Introduction
Peripheral T-cell lymphoma (PTCL) represents a small
heterogeneous group of non-Hodgkin lymphoma (NHL) that accounts for
~10% of NHLs in western countries and ~25% of NHLs in Japan
(1,2).
PTCLs generally have a poor outcome with shorter long-term survival
compared with B-cell lymphomas. For example, the 5-year overall
survival for the World Health Organization (WHO) subtype ‘PTCL-not
otherwise specified’ (PTCL-NOS) is 32%. PTCL remains extremely
difficult to treat as the majority of subtypes become refractory to
even aggressive chemotherapy regimens or relapse, and thus, there
is a requirement for novel treatment modalities. Mogamulizumab is a
humanized immunoglobulin G1 monoclonal antibody that targets CC
chemokine receptor 4 (CCR4). CCR4 is highly expressed by aggressive
PTCLs, particularly adult T-cell leukemia/lymphoma (ATL) and
cutaneous T-cell lymphomas (CTCLs). A phase II study of
mogamulizumab yielded an objective response in 35% of patients and
a complete response in 14%, with a median progression-free survival
(PFS) of 3 months (3). To date,
however, the efficacy of mogamulizumab in patients who become
refractory to chemotherapy following autologous stem cell
transplantation (ASCT) has not been investigated.
The present study reports a patient with PTCL who
became refractory following ASCT and resistant to a salvage
therapy, in whom mogamulizumab showed evident efficacy without
severe adverse event (AE).
Case report
A 49-year-old woman presented with subcutaneous
tumors and erythema from face to trunk with cervical
lymphadenopathy. From a biopsy of the tumor, the patient was
