Introduction
Concurrent chemoradiotherapy (CCRT) is the standard
treatment strategy for locally advanced head and neck squamous cell
carcinoma (HNSCC) (1). High-dose
cisplatin is the most common CCRT regimen with evidence of its
efficacy obtained in randomized clinical trials (2–4); however,
due to severe adverse events, high-dose cisplatin is not applicable
to all patients. Although the use of supportive care has improved
the tolerability of cisplatin, the completion of a high-dose
cisplatin CCRT regimen remains a difficult task. Additionally,
although 100 mg/m2 per 3 weeks is the most certified
dose of cisplatin in randomized clinical trials, this dose has not
been approved in Japan.
Between 2000 and 2010, cetuximab, an anti-epidermal
growth factor receptor (EGFR) antibody, was approved for the
treatment of HNSCCs. Bio-radiotherapy (BRT) has also emerged as a
new treatment option for locally advanced HNSCC (5). Although the superiority or
non-inferiority of BRT compared to CCRT has not yet been
established, BRT has been recognized as a feasible treatment option
and it is becoming more widely used in clinical practice. In light
of the effectiveness of BRT as an alternative treatment, when
clinicians consider administering CCRT, they must select patients
who can tolerate a high-dose cisplatin regimen. When making
decisions regarding the appropriate treatment, it would be
extremely helpful to know the predictive factors of cisplatin
tolerability. The present study retrospectively explored the
predictive factors of high-dose cisplatin tolerance.
Materials and methods
Patient enrollment
Subsequent to obtaining approval from our
institutional review board, the clinical records of newly diagnosed
HNSCC patients who received CCRT with a high-dose cisplatin regimen
were retrospectively reviewed between June 2006 and March 2013 at
the Cancer Institute Hospital of the Japanese Foundation for Cancer
Research.
All the patients were diagnosed pathologically by
histological specimens, and pathological specimens and reports were
reviewed by a pathologist with a specialty in oncology (Yukiko
Sato). For the evaluations of the primary lesions and lymph node
metastases, computed tomography (CT) and magnetic resonance imaging
(MRI) scans of the head and neck were performed in each case. For
the identification of distant metastases and double cancers, chest
CT scans and gastrointestinal fiberscopy were also performed for
all patients.
Distant metastases were excluded from the
indications for CCRT; however, patients with double cancers of the
esophagus and/or stomach were allowed to receive CCRT when the
double malignancies were limited to early stages that could be
treated surgically or endoscopically prior or subsequent to CCRT.
Patients who received induction chemotherapy or neck dissection
prior to CCRT were included in the present analysis; however, cases
of adjuvant CCRT following curative surgical resection were
excluded. The indications for CCRT were discussed and decided upon
by our institute's cancer board, which is comprised of medical
oncologists, oncology radiologists, and head and neck surgeons.
Treatment
For the CCRT, cisplatin 80 mg/m2, which
is the approved dose in Japan, was administered every 3 weeks for a
total of three cycles. During the chemotherapy, the patients
received appropriate antiemetics and hydration. Following the
approval of the antiemetic and neurokinin 1 receptor antagonist
aprepitant in Japan in December 2009, aprepitant or another
neurokinin 1 receptor antagonist was administered perorally or
intravenously to the patients who received CCRT. Elderly patients
and those with reduced organ function received a reduced cisplatin
dose according to the discretion of their physician. In cases in
which one or more severe adverse events were observed during the
CCRT, a skip, delay or dose reduction of the second or third
cisplatin cycle was also allowed.
Radiotherapy was performed as 3-dimensional
conformal radiotherapy (3D-CRT) or intensity-modified radiotherapy
(IMRT) with the conventional fraction: 2–2.12 Gy/fraction, once a
day, five times/week. Prophylactic percutaneous endoscopic
gastrostomy (PEG) was performed unless particular reasons
prohibited it (such as refusal by the patient or past history of
gastrectomy).
Evaluation
The patient cisplatin tolerability was evaluated
according to the cumulative cisplatin dose. Patients who received a
cumulative dose of ≥200 mg/m2 were defined as tolerant
to high-dose cisplatin, and patients who received a cumulative dose
of <200 mg/m2 were defined as intolerant to high-dose
cisplatin. The rate of high-dose cisplatin-tolerant patients was
identified, and predictive factors of high-dose cisplatin tolerance
were investigated using Fisher's exact test and a logistic
regression analysis. The associations between high-dose cisplatin
tolerance and response, and between high-dose cisplatin tolerance
and adverse events were also analyzed using Fisher's exact
test.
