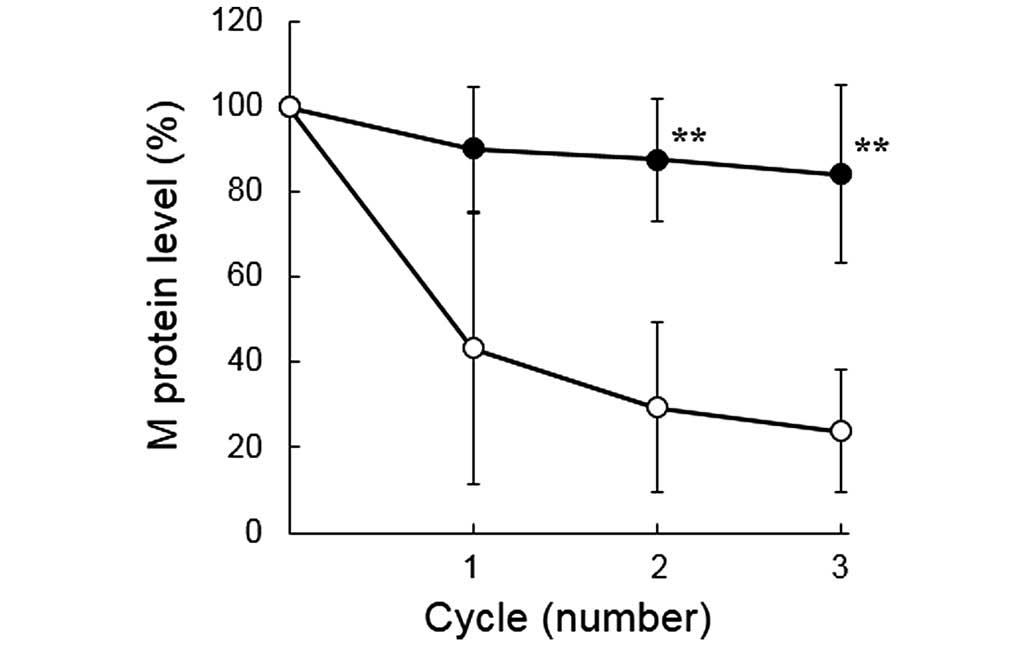
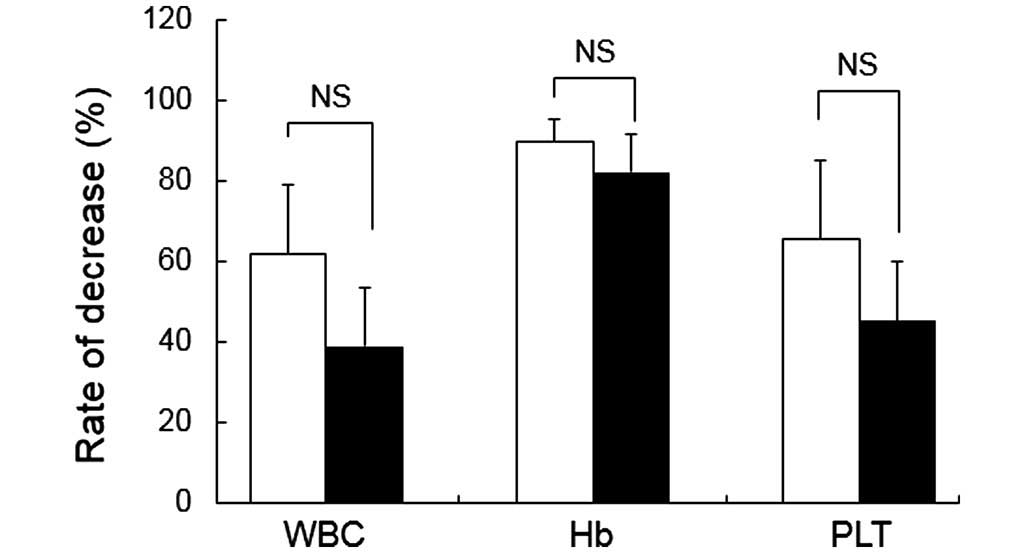
|
1
|
Raab MS, Podar K, Breitkreutz I,
Richardson PG and Anderson KC: Multiple myeloma. Lancet.
