complex and has not been fully elucidated, with no specific
therapeutic targets yet identified.
To better understand the genetic etiology of
osteosarcomas, one recent genome-wide association study (GWAS)
investigated 941 patients with osteosarcoma and 3,291 cancer-free
adult controls of European descent (2). That study identified two significant
loci, one located at 6p21.3, containing the GRM4 gene, which
encodes the metabotropic glutamate receptor 4 (mGluR4), whereas the
other one was located in the gene desert. The study suggested that
mGluR4 may play an important role in the pathogenesis of
osteosarcoma. The amino acid glutamate is a fundamental
extracellular messenger in a number of tissues and functions in
neural and non-neural signaling in bone to maintain structural and
functional homeostasis. Glutamic acid signals through cell surface
glutamate receptors, which have been classified into two types,
ionotropic glutamate receptors (iGluRs) and mGluRs. iGluRs, the
first structure identified in the glutamic acid receptor family,
are classic ligand-gated ion channels, including
N-methyl-D-aspartate and
α-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptors
(3), which allow cations such as
Na+ and Ca2+ to enter the cell. By contrast,
mGluRs are G-protein-coupled receptors that stimulate secondary
messengers, such as inositol triphosphate (IP3), diacylglycerol
(DAG) and cAMP to generate the desired signaling effect (4).
Accumulating evidence has revealed that mGluRs are
involved in cancer pathophysiology, with their mRNA detected in
various cancer cell lines (5).
However, the number of studies investigating the physiological
roles of mGulRs in sarcomas is currently limited. In this
preliminary study, mGluR4 expression in osteosarcoma tissues and
its correlations with clinical characteristics were assessed.
Patients and methods
Patients and tissue specimens
The specimens were collected retrospectively. This
study was approved by the Internal Review Board of the First
Affiliated Hospital of PLA General Hospital (Beijing, China) and
all the pathological specimens (osteosarcomas, giant-cell tumors
and one cerebellar tissue sample) were obtained from the hospital's
department of Pathology database. A total of 58 osteosarcoma
samples (35 from male and 23 from female patients) and 32
giant-cell tumor of bone samples (20 from male and 12 from female
patients) were collected and reviewed by two pathologists. All the
samples originated from definitive surgeries, apart from 2
osteosarcoma samples derived from biopsies. Osteosarcoma patient
demographics, such as age, gender, tumor location, histological
subtype, metastasis and Enneking stage, were analyzed. In
osteosarcoma patients, the mean age at presentation was 19.7 years
(range, 5–61 years) and the tumor sites included the femur (n=32),
tibia and fibula (n=16), humerus (n=6), scapula (n=2), rib (n=1)
and sternum (n=1). The histological subtypes included osteoblastic
(n=42), chondroblastic (n=12), fibroblastic (n=3) and small-cell
(n=1) osteosarcomas. All the patients were regularly followed up,
with a mean of 30.9 months (range, 12–102 months) from 2006 to
2014. The diagnoses were confirmed by histological analysis and
radiographic findings in all the cases; the histological
characteristics included spindle cell proliferation in
osteosarcoma, and the presence of multinucleated giant cells in
giant-cell tumors. Cerebellar tissue was used as a control for
mGRM4 expression evaluated via immunohistochemistry.
Immunohistochemistry
The specimens were fixed in formalin, decalcified in
5% nitric acid for 12 h, embedded in paraffin and sectioned (2 µm).
The sections were dewaxed and hydrated, with a heat-antigen
retrieval method used prior to incubation with 0.01 M sodium
citrate buffer solution (pH 6.0) for 10 min at 90°C. The sections
were then incubated in 3% hydrogen peroxide for 10 min at 37°C to
inactivate endogenous peroxidase, followed by incubation with a
drop of goat serum [1:10 with phosphate-buffered saline (PBS)] for
1 h at 37°C. Each section was then stained with mouse monoclonal
anti-mGluR4 antibody (cat. no. sc-376485; 1:100; Santa Cruz
Biotechnology, USA) and incubated overnight at 4°C. Subsequently,
the sections were incubated with biotin-labeled goat anti-mouse
secondary antibody (cat. no. ZB-2305; 1:100; Zhongshan
Biotechnology, Beijing, China) for 1 h and colorimetrically
detected with horseradish peroxidase-labeled avidin-biotin complex
and diaminobenzidine (Zhongshan Biotechnology) at 37°C for 7 min.
