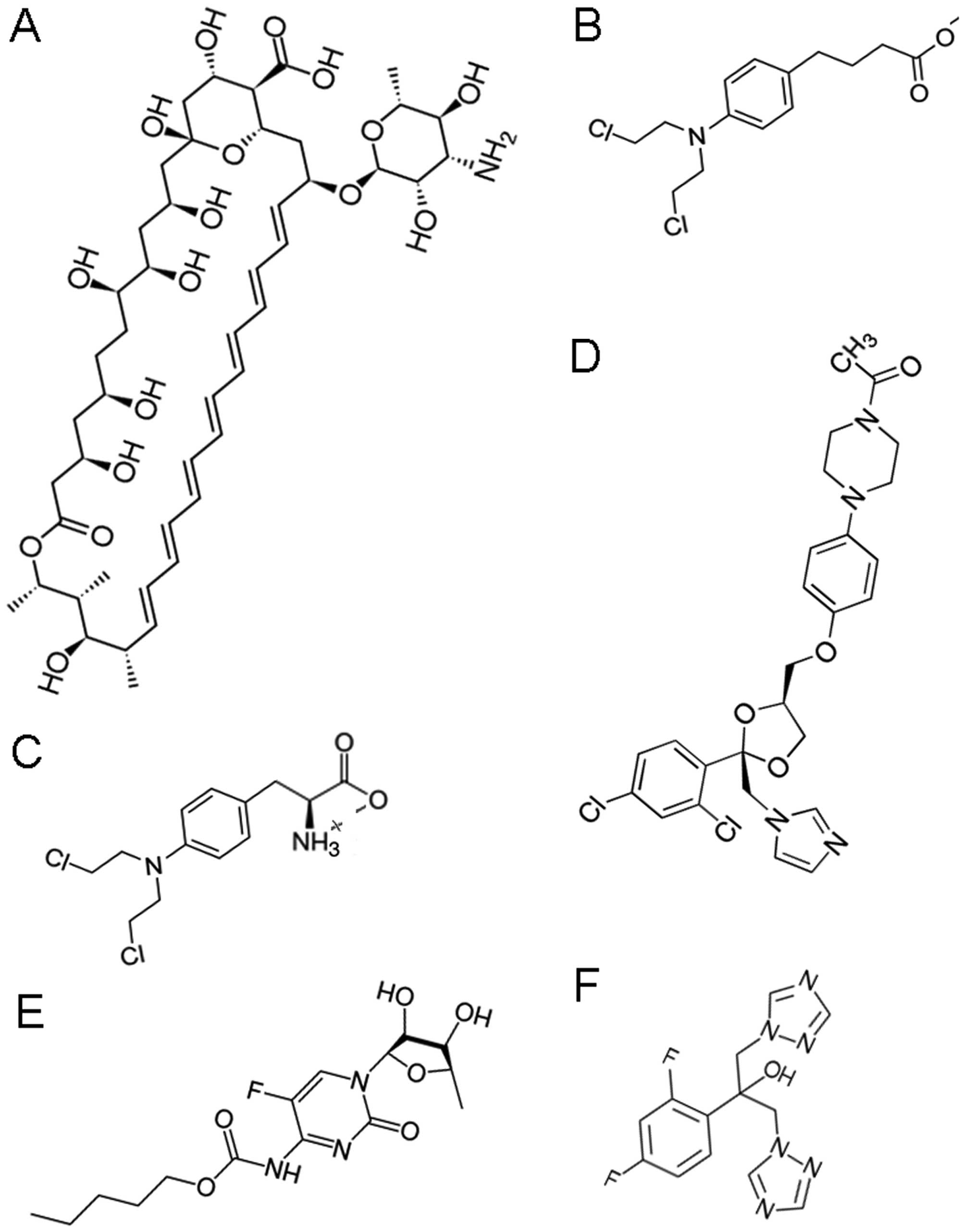
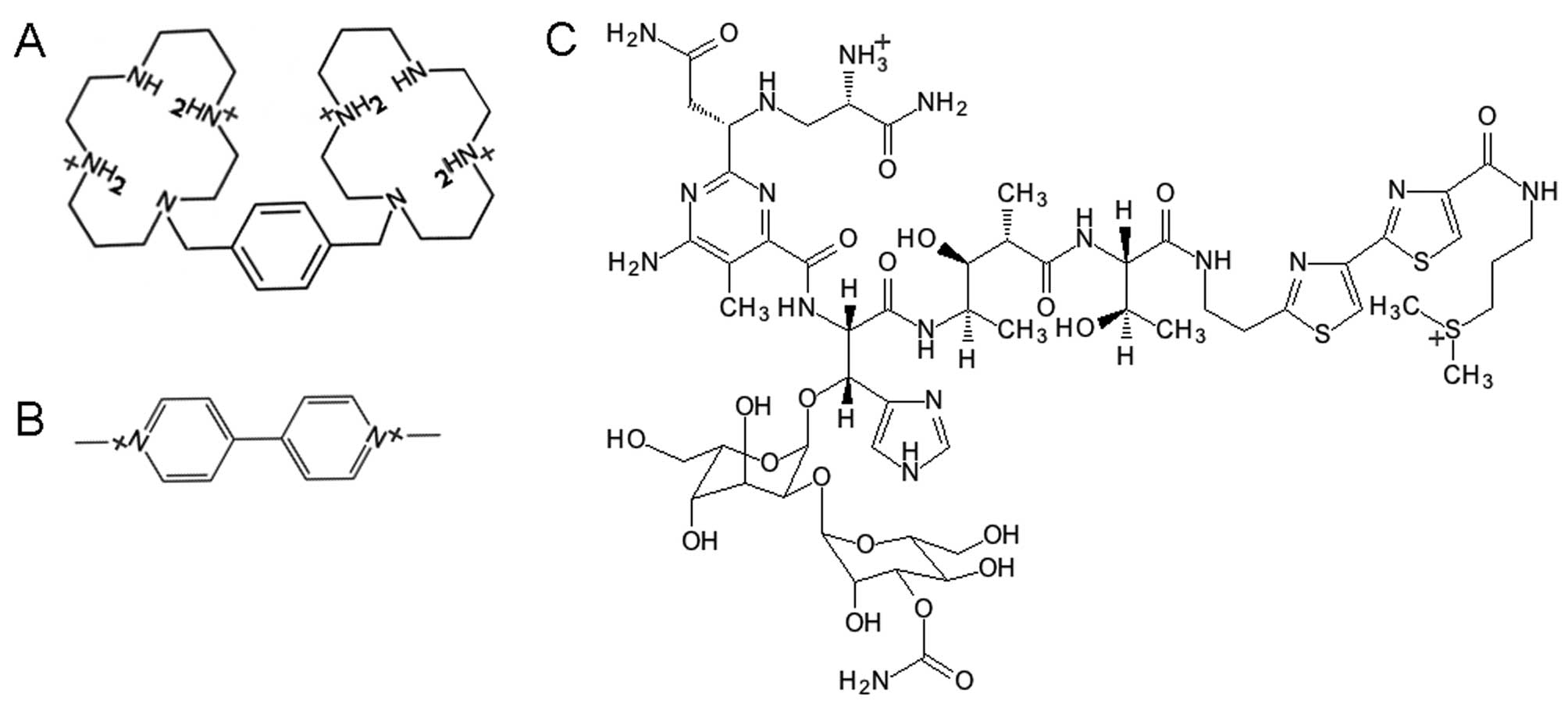
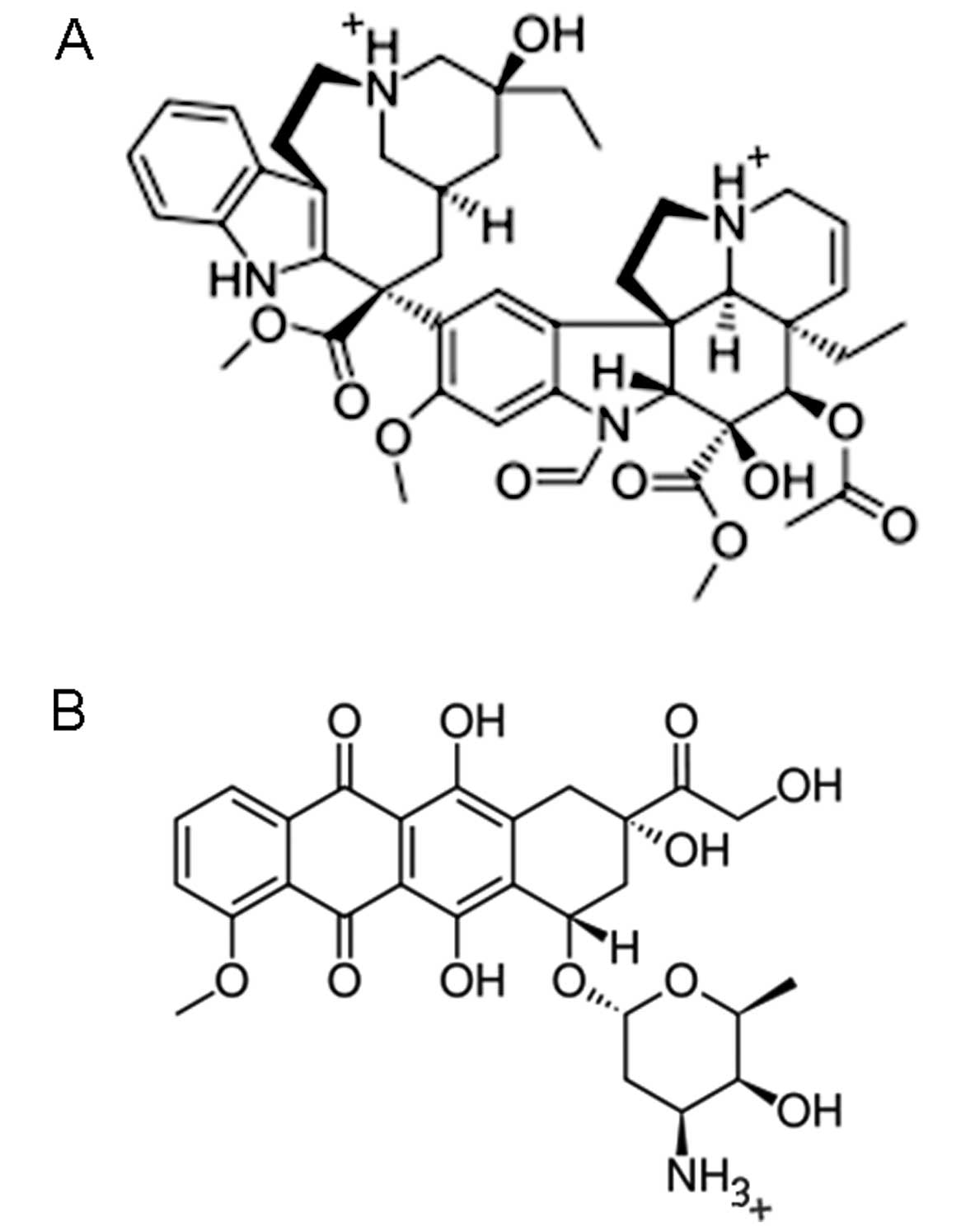
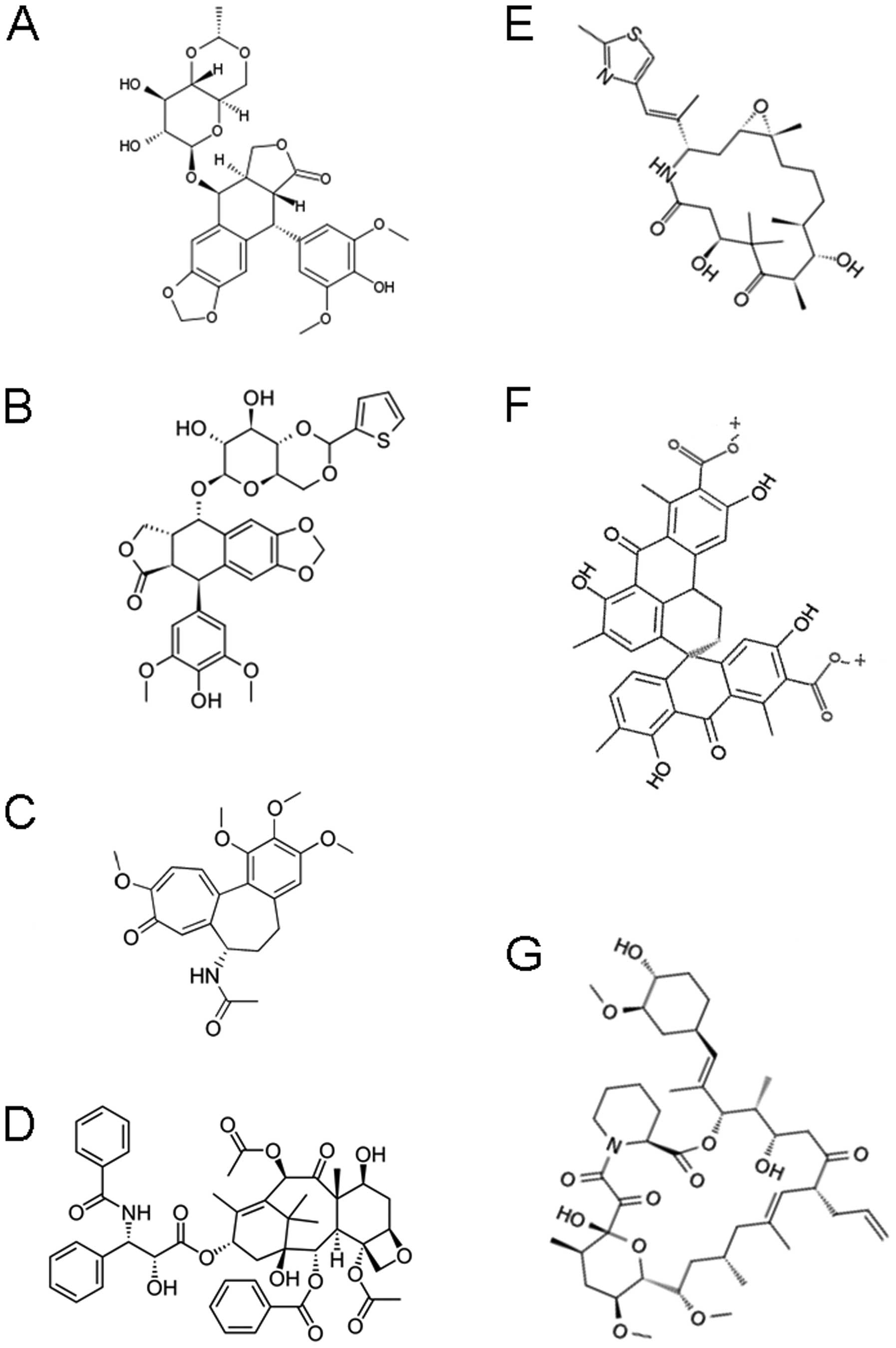
|
1
|
Klassen CD and Watkins IB III: Casarett
and Doull's Essentials of Toxicology. New York, NY: McGraw-Hill.
2010. View Article : Google Scholar
|
|
2
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
Smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
3
|
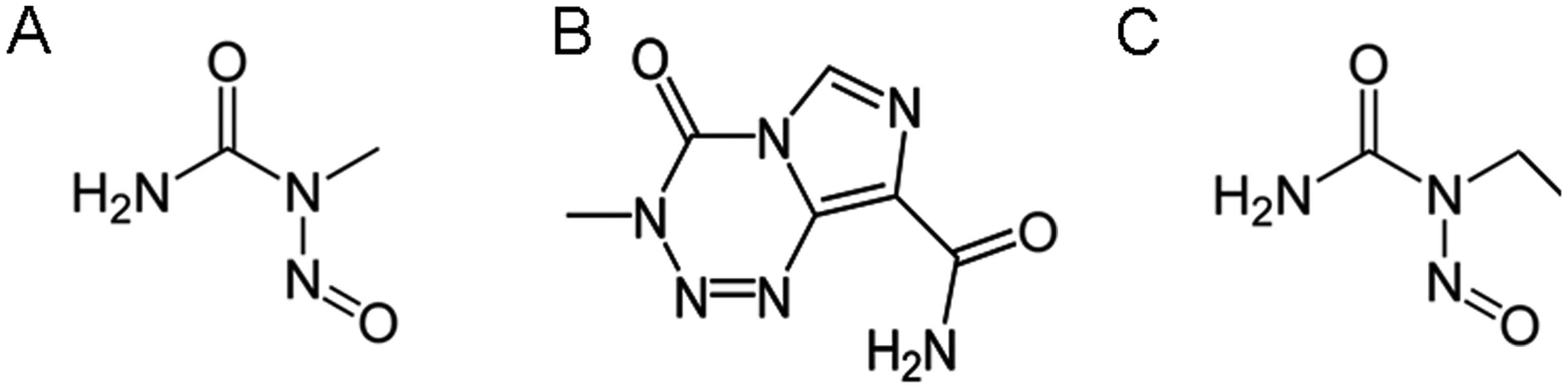
Sarin H: Recent progress towards
development of effective systemic chemotherapy for the treatment of