The patient objective responses were evaluated by
laryngopharyngeal endoscopy, CT scan and/or MRI, based on the
Response Evaluation Criteria In Solid Tumours version 1.1 (6). Adverse events were documented based on
the Common Terminology Criteria for Adverse Events version 4.0
(http://evs.nci.nih.gov/ftp1/CTCAE/About.html).
For prognoses, progression-free survival (PFS) and
overall survival (OS) were estimated by the Kaplan-Meier method.
The association between high-dose cisplatin tolerance and prognoses
was analyzed by Cox regression analyses.
For all statistical analyses, SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA) was used. In all the analyses,
P<0.05 was considered to indicate a statistically significant
difference, and in Fisher's exact test, two-sided P-values were
evaluated.
Results
Patient characteristics
A total of 159 patients were enrolled in the
analyses. The median follow-up time was 36.7 months. The patient
characteristics are shown in Table I.
The median age was 63 years old (range, 21–78 years), and the
majority of patients (86%) were male. Although over half the
patients were stage IV, 30 (19%) of the stage II patients were
included in the study. The median body surface area (BSA) was 1.69
m2 (range, 1.30–2.14 m2), and the BSA values
of 49 (31%) patients were >1.80 m2. The median body
mass index (BMI) was 22.4 kg/m2 (range, 14.9–29.8
kg/m2), and 114 (72%) patients were within the range of
18.5–25.0 kg/m2. Prior to the CCRT induction, 33 (21%)
patients received some anticancer treatments; 23 underwent neck
dissection, and 10 received induction chemotherapy. As the
induction chemotherapy regimen, docetaxel and cisplatin (TP) with
5-fluorouracil were used for 7 patients, TP for 2, and S-1 for 1
patient.
 | Table I.Characteristics of the patients with
head and neck squamous cell carcinoma. |
Table I.
Characteristics of the patients with
head and neck squamous cell carcinoma.
| Characteristics | Variables
(n=159) |
|---|
| Age, years |
|
| Median
(range) | 63 (21–78) |
| >60, n
(%) | 91 (58) |
| Gender, n (%) |
|
| Male | 136 (86) |
|
Female | 23 (14) |
| ECOG performance
status, n (%) |
|
| 0 | 148 (93) |
| 1 | 11 (7) |
| ≥2 | 0 (0) |
| Body surface area,
m2 |
|
| Median
(range) | 1.69
(1.30–2.14) |
|
>1.80, n (%) | 49 (31) |
| Body mass index,
kg/m2 |
|
| Median
(range) | 22.4
(14.9–29.8) |
|
<18.5, n (%) | 14 (9) |
|
18.5–25.0, n (%) | 114 (72) |
| ≥25.0,
n (%) | 31 (19) |
| Primary site, n
(%) |
|
|
Nasopharynx | 20 (12) |
| Oral
cavity | 5 (3) |
|
Oropharynx | 57 (36) |
|
Hypopharynx | 57 (36) |
|
Larynx | 14 (9) |
|
Paranasal sinus/nasal
cavity | 6 (4) |
| T stage, n (%) |
|
| T1 | 8 (5) |
| T2 | 92 (58) |
| T3 | 32 (20) |
| T4 | 27 (17) |
| N stage, n (%) |
|
| N0 | 49 (31) |
| N1 | 22 (14) |
| N2 | 86 (54) |
| N3 | 2 (1) |
| Clinical stage, n
(%) |
|
| II | 30 (19) |
|
III | 33 (21) |
| IV | 96 (60) |
| Laboratory data, n
(%) |
|
|
Albumin, ≥4.0 g/dl | 107 (67) |
| High
creatine clearance, >100 ml/min | 60 (38) |
|
Glycated hemoglobin, >upper
normal limit | 19 (11) |
| Previous therapy, n
(%) |
|
|
Absent | 126 (79) |
|
Present | 33 (21) |
| Surgery
(neck dissection) | 23 (14) |
|
Induction chemotherapy | 10 (6) |
| Year of treatment,
n (%) |
|
|
2006–2009 | 17 (11) |
|
2010 | 48 (30) |
|
2011 | 41 (26) |
|
2012 | 42 (26) |
|
2013 | 11 (7) |
Treatment exposure
The patient treatment details are shown in Table II. Approximately one-half of the 159
patients completed three cycles of cisplatin, and 73 (46%) patients
reached cumulative cisplatin doses that were ≥200 mg/m2;
i.e., they were high-dose cisplatin-tolerant. IMRT was performed
for 64 (40%) patients, and 3D-CRT was administered to 95 (60%)
patients. Two patients failed to complete the pre-planned radiation
therapy; 1 discontinued RT due to a lethal infectious adverse
event, and the other declined the continuation of the therapy. A
neurokinin 1 receptor antagonist (aprepitant or fosaprepitant) was
used for 138 (87%) patients. PEG was conducted for 124 (78%)
patients, and 107 (67%) patients received tube feeding during
and/or following CCRT; this includes patients who received tube
feeding from a nasogastric tube without PEG placement.
 | Table II.Treatment details. |
Table II.