374:324–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harousseau JL and Moreau P: Autologous
hematopoietic stem-cell transplantation for multiple myeloma. N
Engl J Med. 360:2645–2654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimazaki C: Autologous stem cell
transplantation for multiple myeloma: History and future. Int J
Myeloma. 3:55–66. 2013.
|
|
5
|
Rajkumar SV, Blood E, Vesole D, Fonseca R
and Greipp PR: Eastern Cooperative Oncology Group: Phase III
clinical trial of thalidomide plus dexamethasone compared with
dexamethasone alone in newly diagnosed multiple myeloma: A clinical
trial coordinated by the Eastern cooperative oncology group. J Clin
Oncol. 24:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zonder JA, Crowley J, Hussein MA, Bolejack
V, Moore DF Sr, Whittenberger BF, Abidi MH, Durie BG and Barlogie
B: Lenalidomide and high-dose dexamethasone compared with
dexamethasone as initial therapy for multiple myeloma: A randomized
southwest oncology group trial (S0232). Blood. 116:5838–5841. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dimopoulos MA, Chen C, Spencer A,
Niesvizky R, Attal M, Stadtmauer EA, Petrucci MT, Yu Z, Olesnyckyj
M, Zeldis JB, et al: Long-term follow-up on overall survival from
the MM-009 and MM-010 phase III trials of lenalidomide plus
dexamethasone in patients with relapsed or refractory multiple
myeloma. Leukemia. 23:2147–2152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harousseau JL, Attal M, Avet-Loiseau H,
Marit G, Caillot D, Mohty M, Lenain P, Hulin C, Facon T, Casassus
P, et al: Bortezomib plus dexamethasone is superior to vincristine
plus doxorubicin plus dexamethasone as induction treatment prior to
autologous stem-cell transplantation in newly diagnosed multiple
myeloma: Results of the IFM 2005-01 phase III trial. J Clin Oncol.
28:4621–4629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richardson PG, Sonneveld P, Schuster M,
Irwin D, Stadtmauer E, Facon T, Harousseau JL, Ben-Yehuda D, Lonial
S, Goldschmidt H, et al: Extended follow-up of a phase 3 trial in
relapsed multiple myeloma: Final time-to-event results of the APEX
trial. Blood. 110:3557–3560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ria R, Reale A and Vacca A: Novel agents
and new therapeutic approaches for treatment of multiple myeloma.
World J Methodol. 4:73–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harousseau JL, Palumbo A, Richardson PG,
Schlag R, Dimopoulos MA, Shpilberg O, Kropff M, Kentos A, Cavo M,
Golenkov A, et al: Superior outcomes associated with complete
response in newly diagnosed multiple myeloma patients treated with
nonintensive therapy: Analysis of the phase 3 VISTA study of
bortezomib plus melphalan-prednisone versus melphalan-prednisone.
Blood. 116:3743–3750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapoor P, Rajkumar SV, Dispenzieri A,
Gertz MA, Lacy MQ, Dingli D, Mikhael JR, Roy V, Kyle RA, Greipp PR,
et al: Melphalan and prednisone versus melphalan, prednisone and
thalidomide for elderly and/or transplant ineligible patients with
multiple myeloma: A meta-analysis. Leukemia. 25:689–696. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palumbo A, Hajek R, Delforge M, Kropff M,
Petrucci MT, Catalano J, Gisslinger H, Wiktor-Jędrzejczak W,
Zodelava M, Weisel K, et al: Continuous lenalidomide treatment for
newly diagnosed multiple myeloma. N Engl J Med. 366:1759–1769.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palumbo A, Bringhen S, Rossi D, Cavalli M,
Larocca A, Ria R, Offidani M, Patriarca F, Nozzoli C, Guglielmelli
T, et al: Bortezomib-melphalan-prednisone-thalidomide followed by
maintenance with bortezomib-thalidomide compared with
bortezomib-melphalan-prednisone for initial treatment of multiple
myeloma: A randomized controlled trial. J Clin Oncol. 28:5101–5109.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alexanian R, Haut A, Khan AU, Lane M,
McKelvey EM, Migliore PJ, Stuckey WJ Jr and Wilson HE: Treatment
for multiple myeloma. Combination chemotherapy with different