diagnosed with PTCL-NOS, clinical stage IIIA. The patient received
5 cycles of a regimen containing cyclophosphamide, doxorubicin,
vincristine and prednisone, and achieved complete remission (CR).
The patient subsequently received an ASCT using the
ranimustine/etoposide/cytarabine/melphalan regimen. Following ASCT,
the patient remained in CR for 1 month before the skin lesions
worsened without lymphadenopathy. The patient received the
rituximab, etoposide, methylprednisolone, cytarabine and cisplatin
regimen, as a salvage therapy, but the skin lesions remained
refractory. The patient received phototherapy, which was similarly
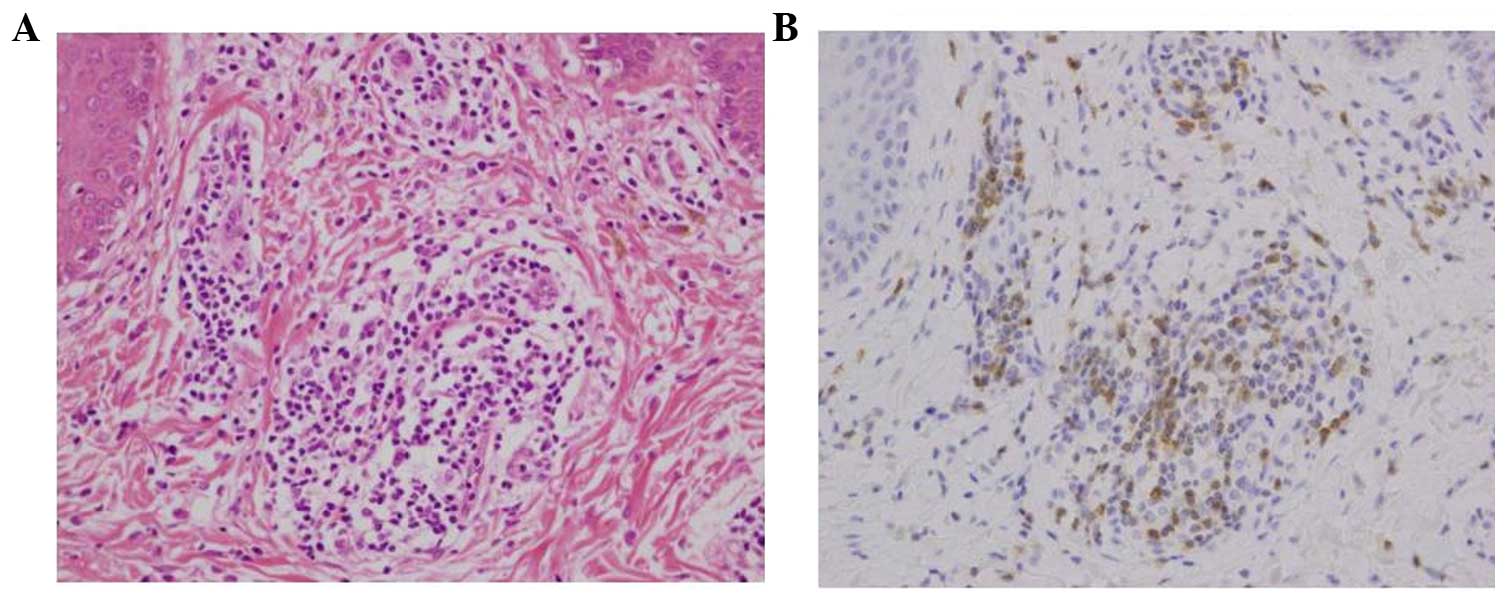
ineffective. Re-biopsy of the skin revealed that it was positive
for CCR4 (Fig. 1). The patient
received mogamulizumab once a week for 8 weeks by intravenous
infusion at 1.0 mg/kg. A grade 1 infusion reaction was observed at
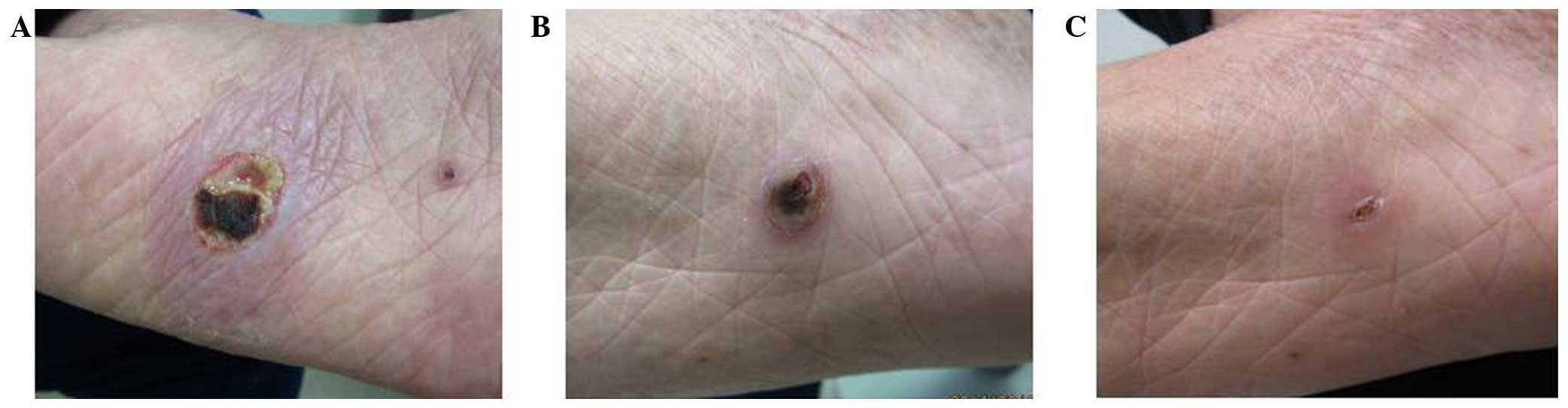
the first dose; however, no other AE was observed. After 5 weeks,
the skin lesions had improved, and after 8 weeks the patient
achieved CR (Fig. 2). The patient
remained in CR for >1 year.
Discussion
According to the WHO classification, PTCL is a
heterogeneous category of mature T-cell neoplasms. The most common
mature T-cell neoplasms are PTCL-NOS, angioimmunoblastic T-cell
lymphoma anaplastic large-cell lymphoma (ALCL) (4). PTCL remains extremely difficult to
treat, as the majority of PTCL subtypes become refractory to even
aggressive chemotherapy regimens or relapse, and thus there is a
medical requirement for novel treatment modalities.