Treatment details.
| Treatments | Patients, n
(%) |
|---|
| Radiation
therapy |
|
|
Radiation style |
|
|
3D-CRT | 95
(60) |
|
IMRT | 64
(40) |
|
Radiation dosage, Gy |
|
|
<60.0 | 2
(1) |
|
60.0–65.9 | 6
(4) |
|
66 | 124 (78) |
|
66.1–72.5 | 27
(17) |
| Chemotherapy |
|
|
Cisplatin cycles |
|
|
1 | 14 (9) |
|
2 | 65
(41) |
|
3 | 80
(50) |
|
Cisplatin cumulative dose,
mg/m2 |
|
|
<160 | 29
(18) |
|
160–199 | 57
(36) |
|
200–240 | 73
(46) |
| Total | 159
(100) |
Predictive factors of high-dose
cisplatin tolerance
The results of our analyses of predictive factors of
high-dose cisplatin tolerance are shown in Table III. By Fisher's exact test, male
gender [odds ratio (OR), 24.75; P<0.001], high BSA (>1.80
m2; OR, 3.17; P=0.002), high BMI (>25.0; OR, 2.57;
P=0.027), and high creatinine clearance (>100 ml/min; OR, 2.01;
P=0.048) were significantly positive predictive factors of
high-dose cisplatin tolerance. Of these factors, only male gender
(OR, 25.00; P=0.005) and high BSA (OR, 2.21; P=0.032) were also
significantly predictive of high-dose cisplatin tolerance by the
logistic regression analysis.
 | Table III.Predictive factors of high-dose
cisplatin tolerance. |
Table III.
Predictive factors of high-dose
cisplatin tolerance.
|
| Fisher's exact
test | Logistic regression
analysis |
|---|
|
|
|
|
|---|
| Variable | Odds ratio | P-value | Odds ratio | P-value |
|---|
| Male | 24.75 | <0.001 | 25 | 0.005 |
| Smoking history
present |
1.35 | 0.46 |
|
|
| Age, ≥61 years |
0.61 | 0.15 |
|
|
| Body surface area,
>1.80 m2 |
3.17 |
0.002 | 2.212 | 0.032 |
| Body mass index,
kg/m2 |
|
|
|
|
|
<18.5 |
0.29 |
0.089 |
|
|
|
≥25.0 |
2.57 |
0.027 |
|
|
| Stage 4 |
0.89 | 0.75 |
|
|
| Albumin, ≥4
g/dl |
1.77 | 0.13 |
|
|
| Glycated
hemoglobin, >upper normal limit |
1.10 | 1.00 |
|
|
| High creatine
clearance, >100 ml/min |
2.01 |
0.048 |
|
|
| Intensity-modified
radiotherapy |
1.19 | 0.63 |
|
|
| Treatment between
2012–2013 |
1.73 | 0.15 |
|
|
Adverse events
The grade 3/4 adverse events during CCRT are shown
in Table IV. One patient succumbed
due to an adverse event on day 30 of the CCRT, due to systemic
sepsis following diarrhea and severe renal failure.
 | Table IV.Grade 3/4 adverse events. |
Table IV.
Grade 3/4 adverse events.