malignant brain tumors. J Transl Med. 7:772009. View Article : Google Scholar : PubMed/NCBI
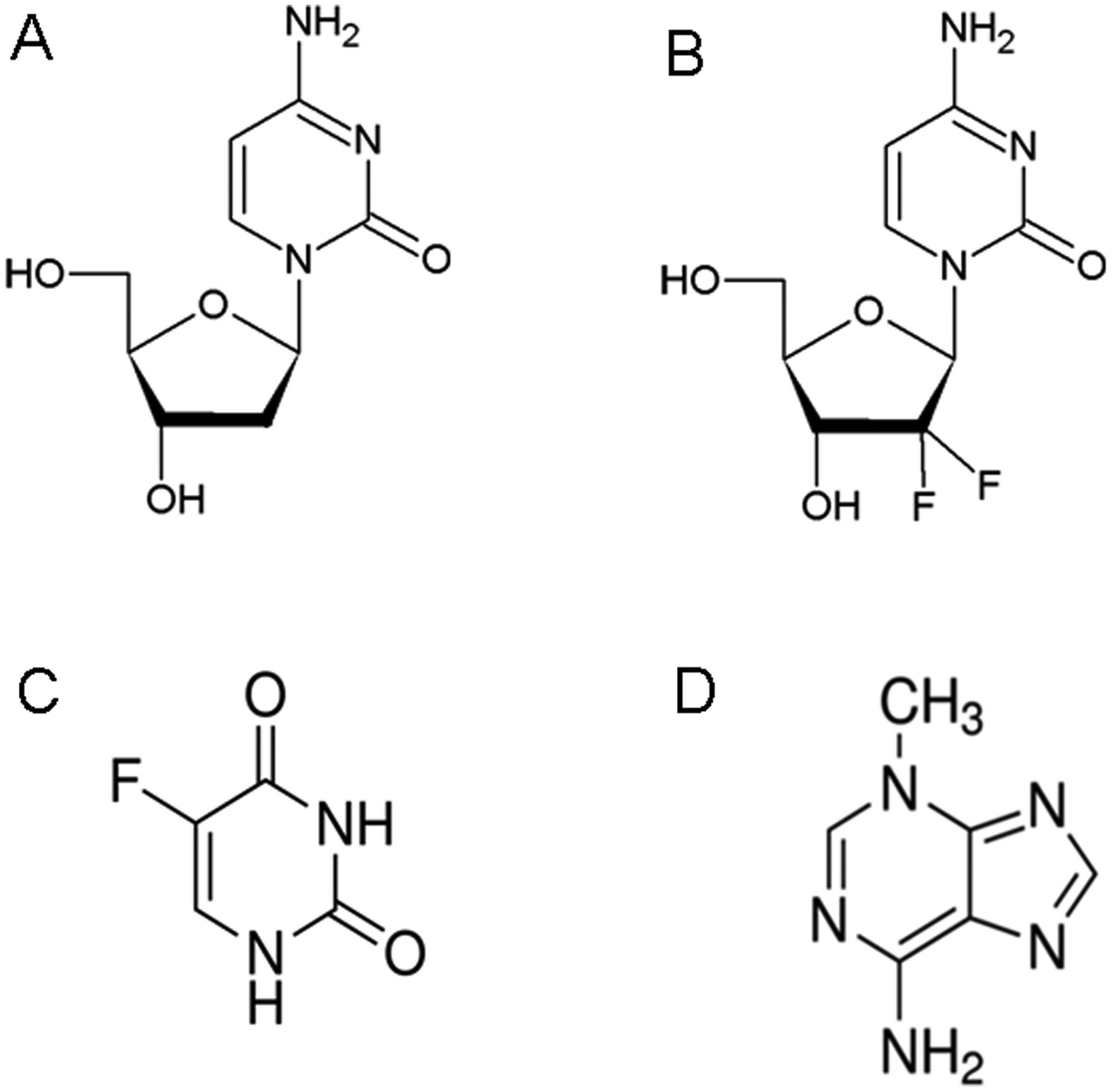
|
|
4
|
Sarin H: Overcoming the challenges in the
effective delivery of chemotherapies to CNS solid tumors. Ther
Deliv. 1:289–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sarin H: On the future development of
optimally-sized lipid-insoluble systemic therapies for CNS solid
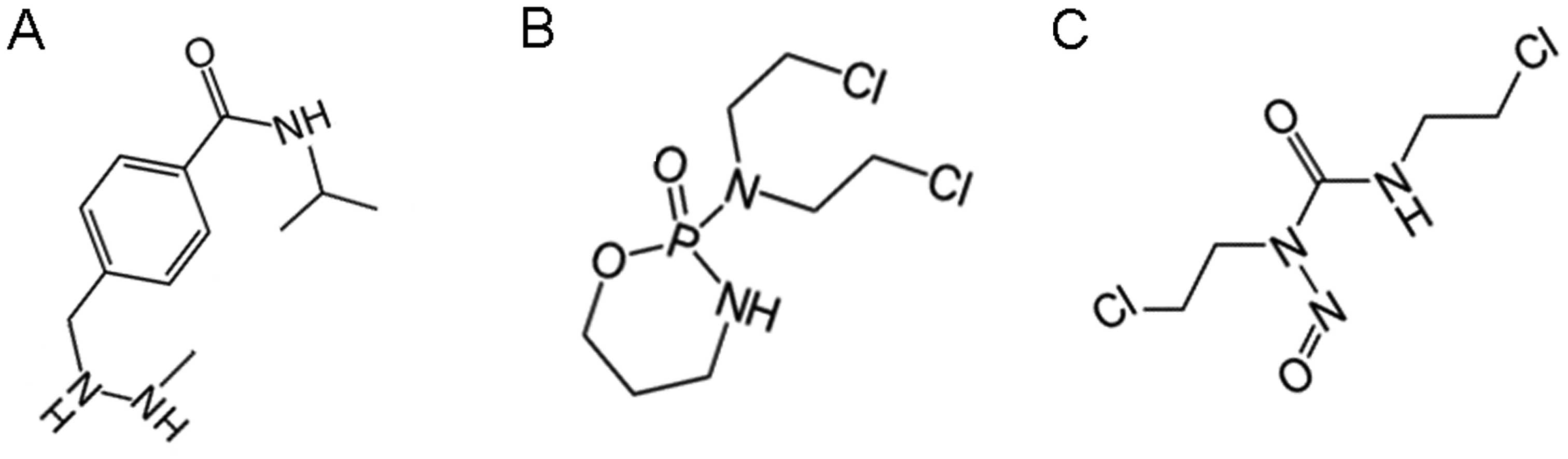
tumors and other neuropathologies. Recent Patents CNS Drug Discov.
5:239–252. 2010. View Article : Google Scholar
|
|
6
|