Mogamulizumab is the first approved
glycol-engineered therapeutic antibody and first approved
monoclonal antibody to target CCR4 (5–7). CCR4 is
principally expressed on regulatory T cells (Tregs) and helper T
cells where it functions to induce homing of these leukocytes to
sites of inflammation. Tregs have an essential role in providing
and maintaining a favorable environment in which tumors can grow.
Mogamulizumab depletes CCR4-positive Tregs, potentially evoking
antitumor immune responses by autologous effector cells. This
ability is highly pertinent as subsets of malignant T cells are
believed to function as CD4-positive Tregs, overexpressing CCR4
(8–10). CCR4 is highly expressed by PTCLs,
particularly ATL and CTCLs. A previous study of 169 biopsies
demonstrated that CCR4 was expressed in the following PTCL
subtypes: ALCL anaplastic large cell kinase (ALK)-negative (67%),
PTCL-NOS (38%), angioimmunoblastic T-cell lymphoma (35%), ALCL
ALK-positive (4.2%), nasal-type natural killer/T-cell lymphoma
(3.7%). Within the PTCL-NOS subtype, CCR4-positive patients had a
significantly poorer prognosis compared to CCR4-negative patients
and multivariate analysis confirmed that CCR4 expression was an
independent unfavorable prognostic factor (11).
Mogamulizumab is developed, owned and manufactured
by Kyowa Hakko Kirin Co., Ltd. (Tokyo, Japan). The drug was
approved in Japan for the treatment of patients with
relapsed/refractory ATL in March 2012 and for the treatment of
relapsed/refractory CCR4-positive PTCL and CTCL in March 2014. This
agent is currently only available in Japan.
In a phase I study conducted in patients with
relapsed ATL and PTCL/CTCL, mogamulizumab was well tolerated at
doses of ≤1.0 mg/kg. The overall response rate (ORR) in the 16
patients included in this study was 31% (12). Similarly, a recent phase I/II
multicenter, dose-escalation study of mogamulizumab in relapsed
patients with CTCL that was conducted in the United States reported
an ORR of 36.8% (13). A Japanese
multicenter phase II study investigating mogamulizumab in patients
previously treated with PTCL or CTCL was published in 2014. In the
37 patients who received mogamulizumab, the ORR was 35%, complete
response was 14% and median PFS was 3 months (3). Based on the results of this phase II
study, mogamulizumab was approved for use of refractory/relapsed
PTCL in Japan.
In the Japanese phase II study, AEs were mild and
reversible. The most common of these were hematological events
(lymphocytopenia, 81%; leukocytopenia, 43%; thrombocytopenia, 8%;
neutropenia, 38%), followed by skin and subcutaneous tissue
disorders (51%) (3). The patient in
the present study had an infusion reaction, which was observed in
24% of patients in the phase II study. Due to the low frequency of
this disease and its endemic features, few physicians have used
this agent. Studies that became the foundation of its approval were
conducted on relatively small cohorts; therefore, there is a
requirement for larger multicenter investigations on the use of
mogamulizumab to treat PTCL. The present case is of particular
interest, as to the best of our knowledge, the use of mogamulizumab
has not been described previously in detail in PTCL cases
refractory following ASCT. Three patients who were refractory
following ASCT were included in the Japanese phase II study;
however, full details of these cases were not included (3). The present case became resistant quickly
following ASCT and remained refractory to a salvage therapy, but
mogamulizumab monotherapy was markedly effective. In our
experience, mogamulizumab has promising efficacy with relapsed PTCL
patients following ASCT.
Mogamulizumab is likely to provide new and promising
treatment options for patients with CCR4-positive T-cell lymphomas;
however, the precise approach may require refinement. For example,
patients may benefit from the use of mogamulizumab in combination
with other agents. Further studies are required to fully understand
the physiological effects of this agent and to determine which
subtypes of T-cell lymphoma may receive optimum benefit from this
agent.