|
|
| Cisplatin
cumulative dose, n (%) |
|
|---|
|
|
|
|
|
|---|
| Adverse events | All patients, n
(%) | ≥200
mg/m2 | <200
mg/m2 | P-value |
|---|
| Hematological |
|
|
|
|
|
Leukocytopenia | 48
(30) | 16 (22) | 32 (37) |
0.024 |
|
Neutropenia | 22
(14) | 7
(10) | 15 (17) | 0.17 |
|
Anemia | 12 (8) | 2 (3) | 10 (12) |
0.039 |
|
Thrombocytopenia | 2
(1) | 0 (0) | 2 (2) | 0.50 |
|
Non-hematological |
|
|
|
|
|
Mucositis | 41
(26) | 16 (22) | 25 (29) | 0.36 |
|
Dysgeusia | 4
(3) | 2 (3) | 2 (2) | 1.00 |
|
Xerostomia | 7
(4) | 2 (3) | 5 (6) | 0.45 |
|
Dermatitis | 2
(1) | 2 (3) | 0 (0) | 0.21 |
| Liver
function disorder | 8
(5) | 3 (4) | 5 (6) | 0.73 |
|
Creatinine increased | 7
(4) | 0 (0) | 7 (8) |
0.016 |
|
Electrolytes disorder | 16
(10) | 8
(11) | 8 (9) | 0.80 |
|
Aspiration pneumonia | 20
(13) | 2 (3) | 18 (20) |
0.001 |
The hematological adverse events tended to be more
severe in the high-dose cisplatin intolerable patients;
leukocytopenia and anemia in particular were observed at a
significantly higher rate in the high-dose cisplatin-intolerant
patients (P=0.024 and P=0.039, respectively). As for the
non-hematological adverse events, increased creatinine and
aspiration pneumonia were observed at a significantly high rate in
the high-dose cisplatin-intolerant patients (P=0.016 and P=0.001,
respectively). Mucositis was observed at a high rate regardless of
the cisplatin tolerance.
Response and prognosis
At the time of the analyses, 21 fatalities and 62
adverse events were observed; these events included 46 progressive
disease (31 local recurrences and/or 17 metastases), 16 secondary
malignancies, 1 fatality due to an adverse event during CCRT, and 1
lethal event unrelated to malignancy following CCRT. The details of
secondary malignancies are as follows: 7 esophageal carcinomas, 4
primary lung carcinomas, 1 cholangiocellular carcinoma, 1
pancreatic carcinoma, 2 colorectal carcinomas, 1 breast carcinoma,
and 1 malignant lymphoma. One patient suffered from esophageal and
lung carcinoma during the follow-up. Two patients succumbed due to
secondary malignancies; one due to lung cancer and the other to
malignant lymphoma.
Following the completion of CCRT, a complete
response (CR) was certified in 118 (74%) patients. Using the
Kaplan-Meier method, the 3-year OS and PFS rates were 80.8 and
55.9%, respectively. The CR rate of the high-dose
cisplatin-tolerant patients was significantly higher than that of
the high-dose cisplatin-intolerant patients (82 vs. 67%, P=0.045).
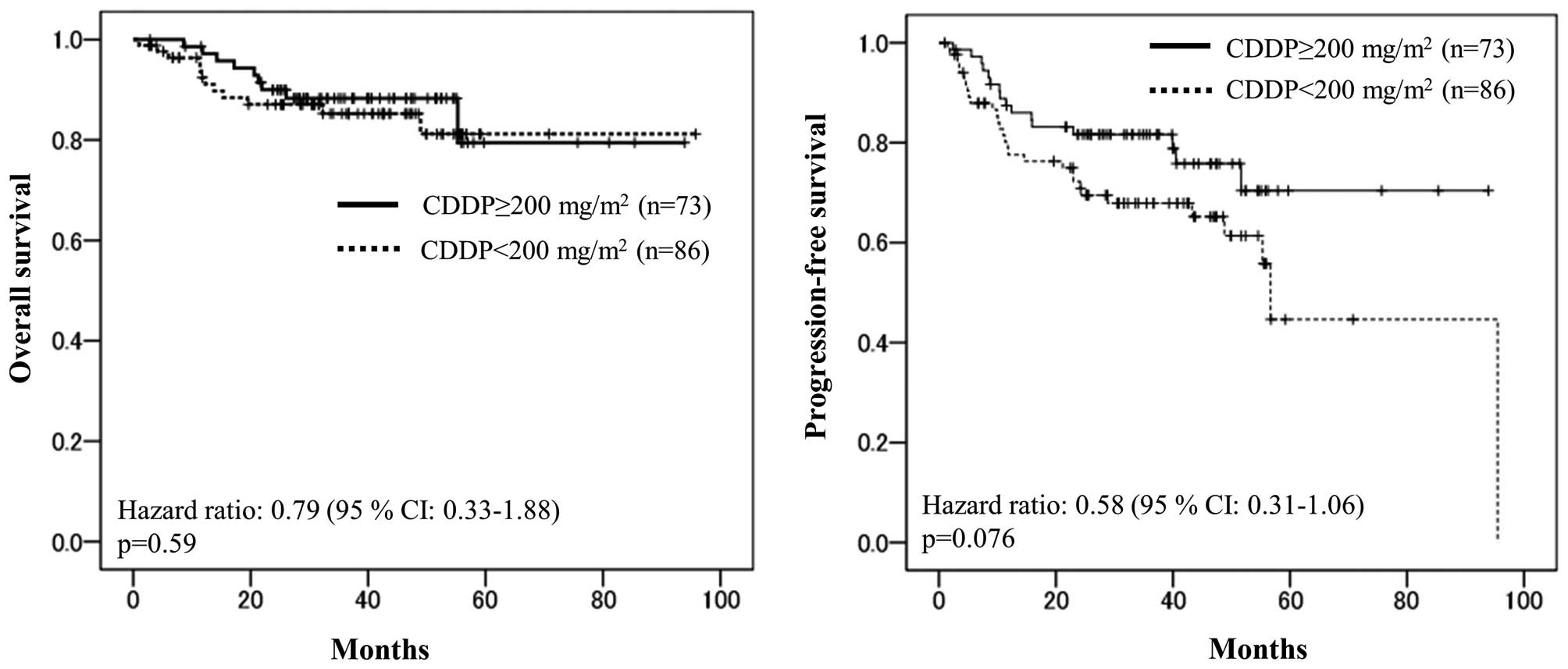
By Cox regression analyses, however, there were no significant
differences in the OS or PFS rates; the 3-year OS values of the
high-dose cisplatin-tolerant/intolerant patients were 79.5 and
81.2%, respectively (hazard ratio=0.79, P=0.59), and the 3-year PFS
values of high-dose cisplatin-tolerant/intolerant patients were
70.4 and 44.6%, respectively (hazard ratio=0.58, P=0.076) (Fig. 1). Although the PFS tended to be longer
in the high-dose cisplatin-tolerant patients, a statistically
significant difference was not observed.
Discussion
For CCRT, the most well-confirmed chemotherapy
regimen in randomized clinical trials (2–4) is