The sections were rinsed 3–5 times with PBS between steps, with the
exception of the last step. The cerebellar tissue was stained in
the same manner with anti-mGluR4 antibodies and hematoxylin, and
displayed a positive result; it was previously demonstrated that
mGluR4 is present in cerebellar granule cell neuroprogenitors
(6).
The immunostaining results were independently
evaluated by two pathologists (Min Zhao and Yiduo Jin) who were
blinded to the clinical data; any discrepancies were resolved by
consensus. The result was calculated as the percentage of
positively stained cells, with only cytoplasmic mGluR4 staining
considered as a positive result. Specimen staining was defined
based on positivity or negativity for mGluR4. Immunostaining
positivity was defined as a staining proportion of tumor cells of
≥10%; when the staining proportion was <10%, immunostaining was
defined as negative. Only strong staining intensity was considered
as positive.
Statistical analysis
All the analyses were conducted using the SPSS
statistical software package, version 19.0 (IBM SPSS, Armonk, NY,
USA). The Chi-squared test was used to analyze differences between
classified variables, with P<0.05 deemed as statistically
significant. A multivariate Cox regression analysis was performed,
including the covariables with a P-value of ≤0.05 in the
Chi-squared test. The Kaplan-Meier method and log-rank test were
used to calculate survival probability.
Results
Expression of mGluR4 in osteosarcoma
and giant-cell tumors
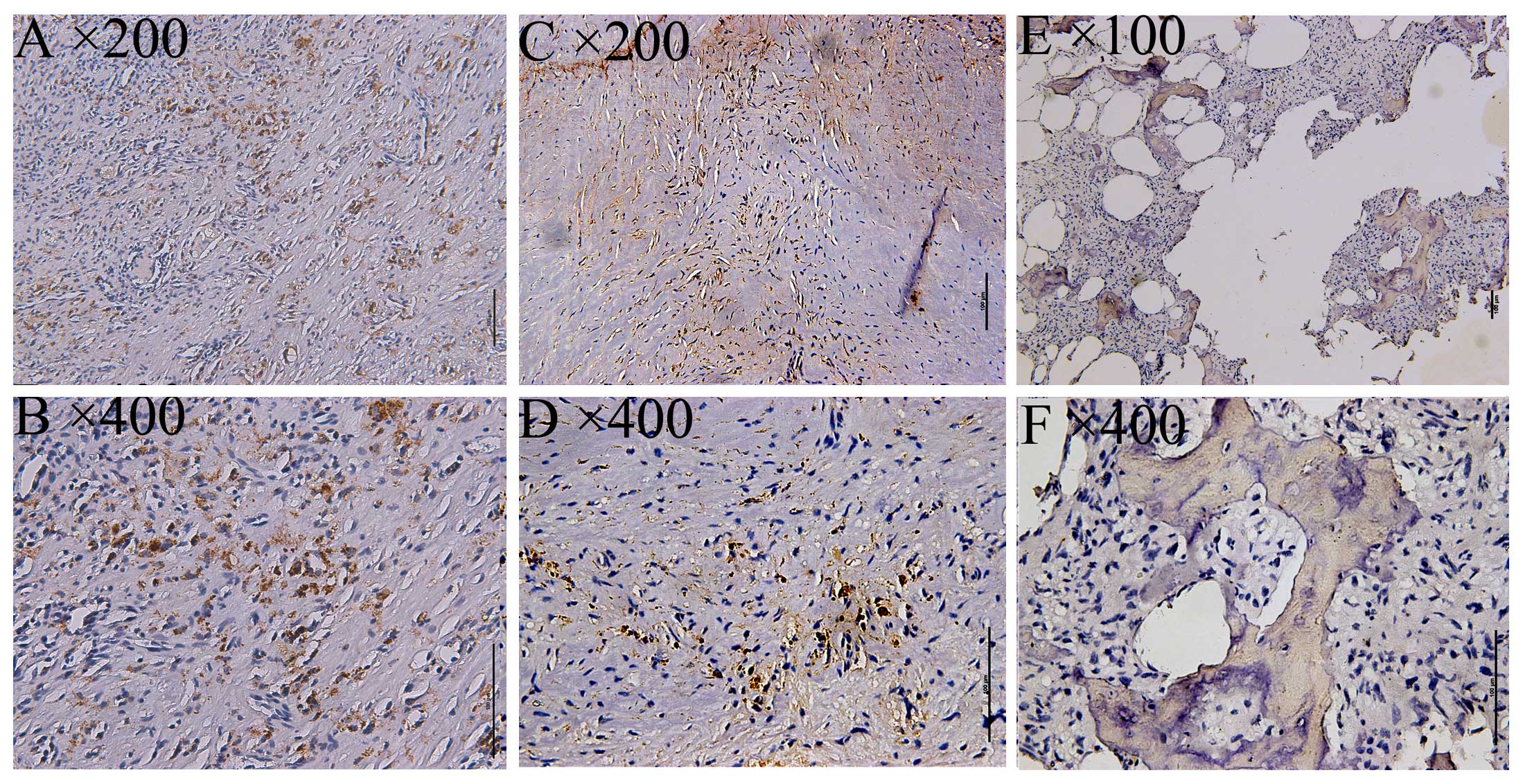
mGlu4 expression as detected by immunohistochemical
staining was examined via imaging with a BX 50–32 scanner (Olympus,
Union City, CA, USA) and analyzed with NIS-Elements D software
(Nikon BX Series Eclipse Ci-E; Nikon, Tokyo, Japan). As a positive
control, a specimen from the cerebellum of a patient with epilepsy
was used, displaying cytoplasmic mGluR4 expression in the
supranuclear portion. Subsequently, mGluR4 expression was
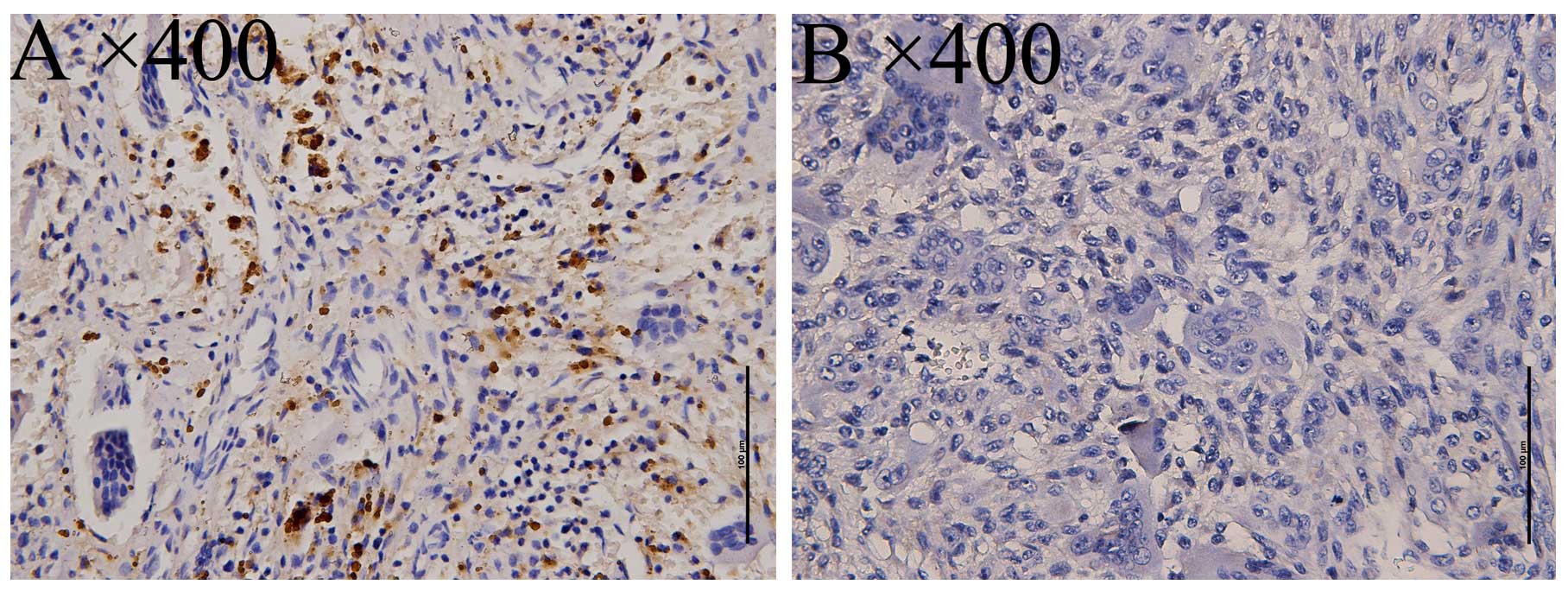
investigated in 58 osteosarcoma specimens, with 12/58 staining
positive (20.69%, Fig. 1), and 32
giant-cell tumors, with 14/32 staining positive (43.75%, Fig. 2).