Sarin H: Effective transvascular delivery
of chemotherapy into cancer cells with imageable nanoparticles in
the 7 to 10 nanometer size range. Current Advances in the Medical
Application of Nanotechnology. Bentham Science Publishers Ltd.
10–24. 2012.
|
|
7
|
Sarin H: Permeation tt n Silico Pharmacol.
3:52015. View Article : Google Scholar
|
|
8
|
Sarin H: Translational theranostic
methodology for diagnostic imaging and the concomitant treatment of
malignant solid tumors. Neurovascular Imaging. 1:32015. View Article : Google Scholar
|
|
9
|
Lee CC, Gillies ER, Fox ME, Guillaudeu SJ,
Fréchet JM, Dy EE and Szoka FC: A single dose of
doxorubicin-functionalized bow-tie dendrimer cures mice bearing
C-26 colon carcinomas. Proc Natl Acad Sci USA. 103:16649–16654.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosier RN, Teot LA, Hicks DG, Schwartz C,
O'Keefe RJ and Puzas JE: Multiple drug resistance in osteosarcoma.
Iowa Orthop J. 15:66–73. 1995.PubMed/NCBI
|
|
12
|
Kleinschmidt-Demasters BK, Kang JS and
Lillehei KO: The burden of radiation-induced central nervous system
tumors, A single institution experience. J Neuropathol Exp Neurol.