high-dose cisplatin, 100 mg/m2 per 3 weeks. However,
even with the extensive evidence of the efficacy of a high-dose
cisplatin regimen, such a regimen has been not applied to all the
HNSCC patients. Additionally, although the feasibility of this
regimen for Japanese patients was also evaluated and affirmed in a
small study (7), the standard dose of
cisplatin has been not approved in Japan.
The major reason for not administering a high-dose
cisplatin regimen is its accompanying high risk of severe adverse
events, resulting in the frequent non-completion of the cisplatin
regimen. CCRT with high-dose cisplatin has been confirmed to
improve patient responses and OS; however, its risk of severe
adverse events is also increased (2–4). It was
also reported that during long follow-ups, severe late toxicities
are also problematic and affect long-term survival (8). However, dose reduction or
discontinuation of high-dose cisplatin based on the risk of adverse
events is not recommended, as the cumulative cisplatin dose and
treatment cycles are correlated with the prognoses of HNSCC
patients receiving CCRT (9).
Based on the review of the results of clinical
trials reported until the early 2000s, the study by Ang (10) suggested that a cumulative cisplatin
dose of 200 mg/m2 would be the threshold for patients to
yield a beneficial antitumor effect from CCRT regardless of the
schedule. Previous studies also suggest that a cumulative dose of
200 mg/m2 is the threshold to experience a benefit from
CCRT (11,12), and certain clinical trials set a total
dose of 200 mg/m2 in their control group (9,13). Based
on these findings, the present study used the cumulative dose of
200 mg/m2 as the target dose.
In the present patient population, the rate of
high-dose cisplatin-tolerant patients was 46%; thus, approximately
one-half of the patients did not reach the provisional cumulative
cisplatin dose of 200 mg/m2 in the clinical practice.
For high-dose cisplatin-intolerant patients, offering more feasible
regimens other than high-dose cisplatin should be considered.
Alternative regimens providing improved prognoses and less toxicity
have been pursued, but as of 2000, there were no other treatment
regimens for CCRT that surpassed the effectiveness of high-dose
cisplatin.
As a new treatment strategy, concomitant cetuximab
treatment and radiotherapy (BRT) was certified to improve the
prognoses of patients with locally advanced HNSCC compared to
radiation only (5). BRT is recognized
as a more feasible regimen compared to CCRT and is now widely
indicated for locally advanced HNSCC patients. However, there is
not enough evidence to conclude the superiority or non-inferiority
of BRT to CCRT. Retrospective studies comparing the prognoses of
patients who underwent CCRT to those who underwent BRT appear to
show that the patients who received CCRT had a longer survival
(14,15), but it is likely that these studies
have the limitation of selection bias, in which BRT may be
preferably administered to patients with poor performance status,
high risks or comorbidities. Recent prospective randomized trials
comparing CCRT and BRT suggest that the prognoses afforded by these
two treatment strategies are nearly equal (16,17).