Association of mGluR4 expression with
clinicopathological characteristics in osteosarcoma
Only cytoplasmic staining was considered to be a
positive result. The correlations of clinicopathological
characteristics (gender, age, tumor size, tumor location,
histological type and Enneking stage) with mGluR4 expression in
osteosarcoma are summarized in Table
I. mGluR4 expression was correlated with gender (P=0.0308), age
(P=0.0489), Enneking stage (P=0.0415) and tumor volume (P=0.02);
there was no significant correlation with tumor location (P=0.9486)
or histological type (P=0.7030). In the multivariate Cox regression
analysis, Enneking stage exerted a statistically significant effect
on survival (P<0.001). Furthermore, mGluR4 immunostaining and
tumor volume did not exert a statistically significant effect on
survival (P=0.092 and 0.789, respectively). The detailed results of
the Chi-squared test and multivariate analysis are summarized in
Tables I and II, respectively.
 | Table I.Expression of mGluR4 according to
clinicopathological characteristics in osteosarcoma. |
Table I.
Expression of mGluR4 according to
clinicopathological characteristics in osteosarcoma.
|
| Expression of
mGluR4 |
|
|---|
|
|
|
|
|---|
| Characteristics | Number of cases
(n=58) | Negative (n=46) | Positive (n=12) | P-value |
|---|
| Gender |
|
|
| 0.0308 |
| Male | 35 | 24 | 11 |
|
|
Female | 23 | 22 | 1 |
|
| Age, years |
|
|
| 0.0489 |
|
<18 | 36 | 32 | 4 |
|
| ≥18 | 22 | 14 | 8 |
|
| Location |
|
|
| 0.9486 |
|
Femur | 32 | 26 | 6 |
|
| Tibia and
fibula | 16 | 12 | 4 |
|
|
Humerus | 6 | 5 | 1 |
|
|
Othera | 4 | 3 | 1 |
|
| Histological
subtype |
|
|
| 0.7030 |
|
Osteoblastic | 42 | 32 | 10 |
|
|
Chondroblastic | 12 | 10 | 2 |
|
|
Small-cell | 1 | 1 | 0 |
|
|
Fibroblastic | 3 | 3 | 0 |
|
| Enneking stage |
|
|
| 0.0415 |
| II | 36 | 25 | 11 |
|
| III | 22 | 21 | 1 |
|
| Mean tumor volume,
cm3 |
| 306.55 | 195.13 | 0.02 |
 | Table II.Multivariate Cox regression
analysis. |
Table II.
Multivariate Cox regression
analysis.