65:204–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verhoef GE: DeW olf-Peeters C, Ferrant A,
Deprez S, Meeus P, Stul M, Zacheé P, Cassiman JJ, Van den Berghe H
and Boogaerts MA: Myelodysplastic syndromes with bone marrow
fibrosis: A myelodysplastic disorder with proliferative features.
Ann Hematol. 63:235–241. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doudican NA, Kumar A, Singh NK, Nair PR,
Lala DA, Basu K, Talawdekar AA, Sultana Z, Tiwari KK, Tyagi A, et
al: Personalization of cancer treatment using predictive
simulation. J Transl Med. 13:432015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pingle SC, Sultana Z, Pastorino S, Jiang
P, Mukthavaram R, Chao Y, Bharati IS, Nomura N, Makale M, Abbasi T,
et al: In silico modeling predicts drug sensitivity of
patient-derived cancer cells. J Transl Med. 12:1282014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peacock JD, Cherba D, Kampfschulte K,
Smith MK, Monks NR, Webb CP and Steensma M: Molecular-guided
therapy predictions reveal drug resistance phenotypes and treatment
alternatives in malignant peripheral nerve sheath tumors. J Transl
Med. 11:2132013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarin H: Pressuromodulation a. a Transl
Med. 13:3722015. View Article : Google Scholar
|
|
18
|
Newlands ES, Stevens MF, Wedge SR,
Wheelhouse RT and Brock C: Temozolomide: A review of its discovery,
chemical properties, pre-clinical development and clinical trials.
Cancer Treat Rev. 23:35–61. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson RE, Campbell RJ and Laws ER Jr:
The cytotoxic effect of ethylnitrosourea on the developing rat
cerebellum. Histopathology. Acta Neuropathol. 55:257–261. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolarić K, Roth A and Fuss V: Combination
chemotherapy with 1-methyl-1-nitrosourea and cyclophosphamide in
metastatic melanoma. Tumori. 64:89–94. 1978.PubMed/NCBI
|
|
21
|
An Q, Robins P, Lindahl T and Barnes DE:
5-Fluorouracil incorporated into DNA is excised by the Smug1 DNA
glycosylase to reduce drug cytotoxicity. Cancer Res. 67:940–945.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tentori L, Forini O, Fossile E, Muzi A,
Vergati M, Portarena I, Amici C, Gold B and Graziani G:
N3-methyladenine induces early poly(ADP-ribosylation), reduction of
nuclear factor-kappa B DNA binding ability, and nuclear
up-regulation of telomerase activity. Mol Pharmacol. 67:572–581.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paine PL, Moore LC and Horowitz SB:
Nuclear envelope permeability. Nature. 254:109–114. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rostovtseva T and Colombini M: VDAC
channels mediate and gate the flow of ATP: Implications for the
regulation of mitochondrial function. Biophys J. 72:1954–1962.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colombini M, Yeung CL, Tung J and König T:
The mitochondrial outer membrane channel, VDAC, is regulated by a
synthetic polyanion. Biochim Biophys Acta. 905:279–286. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nobel PS: Mitochondrial permeability for
alcohols aldoses, and amino acids. J Membr Biol. 12:287–299. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Griffin RJ, Arris CE, Bleasdale C, Boyle
FT, Calvert AH, Curtin NJ, Dalby C, Kanugula S, Lembicz NK, Newell
DR, et al: Resistance-modifying agents. 8. Inhibition of
O(6)-alkylguanine-DNA alkyltransferase by O(6)-alkenyl-,
O(6)-cycloalkenyl-, and O(6)-(2-oxoalkyl)guanines and potentiation
of temozolomide cytotoxicity in vitro by
O(6)-(1-cyclopentenylmethyl)guanine. J Med Chem. 43:4071–4083.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Long L and Dolan ME: Role of cytochrome
P450 isoenzymes in metabolism of O(6)-benzylguanine: Implications
for dacarbazine activation. Clin Cancer Res. 7:4239–4244.
2001.PubMed/NCBI
|
|
30
|
Ortiz de and Montellano PR: Cytochrome
P450-activated prodrugs. Future Med Chem. 5:213–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meyer RP, Podvinec M and Meyer UA:
Cytochrome P450 CYP1A1 accumulates in the cytosol of kidney and
brain and is activated by heme. Mol Pharmacol. 62:1061–1067. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sangar MC, Anandatheerthavarada HK, Tang
W, Prabu SK, Martin MV, Dostalek M, Guengerich FP and Avadhani NG:
Human liver mitochondrial cytochrome P450 2D6 - individual
variations and implications in drug metabolism. FEBS J.
276:3440–3453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pletsa V, Valavanis C, van Delft JH,
Steenwinkel MJ and Kyrtopoulos SA: DNA damage and mutagenesis
induced by procarbazine in lambda lacZ transgenic mice, Evidence
that bone marrow mutations do not arise primarily through miscoding
by O6-methylguanine. Carcinogenesis. 18:2191–2196. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crook TR, Souhami RL and McLean AE:
Cytotoxicity, DNA cross-linking, and single strand breaks induced
by activated cyclophosphamide and acrolein in human leukemia cells.
Cancer Res. 46:5029–5034. 1986.PubMed/NCBI
|
|
35
|
Weber GF and Waxman DJ: Denitrosation of
the anti-cancer drug 1,3-bis(2-chloroethyl)-1-nitrosourea catalyzed
by microsomal glutathione S-transferase and cytochrome P450
monooxygenases. Arch Biochem Biophys. 307:369–378. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Linfoot PA, Gray JW, Dean PN, Marton LJ
and Deen DF: Effect of cell cycle position on the survival of 9L
cells treated with nitrosoureas that alkylate, cross-link, and
carbamoylate. Cancer Res. 46:2402–2406. 1986.PubMed/NCBI
|
|
37
|
Doroshenko N and Doroshenko P: The
glutathione reductase inhibitor carmustine induces an influx of
Ca2+ in PC12 cells. Eur J Pharmacol. 497:17–24. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kehrer JP: The effect of BCNU (carmustine)
on tissue glutathione reductase activity. Toxicol Lett. 17:63–68.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
FitzGerald GB, Bauman C, Hussoin MS and
Wick MM: 2,4-Dihydroxybenzylamine: A specific inhibitor of
glutathione reductase. Biochem Pharmacol. 41:185–190. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Babson JR and Reed DJ: Inactivation of
glutathione reductase by 2-chloroethyl nitrosourea-derived
isocyanates. Biochem Biophys Res Commun. 83:754–762. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bizzozero OA and Ziegler JL: DeJ esus G
and Bolognani F: Acute depletion of reduced glutathione causes
extensive carbonylation of rat brain proteins. J Neurosci Res.