However, in those prospective studies, the patients in the CCRT
arms had relatively low compliance to the chemotherapy. It is quite
possible that the equality of CCRT and BRT in these randomized
studies was influenced by the differences in the treatment
feasibility. To evaluate and decide whether CCRT with high-dose
cisplatin or BRT is the better treatment strategy, high-dose
cisplatin-tolerant patients must be identified, and the patient
responses and prognoses following CCRT and BRT must be compared. As
adding cetuximab to CCRT was shown to not contribute to an
improvement of OS in the randomized trial RTOG 0522 (13), it is apparent that CCRT and BRT should
not be administered to the same patients.
In current decisions of the indications for CCRT, in
light of BRT as a feasible treatment option, we would like to
predict the patients' tolerance to high-dose cisplatin, as well as
their responses and prognoses. In the present retrospective study,
male gender and BSA were significantly predictive for cisplatin
tolerance. The male-female ratio of the HNSCC patients was highly
biased, but considering the differences of the absolute number of
patients, there is a clinical study that suggests female HNSCC
patients tended to have poor prognoses (18). Differences of tolerability to
high-dose cisplatin may be one of the reasons for the poor response
among female HNSCC patients.
Associations between body weight and the incidence
of several types of cancer have been described (19). Body weight is also suggested to be
associated with prognoses in certain malignancies. Being overweight
is suggested to be a poor prognostic factor in breast cancer
patients (20), whereas for lung
cancer patients and malignant lymphoma patients, there are certain
studies indicating that overweight and even obesity is associated
with a better prognoses (21,22). HNSCC patients with high BMIs have been
shown to be associated with longer survival (23,24), and
similar data were also reported for Japanese HNSCC patients
(25).
In the present study, in addition to BSA, clinical
parameters associated with the body mass and body weight, such as
creatinine clearance and BMI were significant predictors of
cisplatin tolerance by Fisher's exact test. Body weight and BMI
reflect numerous patient background factors, such as nutrition,
dietary habits, total mass of fat and/or muscle, and genetic
factors. Forming conclusions regarding the associations between
body weight and survival in HNSCC patients is difficult, but as a
tool for evaluating the feasibility of treatments, body weight
could be a useful factor as it is extremely easy to measure.
As for adverse events, high rates of hematological
adverse events, renal dysfunction and aspiration pneumonia were
observed in the cisplatin-intolerant patients. In previous studies,
grade 3/4 hematological adverse events were observed in 29–50% of
patients receiving CCRT with high-dose cisplatin (2–4,6). In the present series, there were no
clear differences in hematological adverse events between the
patients who were cisplatin-tolerant or -intolerant.
Aspiration pneumonia is a problematic adverse event
among HNSCC patients, which is associated with dysphagia. Chen
et al (26) examined the
clinical courses of 595 HNSCC patients who received CCRT, and
identified that the incidence of aspiration pneumonia during CCRT
was 7%. The risk of aspiration pneumonia remains high following
CCRT completion. Hunter et al (27) reported that the cumulative incidence
of aspiration pneumonia of oropharyngeal cancer patients following
CCRT was 20% with the median follow-up time of 49 months. Mortensen
et al (28) estimated the
incidence of aspiration pneumonia in HNSCC patients treated with
CCRT using the DAHANCA database, and reported the incidence rate of
29 per 1,000 person-years and the incidence proportion of 5.3% in
the first year after radiotherapy. In the present study, the
incidence of aspiration pneumonia was 12%, which is relatively
higher compared to the values in the previous studies. To complete
a pre-planned cisplatin regimen, the prevention of aspiration
pneumonia is extremely important. Weight loss and BMI were
suggested to be associated with aspiration in patients treated for
head and neck cancer (29).
The high-dose cisplatin-tolerant patients in the
present study showed a high CR rate; however, there were no
significant differences in the tolerant versus intolerant patient
prognoses, in neither the PFS nor the OS. The present study has
certain limitations regarding the evaluation of prognoses following
CCRT: Short follow-up times, the stages included, and the lack of
an evaluation of the patients' human papilloma virus (HPV) status.
The median follow-up time in the study was 37.6 months, <5
years; which may not be enough time to evaluate long-term
prognoses. The inclusion of stage II patients may confuse the
evaluation of prognoses. HPV infection has been known to affect the
incidence and prognosis of HNSCC, particularly oropharyngeal cancer
(30). The present study included 57
(36%) oropharyngeal cancer patients; however, the HPV status of
these patients was not certified. In numerous prospective studies,
HPV infection has been proven to be the better prognostic factor,
as HPV-related HNSCC patients have good responses to radiotherapy
and chemotherapy. In certain clinical trials, the influence of HPV
status on prognosis was greater than that of the treatment
interventions (31).