| Factors | B | SE | Wald | df | Sig. | Exp(B) | 95.0% CI |
|---|
| Gender | 0.430 | 0.503 | 0.732 | 1 |
0.392 | 0.650 | 0.243–1.743 |
| Age | 0.017 | 0.021 | 0.656 | 1 |
0.418 | 0.983 | 0.943–1.025 |
| Tumor volume | 0.000 | 0.001 | 0.072 | 1 |
0.789 | 1.000 | 0.998–1.003 |
| Enneking stage | 3.096 | 0.821 | 14.209 | 1 | <0.001 | 22.111 | 4.420–110.601 |
| mGluR4
immunostaining | 2.011 | 1.195 | 2.830 | 1 |
0.092 | 7.467 | 0.718–77.695 |
Prognostic value of mGluR4 for
patients with osteosarcoma
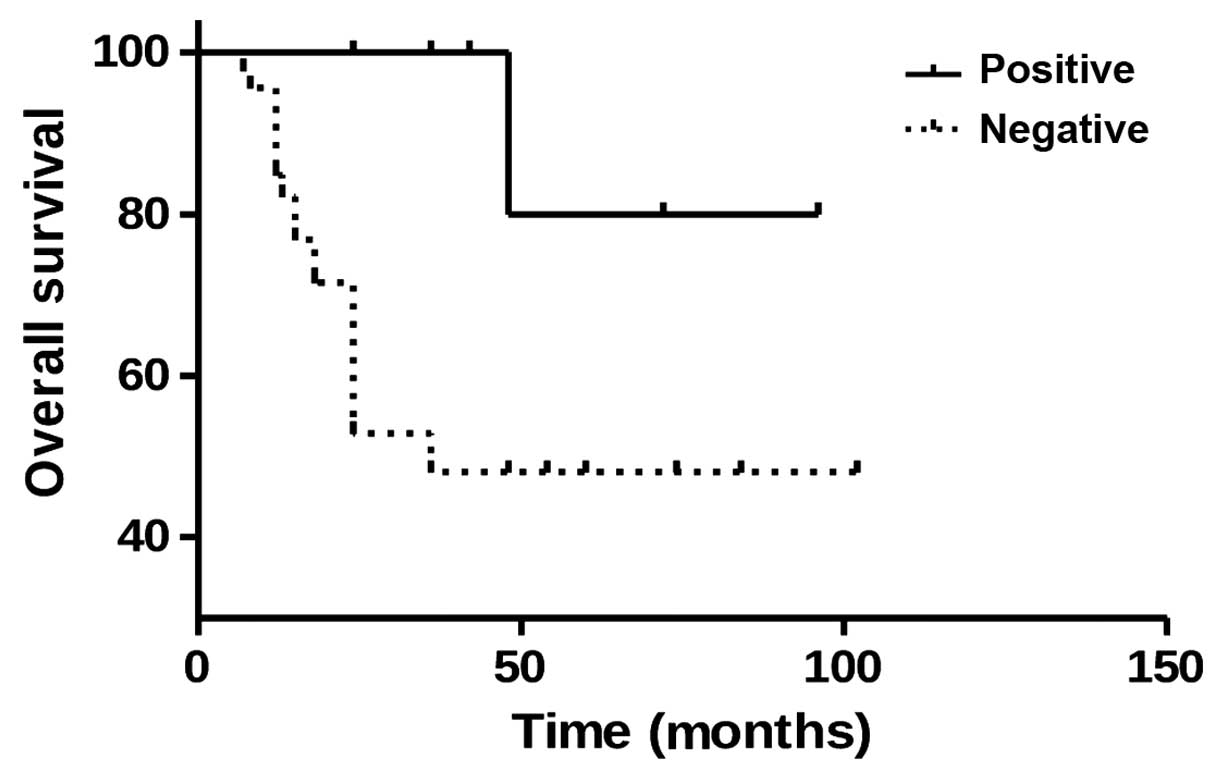
To elucidate whether mGluR4 signaling affects
patient prognosis, mGluR4 expression and patient survival were
investigated using the Kaplan-Meier survival analysis. Of the 12
osteosarcoma patients with positive mGluR4 expression, 11 survived,
while 19 of the 46 patients with negative mGluR4 expression
succumbed to the disease. Furthermore, the log-rank analysis
revealed a higher survival rate in osteosarcoma patients with
positive mGluR4 expression compared with those with negative
expression (P=0.0122, Fig. 3). These
findings suggest that mGluR4 positivity is correlated with a
favorable prognosis in patients with osteosarcoma.
Discussion
There is an urgent need for identifying more
osteosarcoma biomarkers. The implication of mGluR4 in osteosarcoma
was revealed by a GWAS study (2).
Therefore, the present study was conducted to investigate the role
of mGluR4 in osteosarcoma development and prognosis. mGluR1 was
initially discovered in mouse brain 20 years ago, with a total of 7
different subtypes identified to date (7). These subtypes are further divided into
three functional subgroups based on their sequence homologies,
signal transduction profiles and pharmacological properties as
follows: Group I (mGluR1 and mGluR5), which is coupled to
phospholipase C via Gq/11 proteins, thus leading to
phosphoinositide hydrolysis and the generation of IP3 and DAG;
group II (mGluR2 and mGluR3), which negatively regulates adenylate
cyclase (AC) in a recombinant system; and group III (mGluR4,
mGluR6, mGluR7 and mGluR8), which also negatively regulates AC,
reduces cAMP formation and may be activated by
L-2-amino-4-phosphonobutyric acid (4,8).