83:656–667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Street JC, Mahmood U, Matei C and Koutcher
JA: In vivo and in vitro studies of cyclophosphamide chemotherapy
in a mouse mammary carcinoma by 31P NMR spectroscopy. NMR Biomed.
8:149–158. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Street JC and Koutcher JA: Effect of
radiotherapy and chemotherapy on composition of tumor membrane
phospholipids. Lipids. 32:45–49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jilani K and Lang F: Carmustine-induced
phosphatidylserine translocation in the erythrocyte membrane.
Toxins (Basel). 5:703–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Westphal M, Hilt DC, Bortey E, Delavault
P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J and Ram Z: A
phase 3 trial of local chemotherapy with biodegradable carmustine
(BCNU) wafers (Gliadel wafers) in patients with primary malignant
glioma. Neuro Oncol. 5:79–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lambros MP and Rahman YE: Effects of
cyclosporin A on model lipid membranes. Chem Phys Lipids.
131:63–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Birraux J, Kirby JA, Thomason JM and
Taylor JJ: The effect of cyclosporin on cell division and apoptosis
in human oral keratinocytes. J Periodontal Res. 41:297–302. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bokemeyer D, Kramer HJ and Meyer-Lehnert
H: Atrial natriuretic peptide blunts the cellular effects of
cyclosporine in smooth muscle. Hypertension. 21:166–172. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Raymond MA, Mollica L, Vigneault N,
Désormeaux A, Chan JS, Filep JG, Hébert MJ, et al: Blockade of the
apoptotic machinery by cyclosporin A redirects cell death toward
necrosis in arterial endothelial cells: regulation by reactive
oxygen species and cathepsin D. FASEB J. 17:515–517.
2003.PubMed/NCBI
|
|
50
|
Laursen M, Yatime L, Nissen P and Fedosova
NU: Crystal structure of the high-affinity
Na+K+-ATPase-ouabain complex with
Mg2+ bound in the cation binding site. Proc Natl Acad
Sci USA. 110:10958–10963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu J, Kesiry R, Periyasamy SM, Malhotra
D, Xie Z and Shapiro JI: Ouabain induces endocytosis of
plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent
mechanism. Kidney Int. 66:227–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suwalsky M, Hernandez P, Villena F and
Sotomayor CP: The anticancer drug chlorambucil interacts with the
human erythrocyte membrane and model phospholipid bilayers. Z
Naturforsch C. 54:1089–1095. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Krigel R, Liebes LF, Pelle E and Silber R:
Chlorambucil therapy in hairy cell leukemia, Effects on lipid
composition and lymphocyte subpopulations. Blood. 60:272–275.
1982.PubMed/NCBI
|
|
54
|
Matsura T, Kai M, Jiang J, Babu H, Kini V,
Kusumoto C, Yamada K and Kagan VE: Endogenously generated hydrogen
peroxide is required for execution of melphalan-induced apoptosis
as well as oxidation and externalization of phosphatidylserine.
Chem Res Toxicol. 17:685–696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tilby MJ, Lawley PD and Farmer PB:
Alkylation of DNA by melphalan in relation to immunoassay of
melphalan-DNA adducts, Characterization of mono-alkylated and
cross-linked products from reaction of melphalan with dGMP and GMP.
Chem Biol Interact. 73:183–194. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Van den Driessche B and Lemière F: VanD
ongen W and Esmans EL: Alkylation of DNA by melphalan:
Investigation of capillary liquid chromatography-electrospray
ionization tandem mass spectrometry in the study of the adducts at
the nucleoside level. J Chromatogr B Analyt Technol Biomed Life
Sci. 785:21–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rodriguez RJ and Acosta D Jr: Inhibition
of mitochondrial function in isolated rate liver mitochondria by
azole antifungals. J Biochem Toxicol. 11:127–131. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Maté MJ, Ortiz-Lombardía M, Boitel B,
Haouz A, Tello D, Susin SA, Penninger J, Kroemer G and Alzari PM:
The crystal structure of the mouse apoptosis-inducing factor AIF.
Nat Struct Biol. 9:442–446. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
59
|
Daugas E, Susin SA, Zamzami N, Ferri KF,
Irinopoulou T, Larochette N, Prévost MC, Leber B, Andrews D,
Penninger J, et al: Mitochondrio-nuclear translocation of AIF in
apoptosis and necrosis. FASEB J. 14:729–739. 2000.PubMed/NCBI
|
|
60
|
Lewis EM, Wilkinson AS, Davis NY, Horita
DA and Wilkinson JC: Nondegradative ubiquitination of apoptosis
inducing factor (AIF) by X-linked inhibitor of apoptosis at a
residue critical for AIF-mediated chromatin degradation.
Biochemistry. 50:11084–11096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wei Y, Fox T, Chambers SP, Sintchak J,
Coll JT, Golec JM, Swenson L, Wilson KP and Charifson PS: The
structures of caspases-1, −3, −7 and −8 reveal the basis for
substrate and inhibitor selectivity. Chem Biol. 7:423–432. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mitochondria - specificity in membrane targeting
for death. Biochim Biophys Acta. 1813:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cho Y, Gorina S, Jeffrey PD and Pavletich
NP: Crystal structure of a p53 tumor suppressor-DNA complex,
Understanding tumorigenic mutations. Science. 265:346–355. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Billen LP, Shamas-Din A and Andrews DW:
Bid A Bax-like BH3 protein. Oncogene. 27((Suppl 1)): S93–S104.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chipuk JE and Green DR: PUMA cooperates
with direct activator proteins to promote mitochondrial outer
membrane permeabilization and apoptosis. Cell Cycle. 8:2692–2696.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nakano K and Vousden KH: PUMA a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Loose DS, Kan PB, Hirst MA, Marcus RA and
Feldman D: Ketoconazole blocks adrenal steroidogenesis by
inhibiting cytochrome P450-dependent enzymes. J Clin Invest.