References
|
1
|
No authors: listed: T he World Health
Organization classification of malignant lymphomas in Japan:
Incidence of recently recognized entities. Histopathology. Pathol
Int. 50:696–702. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aoki R, Karube K, Sugita Y, Nomura Y,
Shimizu K, Kimura Y, Hashikawa K, Suefuji N and Kikuchi M:
Distribution of malignant lymphoma in Japan, Analysis of 2260
cases, 2001-2006. Pathol Int. 58:174–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogura M, Ishida T, Hatake K, Taniwaki M,
Ando K, Tobinai K, Fujimoto K, Yamamoto K, Miyamoto T and Uike N:
Multicenter phase II study of mogamulizumab (KW-0761), a
defucosylated anti-cc chemokine receptor 4 antibody, in patients
with relapsed peripheral T-cell lymphoma and cutaneous T-cell
lymphoma. J Clin Oncol. 32:1157–1163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swerdlow SH, Campo E, Harris NL, et al:
WHO Classification of Tumours of Haematolopoietic and Lymphoid
Tissues (4th). Lyon: IARC. 2008.
|
|
5
|
Imai T, Nagira M, Takagi S, Kakizaki M,
Nishimura M, Wang J, Gray PW, Matsushima K and Yoshie O: Selective
recruitment of CCR4-bearing Th2 cells toward antigen-presenting
cells by the CC chemokines thymus and activation-regulated
chemokine and macrophage-derived chemokine. Int Immunol. 11:81–88.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niwa R, Shoji-Hosaka E, Sakurada M,
Shinkawa T, Uchida K, Nakamura K, Matsushima K and Ueda R:
Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with
enhanced antibody-dependent cellular cytotoxicity shows potent
therapeutic activity to T-cell leukemia and lymphoma. Cancer Res.
64:2127–2133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishii T, Ishida T, Utsunomiya A, Inagaki
A, Yano H, Komatsu H, Iida S, Imada K, Uchiyama T, Akinaga S, et
al: Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761
as a novel immunotherapeutic agent for adult T-cell
leukemia/lymphoma. Clin Cancer Res. 16:1520–1531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohshima K, Karube K, Kawano R, Tsuchiya T,
Suefuji H, Yamaguchi T, Suzumiya J and Kikuchii M: Classification
of distinct subtypes of peripheral T-cell lymphoma unspecified,
identified by chemokine and chemokine receptor expression, Analysis
of prognosis. Int J Oncol. 25:605–613. 2004.PubMed/NCBI
|
|
9
|
Nakagawa M, Nakagawa-Oshiro A, Karnan S,
Tagawa H, Utsunomiya A, Nakamura S, Takeuchi I, Ohshima K and Seto
M: Array comparative genomic hybridization analysis of PTCL-U
reveals a distinct subgroup with genetic alterations similar to
lymphoma-type adult T-cell leukemia/lymphoma. Clin Cancer Res.
15:30–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishida T and Ueda R: Immunopathogenesis of
lymphoma: Focus on CCR4. Cancer Sci. 102:44–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishida T, Inagaki H, Utsunomiya A,
Takatsuka Y, Komatsu H, Iida S, Takeuchi G, Eimoto T, Nakamura S
and Ueda R: CXC chemokine receptor 3 and CC chemokine receptor 4
expression in T-cell and NK-cell lymphomas with special reference
to clinicopathological significance for peripheral T-cell lymphoma,
unspecified. Clin Cancer Res. 10:5494–5500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto K, Utsunomiya A, Tobinai K,
Tsukasaki K, Uike N, Uozumi K, Yamaguchi K, Yamada Y, Hanada S,
Tamura K, et al: Phase I study of KW-0761, a defucosylated
humanized anti-CCR4 antibody, in relapsed patients with adult
T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin
Oncol. 28:1591–1598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duvic M, Pinter-Brown L, Foss FM, Sokol L,
Jorgensen JL, et al: Phase 1/2 study of mogamulizumab, a
defucosylated anti-CCR4 antibody, in previously treated patients
with cutaneous T-cell lymphoma. Blood. 125:1883–1889. 2015.
View Article : Google Scholar : PubMed/NCBI
|