When planning treatment strategies for HNSCC
patients, to improve patient prognoses and qualities of life, the
appropriate treatment strategy should be selected based on the
prognostic and predictive factors of each treatment. The present
findings suggest that parameters associated with the patient body
mass and weight may also be predictive factors of a high-dose
cisplatin CCRT regimen. It would be worthwhile to further
investigate such parameters when assessing the feasibility of and
indications for CCRT.
Acknowledgements
The authors would like to thank all the staff
members of the Departments of Head and Neck Surgery at the Cancer
Institute Hospital of the Japanese Foundation for Cancer Research
for diagnosing and treating the patients enrolled in the present
study.
References
|
1
|
Brizal DM and Esclamado R: Concurrent
chemoradiotherapy for locally advanced, nonmetastatic, squamous
carcinoma of the head and neck: Consensus, controversy and
conundrum. J Clin Oncol. 24:2612–2617. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adelstein DJ, Li Y, Adams GL, Wagner H Jr,
Kish JA, Ensley DF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemothardiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Sarraf M, LeBlanc M, Giri PG, Fu KK,
Cooper J, Vuong T, Forastiere AA, Adams G, Sakr WA, Schuller DE and
Ensley JF: Chemoradiotherapy versus radiotherapy in patients with
advanced nasopharyngeal cancer: Phase III Randomized Intergroup
Study 0099. J Clin Oncol. 16:1310–1317. 1998.PubMed/NCBI
|
|
4
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, et al: New response evaluation
criteria in solid tumours: Revised RECIST guideline (version 1.1).
Eur J Cancer. 45:226–247. 2009. View Article : Google Scholar
|
|
7
|
Zenda S, Onozawa Y, Tahara M, Kawashima M,
Shikama N, Sasaki S and Boku N: Feasibility study of single agent
cisplatin and concurrent radiotherapy in japanese patients with
squamous cell carcinoma of the head and neck: Preliminary results.
Jpn J Clin Oncol. 37:725–729. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forastiere AA, Zhang Q, Weber RS, Maor MH,
Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, et
al: Long-term results of RTOG 91-11: A comparison of three
nonsurgical treatment strategies to preserve the larynx in patients
with locally advanced larynx cancer. J Clin Oncol. 31:845–852.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS,
Rosenthal DI, Soulieres D, Kim H, Silverman C, Raben A, Galloway
TJ, et al: Randomized phase III trial to test accelerated versus
standard fractionation in combination with concurrent cisplatin for
head and neck carcinomas in the radiation therapy oncology group
0129 trial: Long-term report of efficacy and toxicity. J Clin
Oncol. 32:3858–3866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ang KK: Concurrent radiation chemotherapy
for locally advanced head and neck carcinoma: Are we addressing
burning subjects? J Clin Oncol. 22:4657–4659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Loong HH, Ma BB, Leung SF, Mo F, Hui EP,
Kam MK, Chan SL, Yu BK and Chan AT: Prognostic significance of the
total dose of cisplatin administered during concurrent
chemoradiotherapy in patients with locoregionally advanced
nasopharyngeal carcinoma. Radiother Oncol. 104:300–304. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghi MG, Paccagnella A, Floriani I and
Garavaglia D: Concomitant chemoradiation in locally advanced head
and neck squamous cell carcinoma: A literature-based meta-analysis
on the platinum concomitant chemotherapy. J Clin Oncol. 29(Suppl):
55342011.
|
|
13
|
Ang KK, Zhang QE, Rosenthal DI, Nguyen-Tan
P, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar
AK, et al: Randomized phase III trial of concurrent accelerated
radiation plus cisplatin with or without cetuximab for stage iii to
iv head and neck carcinomas: RTOG 0522. J Clin Oncol. 32:2940–2950.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koutcher L, Sherman E, Fury M, Wolden S,
Zhang Z, Mo Q, Stewart L, Schupak K, Gelblum D, Wong R, et al:
Concurrent cisplatin and radiation versus cetuximab and radiation
for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol
Phys. 81:915–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ley J, Mehan P, Wildes TM, Thorstad W, Gay
HA, Michel L, Nussenbaum B, Trinkaus K and Adkins D: Cisplatin
versus cetuximab given concurrently with definitive radiation
therapy for locally advanced head and neck squamous cell carcinoma.