melphalan dose regimens. JAMA. 208:1680–1685. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Combination chemotherapy versus melphalan
plus prednisone as treatment for multiple myeloma: An overview of
6,633 patients from 27 randomized trials. Myeloma Trialists'
Collaborative Group. J Clin Oncol. 16:3832–3842. 1998.PubMed/NCBI
|
|
17
|
Ma MH, Yang HH, Parker K, Manyak S,
Friedman JM, Altamirano C, Wu ZQ, Borad MJ, Frantzen M, Roussos E,
et al: The proteasome inhibitor PS-341 markedly enhances
sensitivity of multiple myeloma tumor cells to chemotherapeutic
agents. Clin Cancer Res. 9:1136–1144. 2003.PubMed/NCBI
|
|
18
|
Mitsiades N, Mitsiades CS, Richardson PG,
Poulaki V, Tai YT, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph
M, et al: The proteasome inhibitor PS-341 potentiates sensitivity
of multiple myeloma cells to conventional chemotherapeutic agents:
Therapeutic applications. Blood. 101:2377–2380. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woodhouse KW, Hamilton P, Lennard A and
Rawlins MD: The pharmacokinetics of melphalan in patients with
multiple myeloma: An intravenous/oral study using a conventional
dose regimen. Eur J Clin Pharmacol. 24:283–285. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bosanquet AG and Gilby ED:
Pharmacokinetics of oral and intravenous melphalan during routine
treatment of multiple myeloma. Eur J Cancer Clin Oncol. 18:355–362.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ehrsson H, Eksborg S, Osterborg A,
Mellstedt H and Lindfors A: Oral melphalan
pharmacokinetics-relation to dose in patients with multiple
myeloma. Med Oncol Tumor Pharmacother. 6:151–154. 1989.PubMed/NCBI
|
|
22
|
Sviland L, Robinson A, Proctor SJ and
Bateman DN: Interaction of cimetidine with oral melphalan. A
pharmacokinetic study. Cancer Chemother Pharmacol. 20:173–175.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alberts DS, Chang SY, Chen HS, Evans TL
and Moon TE: Oral melphalan kinetics. Clin Pharmacol Ther.
26:737–745. 1979.PubMed/NCBI
|
|
24
|
Tabibi SE and Cradock JC: Stability of
melphalan in infusion fluids. Am J Hosp Pharm. 41:1380–1382.
1984.PubMed/NCBI
|
|
25
|
Durie BG, Harousseau JL, Miguel JS, Bladé
J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J,
Sonneveld P, et al: International Myeloma Working Group:
International uniform response criteria for multiple myeloma.
Leukemia. 20:1467–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.0, U.S. Department of Health and Human
Sciences. National Institutes of Health. National Cancer Institute
Published. May 28–2009.v4.03: June 14, 2010.
|
|
27
|
Quinn DI, Nemunaitis J, Fuloria J, Britten
CD, Gabrail N, Yee L, Acharya M, Chan K, Cohen N and Dudov A:
Effect of the cytochrome P450 2C19 inhibitor omeprazole on the
pharmacokinetics and safety profile of bortezomib in patients with
advanced solid tumours, non-Hodgkin's lymphoma or multiple myeloma.
Clin Pharmacokinet. 48:199–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsukaguchi M, Shibano M, Matsuura A and
Mukai S: The protective effects of lafutidine for bortezomib
induced peripheral neuropathy. J Blood Med. 4:81–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dammann HG, Burkhardt F and Wolf N: The
effects of oral rabeprazole on endocrine and gastric secretory
function in healthy volunteers. Aliment Pharmacol Ther.
13:1195–1203. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adachi K, Katsube T, Kawamura A, Takashima
T, Yuki M, Amano K, Ishihara S, Fukuda R, Watanabe M and Kinoshita
Y: CYP2C19 genotype status and intragastric pH during dosing with
lansoprazole or rabeprazole. Aliment Pharmacol Ther. 14:1259–1266.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bosanquet AG and Gilby ED: Comparison of
the fed and fasting states on the absorption of melphalan in
multiple myeloma. Cancer Chemother Pharmacol. 12:183–186. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reece PA, Kotasek D, Morris RG, Dale BM
and Sage RE: The effect of food on oral melphalan absorption.
Cancer Chemother Pharmacol. 16:194–197. 1986. View Article : Google Scholar : PubMed/NCBI
|