71:1495–1499. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Greenblatt DJ, Zhao Y, Venkatakrishnan K,
Duan SX, Harmatz JS, Parent SJ, Court MH and von Moltke LL:
Mechanism of cytochrome P450-3A inhibition by ketoconazole. J Pharm
Pharmacol. 63:214–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ho YS, Tsai PW, Yu CF, Liu HL, Chen RJ and
Lin JK: Ketoconazole-induced apoptosis through P53-dependent
pathway in human colorectal and hepatocellular carcinoma cell
lines. Toxicol Appl Pharmacol. 153:39–47. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang YJ, Jeng JH, Chen RJ, Tseng H, Chen
LC, Liang YC, Lin CH, Chen CH, Chu JS, Ho WL, et al: Ketoconazole
potentiates the antitumor effects of nocodazole: In vivo therapy
for human tumor xenografts in nude mice. Mol Carcinog. 34:199–210.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Pascual A, García I, Conejo C and Perea
EJ: Uptake and intracellular activity of fluconazole in human
polymorphonuclear leukocytes. Antimicrob Agents Chemother.
37:187–190. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ciccolini J, Fina F, Bezulier K,
Giacometti S, Roussel M, Evrard A, Cuq P, Romain S, Martin PM and
Aubert C: Transmission of apoptosis in human colorectal tumor cells
exposed to capecitabine, Xeloda, is mediated via Fas. Mol Cancer
Ther. 1:923–927. 2002.PubMed/NCBI
|
|
75
|
Baltch AL, Smith RP, Ritz WJ, Bopp LH and
Michelsen PB: Intracellular activity of voriconazole, fluconazole,
and itraconazole against Candida albicans in human monocytes
with and without activation by GM-CSF and TNF-alpha. J Appl Res.
5:42005.
|
|
76
|
Murphy JW, Cho Y, Sachpatzidis A, Fan C,
Hodsdon ME and Lolis E: Structural and functional basis of CXCL12
(stromal cell-derived factor-1 α) binding to heparin. J Biol Chem.
282:10018–10027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Debnath B, Xu S, Grande F, Garofalo A and
Neamati N: Small molecule inhibitors of CXCR4. Theranostics.
3:47–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hatse S, Princen K and Bridger G: DeC
lercq E and Schols D: Chemokine receptor inhibition by AMD3100 is
strictly confined to CXCR4. FEBS Lett. 527:255–262. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Pron G, Belehradek J Jr and Mir LM:
Identification of a plasma membrane protein that specifically binds
bleomycin. Biochem Biophys Res Commun. 194:333–337. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pron G, Belehradek J Jr, Orlowski S and
Mir LM: Involvement of membrane bleomycin-binding sites in
bleomycin cytotoxicity. Biochem Pharmacol. 48:301–310. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Krishan A and Whitlock S:
Bleomycin-induced fine structural alterations in cultured mouse
fibroblasts and human lymphocytes of neoplastic origin. Cancer Res.
33:777–785. 1973.PubMed/NCBI
|
|
82
|
Pron G, Mahrour N, Orlowski S, Tounekti O,
Poddevin B, Belehradek J Jr and Mir LM: Internalisation of the
bleomycin molecules responsible for bleomycin toxicity: A
receptor-mediated endocytosis mechanism. Biochem Pharmacol.
57:45–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ascierto ML, Kmieciak M, Idowu MO, Manjili
R, Zhao Y, Grimes M, Dumur C, Wang E, Ramakrishnan V, Wang XY, et
al: A signature of immune function genes associated with
recurrence-free survival in breast cancer patients. Breast Cancer
Res Treat. 131:871–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen L, Huang Z, Yao G, Lyu X, Li J, Hu X,
Cai Y, Li W, Li X and Ye C: The expression of CXCL13 and its
relation to unfavorable clinical characteristics in young breast
cancer. J Transl Med. 13:1682015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Stamatopoulos B, Meuleman N, De Bruyn C,
Pieters K, Mineur P, Le Roy C, Saint-Georges S, Varin-Blank N,
Cymbalista F, Bron D, et al: AMD3100 disrupts the cross-talk
between chronic lymphocytic leukemia cells and a mesenchymal
stromal or nurse-like cell-based microenvironment: Pre-clinical
evidence for its association with chronic lymphocytic leukemia
treatments. Haematologica. 97:608–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Chen LJ, Ye H, Zhang Q, Li FZ, Song LJ,
Yang J, Mu Q, Rao SS, Cai PC, Xiang F, et al: Bleomycin induced
epithelial-mesenchymal transition (EMT) in pleural mesothelial
cells. Toxicol Appl Pharmacol. 283:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wu Z, Yang L, Cai L, Zhang M, Cheng X,
Yang X and Xu J: Detection of epithelial to mesenchymal transition
in airways of a bleomycin induced pulmonary fibrosis model derived
from an alpha-smooth muscle actin-Cre transgenic mouse. Respir Res.
8:12007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yamada A, Aki T, Unuma K, Funakoshi T and
Uemura K: Paraquat induces epithelial-mesenchymal transition-like
cellular response resulting in fibrogenesis and the prevention of
apoptosis in human pulmonary epithelial cells. PLoS One.
10:e01201922015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dik WA, McAnulty RJ, Versnel MA, Naber BA,
Zimmermann LJ, Laurent GJ and Mutsaers SE: Short course
dexamethasone treatment following injury inhibits bleomycin induced
fibrosis in rats. Thorax. 58:765–771. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Domanska UM, Timmer-Bosscha H, Nagengast
WB, Munnink Oude TH, Kruizinga RC, Ananias HJ, Kliphuis NM, Huls G,
De Vries EG, de Jong IJ, et al: CXCR4 inhibition with AMD3100
sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia.
14:709–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ujwal R, Cascio D, Colletier JP, Faham S,
Zhang J, Toro L, Ping P and Abramson J: The crystal structure of
mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into
metabolite gating. Proc Natl Acad Sci USA. 105:17742–17747. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Catterall WA: Functional subunit structure
of voltage-gated calcium channels. Science. 253:1499–1500. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Barańska W, Kujawa M and Kujawska E:
Influence of vincristine on the Golgi apparatus in
preimplantation development of the mouse embryo. Gegenbaurs Morphol
Jahrb. 134:175–184. 1988.PubMed/NCBI
|
|
94
|
Kujawa M, Ochocka M and Moskalewski S:
Influence of vincristine on the Golgi complex of leukaemic
lymphoblasts. Folia Haematologica. 107:193–203. 1980.PubMed/NCBI
|
|
95
|
Carré M, André N, Carles G, Borghi H,
Brichese L, Briand C and Braguer D: Tubulin is an inherent
component of mitochondrial membranes that interacts with the
voltage-dependent anion channel. J Biol Chem. 277:33664–33669.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Groninger E, Meeuwsen-De Boer GJ, De Graaf
SS, Kamps WA and De Bont ES: Vincristine induced apoptosis in acute
lymphoblastic leukaemia cells: A mitochondrial controlled pathway
regulated by reactive oxygen species? Int J Oncol. 21:1339–1345.