The function of mGluR4 has been widely investigated
in the central nervous system, with the interference of glutamate
signaling in pediatric CNS tumors shown to suppress tumor growth
(9). In a previous study, mGluR4
expression was enhanced using
N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide
(PHCCC) and found proliferation to be inhibited, while
differentiation was promoted in cerebellar granule cell
neuroprogenitors (10). In agreement
with those findings, the present data demonstrated that mGluR4 may
promote differentiation to maintain mature cells. Furthermore,
another study demonstrated that mGluR4 inhibits proliferation and
promotes the differentiation of cerebellar granule cell
neuroprogenitors, as illustrated by positive mGluR4 staining,
contributing to a favorable prognosis (11). Moreover, enhanced mGluR4 expression
following PHCCC treatment was beneficial in medulloblastomas, and
was found to be negatively correlated with neural tube cell tumor
progression. Thus, the stimulation of mGlu4 expression may be used
to treat Parkinson's disease in addition to medulloblastomas
(12). Additionally, these findings
suggest that mGluR4 may be considered as a phenotypic marker of
medulloblastomas of lower malignant potential, such as the nodular
desmoplastic histotype, or may negatively regulate tumor
growth.
However, Chang et al (13) reported that mGluR4 overexpression may
lead to 5-fluorouracil tolerance, as this outcome was observed in
54% of malignant colorectal carcinoma cases and was correlated with
a poor prognosis. Another study investigating mGluR1/5/4 protein
and gene expression in osteosarcomas reported varying expression
levels among these receptors (14).
While mGluR1 and mGluR5 were not statistically significant, mGluR4
was correlated with Enneking stage, tumor metastasis and poor
prognosis in osteosarcoma patients.
According to our results, mGluR4 exhibited strong
positive staining in cerebellar tissue, with a positive rate of
20.69% (12/58) in osteosarcomas and 43.75% (14/32) in giant-cell
tumors of bone, which is a benign tumor. Furthermore, tumors with
elevated mGluR4 expression tended to be less agressive, suggesting
a good prognosis. The statistical analysis revealed that mGluR4
expression is correlated with gender (P=0.0308), age (P=0.0489),
Enneking stage (P=0.0415) and tumor volume (P=0.02) in
osteosarcoma. The survival curves revealed a significantly higher
survival rate in patients with positive mGluR4 expression compared
with those with negative expression (P=0.0122). This correlation
analysis was in accordance with our expectations, with the
exception of gender. Due to the limited sample size, it would
appear that gender is not really significant. A multivariate Cox
regression analysis was performed and Enneking stage exerted a
statistically significant effect on survival (P<0.01), suggesting
that Enneking stage is the most important predictor of
survival.
Glutamate signaling is involved in a wide variety of
processes during normal bone formation, including cell
differentiation and growth (15,16). The
concentration of glutamate is regulated by various types of cells
in the bone environment (17). Since
glutamate signaling plays an important role in maintaining skeletal
homeostasis, it was hypothesized that this mechanism may stimulate
metastatic tumor cells to differentiate, reducing the malignant
potential (10,11,18). A
positive mGluR4 expression was found to be correlated with a
positive prognosis and, therefore, may serve as a useful predictive
indicator. While glutamate signaling appears to be promising,
further molecular and genetic experimentation is required. While
neoadjuvant chemotherapy has improved survival in patients with
osteosarcoma, an effective targeted therapy is not yet available.
Our findings taken together with those of previous studies indicate
that the glutamate signaling pathway may serve as an important
therapeutic target for osteosarcomas (19). However, further investigations are
required to determine the potential of mGluR4 as a prognostic
tool.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savage SA, Mirabello L, Wang Z,
Gastier-Foster JM, Gorlick R, Khanna C, Flanagan AM, Tirabosco R,
Andrulis IL, Wunder JS, et al: Genome-wide association study
identifies two susceptibility loci for osteosarcoma. Nat Genet.