Oncology. 85:290–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lefebvre JL, Pointreau Y, Rolland F,
Alfonsi M, Baudoux A, Sire C, de Raucourt D, Malard O, Degardin M,
Tuchais C, et al: Induction chemotherapy followed by either
chemoradiotherapy or bioradiotherapy for larynx preservation: The
TREMPLIN randomized phase II study. J Clin Oncol. 31:853–859. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghi MG, Paccagnella A, Ferrari D, Foa P,
Rocca MC, Verri E, Maiello E, Azzaello G, D'Ambrosio C, Casanova C,
et al: A phase II–III study comparing concomitant chemoradiotherapy
(CRT) versus cetuximab/RT (CET/RT) with or without induction
docetaxel/cisplatin/5-fluorouracil (TPF) in locally advanced head
and neck squamous cell carcinoma (LASCCHN): Efficacy results
(NCT0186826). J Clin Oncol. 31(suppl): abstr. 60032013.
|
|
18
|
Meyer F, Fortin A, Wang CS, Liu G and
Bairati I: Predictors of severe acute and late toxicities in
patients with localized head-and-neck cancer treated with radiation
therapy. Int J Radiat Oncol Biol Phys. 82:1454–1462. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhaskaran K, Douglas I, Forbes H,
dos-Santos-Silvia I, Leon DA and Smeeth L: Body-mass index and risk
of 22 specific cancers: A population-based cohort study of 5.24
million UK adults. Lancet. 384:755–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sparano JA, Wang M, Zhao F, Stearns V,
Martion S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW, et
al: Obesity at diagnosis is associated with inferior outcomes in
hormone receptor-positive operable breast cancer. Cancer.
118:5937–5946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weiss L, Melchard T, Habringer S,
Boekstegers A, Hufnagl C, Neureiter D, Hopfinger G, Greil R and
Egle A: Increased body mass index is associated with improved
overall survival in diffuse large B-cell lymphoma. Ann Oncol.
25:171–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dahlberg SE, Schiller JH, Bonomi PB,
Sanler AB, Brahmer JR, Ramalingam SS and Johnson DH: Body mass
index and its association with clinical outcomes for advanced
non-small-cell lung cancer patients enrolled on Eastern cooperative
oncology group clinical trials. J Thorac Oncol. 8:1121–1127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arthur AE, Peterson KE, Rozek LS, Tayloe
JM, Light E, Chepeha DB, Hébert JR, Terrell JE, Wolf GT and Duffy
SA: UM Head and Neck SPORE Program: Pretreatment dietary patterns,
weight status and head and neck squamous cell carcinoma prognosis.
Am J Clin Nutr. 97:360–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McRackan TR, Watkins JM, Herrin AE,
Garrett-Mayer EM, Sharma AK, Day TA and Gillespie MB: Effect of
body mass index on chemoradiation outcomes in head and neck cancer.
Laryngoscope. 118:1180–1185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takenaka Y, Takemoto N, Nakahara S,
Yamamoto Y, Yasui T, Hanamoto A, Fukusumi T, Michiba T, Cho H,
Yamamoto M and Inohara H: Prognostic significance of body mass
index before treatment of head and neck cancer. Head and Neck.
37:1518–1523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen SW, Yang SN, Liang JA and Lin FJ: The
outcome and prognostic factors in patients with aspiration
pneumonia during concurrent chemoradiotherapy for head and neck
cancer. Eur J Cancer Care (Engl). 19:631–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hunter KU, Lee OE, Lyden TH, Haxer MJ,
Feng FY, Schipper M, Worden F, Prince ME, McLean SA, Wolf GT, et
al: Aspiration pneumonia after chemo-intensity-modulated radiation
therapy of oropharyngeal carcinoma and its clinical and
dysphagia-related predictors. Head and Neck. 36:120–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mortensen HR, Jensen K and Grau C:
Aspiration pneuonia in patients treated with radiotherapy for head
and neck cancer. Acta Oncologica. 52:270–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ottosson S, Lindblom U, Wahlberg P,
Nilsson P, Kjellén E, Zackrisson B, Levring Jäghagen E and Laurell
G: Weight loss and body mass index in relation to aspiration in
patients treated for head and neck cancer: A long-term follow-up.
Support Care Cancer. 22:2361–2369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Posner MR, Lorch JH, Goloubeva O, Tan M,
Schumaker LM, Sarlis NJ, Haddad RI and Cullen KJ: Survival and
human papillomavirus in oropharynx cancer in TAS 324: A subset
analysis from an international phase III trial. Ann Oncol.
22:1071–1077. 2011. View Article : Google Scholar : PubMed/NCBI
|