45:799–803. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohtani Y, Harada T, Funasaka Y, Nakao K,
Takahara C, Abdel-Daim M, Sakai N, Saito N, Nishigori C and Aiba A:
Metabotropic glutamate receptor subtype-1 is essential for in vivo
growth of melanoma. Oncogene. 27:7162–7170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mathiesen JM, Svendsen N, Bräuner-Osborne
H, Thomsen C and Ramirez MT: Positive allosteric modulation of the
human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and
MPEP. Br J Pharmacol. 138:1026–1030. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teh J and Chen S: mGlu receptors and
cancerous growth. Wiley Interdiscip Rev Membre Transp Signal.
1:211–220. 2012. View
Article : Google Scholar
|
|
6
|
Ye ZC and Sontheimer H: Glioma cells
release excitotoxic concentrations of glutamate. Cancer Res.
59:4383–4391. 1999.PubMed/NCBI
|
|
7
|
Ryo Y, Miyawaki A, Furuichi T and
Mikoshiba K: Expression of the metabotropic glutamate receptor
mGluR1 alpha and the ionotropic glutamate receptor GluR1 in the
brain during the postnatal development of normal mouse and in the
cerebellum from mutant mice. J Neurosci Res. 36:19–32. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dorsam RT and Gutkind JS:
G-protein-coupled receptors and cancer. Nat Rev Cancer. 7:79–94.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brocke KS, Staufner C, Luksch H, Geiger
KD, Stepulak A, Marzahn J, Schackert G, Temme A and Ikonomidou C:
Glutamate receptors in pediatric tumors of the central nervous
system. Cancer Biol Ther. 9:455–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Canudas AM, Di Giorgi-Gerevini V,
Iacovelli L, Nano G, D'Onofrio M, Arcella A, Giangaspero F, Busceti
C, Ricci-Vitiani L, Battaglia G, et al: PHCCC, a specific enhancer
of type 4 metabotropic glutamate receptors, reduces proliferation
and promotes differentiation of cerebellar granule cell
neuroprecursors. J Neurosci. 24:10343–10352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iacovelli L, Arcella A, Battaglia G,
Pazzaglia S, Aronica E, Spinsanti P, Caruso A, De Smaele E, Saran
A, Gulino A, et al: Pharmacological activation of mGlu4
metabotropic glutamate receptors inhibits the growth of
medulloblastomas. J Neurosci. 26:8388–8397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Poul E, Boléa C, Girard F, Poli S,
Charvin D, Campo B, Bortoli J, Bessif A, Luo B, Koser AJ, et al: A
potent and selective metabotropic glutamate receptor 4 positive
allosteric modulator improves movement in rodent models of
Parkinson's disease. J Pharmacol Exp Ther. 343:167–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang HJ, Yoo BC, Lim SB, Jeong SY, Kim WH
and Park JG: Metabotropic gluamate receptor 4 expression in
colorectal carcinoma and its prognostic significance. Clin Cancer
Res. 11:3288–3295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang W, Maolin H, Jinmin Z and Zhe W: High
expression of metabotropic glutamate receptor 4: Correlation with
clinicopathologic characteristics and prognosis of osteosarcoma. J
Cancer Res Clin Oncol. 140:419–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spencer GL, McGrath CJ and Genever PG:
Current perspectives on NMDA-type glutamate signaling in bone. Int
J Biochem Cell Biol. 39:1089–1104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stepulak A, Luksch H, Gebhardt C,
Uckermann O, Marzahn J, Sifringer M, Rzeski W, Staufner C, Brocke
KS, Turski L and Ikonomidou C: Expression of glutamate receptor
subunits in human cancers. Histochem Cell Biol. 132:435–445. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takarada-Iemata M, Takarada T, Nakamura Y,
Nakatani E, Hori O and Yoneda Y: Glutamate preferentially
suppresses osteoblastogenesis than adipogenesis through the
cystine/glutamate antiporter in mesenchymal stem cells. J Cell
Physiol. 226:652–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seidlitz EP, Sharma MK and Singh G:
Extracellular glutamate alters mature osteoclast and osteoblast
functions. Can J Physiol Pharmacol. 88:929–936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seidlitz EP, Sharma MK, Saikali Z, Ghert M
and Singh G: Cancer cell line release glutamate into the
extracellular environment. Clin Exp Metastasis. 26:781–787. 2009.
View Article : Google Scholar : PubMed/NCBI
|