2002.PubMed/NCBI
|
|
97
|
Eom YW, Kim MA, Park SS, Goo MJ, Kwon HJ,
Sohn S, Kim WH, Yoon G and Choi KS: Two distinct modes of cell
death induced by doxorubicin, Apoptosis and cell death through
mitotic catastrophe accompanied by senescence-like phenotype.
Oncogene. 24:4765–4777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Gamen S, Anel A, Lasierra P, Alava MA,
Martinez-Lorenzo MJ, Piñeiro A and Naval J: Doxorubicin-induced
apoptosis in human T-cell leukemia is mediated by caspase-3
activation in a Fas-independent way. FEBS Lett. 417:360–364. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kuznetsov AV, Margreiter R, Amberger A,
Saks V and Grimm M: Changes in mitochondrial redox state, membrane
potential and calcium precede mitochondrial dysfunction in
doxorubicin-induced cell death. Biochim Biophys Acta.
1813:1144–1152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Mizutani H, Tada-Oikawa S, Hiraku Y,
Kojima M and Kawanishi S: Mechanism of apoptosis induced by
doxorubicin through the generation of hydrogen peroxide. Life Sci.
76:1439–1453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang S, Konorev EA, Kotamraju S, Joseph J,
Kalivendi S and Kalyanaraman B: Doxorubicin induces apoptosis in
normal and tumor cells via distinctly different mechanisms.
Histopathology. J Biol Chem. 279:25535–25543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Golomb E, Hill MR, Brown RG and Keiser HR:
Ouabain enhances the mitogenic effect of serum in vascular smooth
muscle cells. Am J Hypertens. 7:69–74. 1994.PubMed/NCBI
|
|
103
|
Kanai R, Ogawa H, Vilsen B, Cornelius F
and Toyoshima C: Crystal structure of a Na+-bound
Na+, K+-ATPase preceding the E1P state.
Nature. 502:201–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Aller SG, Yu J, Ward A, Weng Y,
Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL,
et al: Structure of P-glycoprotein reveals a molecular basis for
poly-specific drug binding. Science. 323:1718–1722. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sehested M, Jensen PB, Skovsgaard T,
Bindslev N, Demant EJ, Friche E and Vindeløv L: Inhibition of
vincristine binding to plasma membrane vesicles from
daunorubicin-resistant Ehrlich ascites cells by multidrug
resistance modulators. Br J Cancer. 60:809–814. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Srivalli KMR and Lakshmi P: Overview of
P-glycoprotein inhibitors: A rational outlook. Braz J Pharm Sci.
48:353–367. 2012. View Article : Google Scholar
|
|
107
|
Chen C, Ke J, Zhou XE, Yi W, Brunzelle JS,
Li J, Yong EL, Xu HE and Melcher K: Structural basis for molecular
recognition of folic acid by folate receptors. Nature. 500:486–489.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Nandini-Kishore SG and Frazier WA:
[3H]Methotrexate as a ligand for the folate receptor of
Dictyostelium discoideum. Proc Natl Acad Sci USA.
78:7299–7303. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Herman S, Zurgil N and Deutsch M: Low dose
methotrexate induces apoptosis with reactive oxygen species
involvement in T lymphocytic cell lines to a greater extent than in
monocytic lines. Inflamm Res. 54:273–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ramadan AA, Yousif WB and Ali AM: The
effect of methotrexate (MTX) on the small intestine of the mouse.
IV. The Golgi apparatus, phosphatases and esterases. Funct
Dev Morphol. 2:111–119. 1992.PubMed/NCBI
|
|
111
|
Ramadan AA, Yousif WB and Ali AM: The
effect of methotrexate (MTX) on the small intestine of the mouse.
Histopathology. Funct Dev Morphol. 2:3–9. 1992.PubMed/NCBI
|
|
112
|
Pritchard DM, Bower L, Potten CS, Jackman
AL and Hickman JA: The importance of p53-independent apoptosis in
the intestinal toxicity induced by raltitrexed (ZD1694, Tomudex):
genetic differences between BALB/c and DBA/2 mice. Clin Cancer Res.
6:4389–4395. 2000.PubMed/NCBI
|
|
113
|
Xue S, Chen YX, Qin SK, Yang AZ, Wang L,
Xu HJ and Geng HY: Raltitrexed induces mitochondrial mediated
apoptosis in SGC7901 human gastric cancer cells. Mol Med Rep.
10:1927–1934. 2014.PubMed/NCBI
|
|
114
|
Chattopadhyay S, Moran RG and Goldman ID:
Pemetrexed Biochemical and cellular pharmacology, mechanisms, and
clinical applications. Mol Cancer Ther. 6:404–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Fischer CD: daC osta M and Rothenberg SP:
The heterogeneity and properties of folate binding proteins from
chronic myelogenous leukemia cells. Blood. 46:855–867.
1975.PubMed/NCBI
|
|
116
|
Fischer CD: DaC osta M and Rothenberg SP:
Properties of purified folate-binding proteins from chronic
myelogenous leukemia cells. Biochim Biophys Acta. 543:328–339.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Barford PA, Blair JA and Malghani MA: The
effect of methotrexate on folate metabolism in the rat. Br J
Cancer. 41:816–820. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ohbayashi M, Kubota S, Kawase A, Kohyama
N, Kobayashi Y and Yamamoto T: Involvement of
epithelial-mesenchymal transition in methotrexate-induced pulmonary
fibrosis. J Toxicol Sci. 39:319–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shprung T and Gozes I: A novel method for
analyzing mitochondrial movement: inhibition by paclitaxel in a
pheochromocytoma cell model. J Mol Neurosci. 37:254–262. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Foland TB, Dentler WL, Suprenant KA, Gupta
ML Jr and Himes RH: Paclitaxel-induced microtubule stabilization
causes mitotic block and apoptotic-like cell death in a
paclitaxel-sensitive strain of Saccharomyces cerevisiae.
Yeast. 22:971–978. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Salido M, Gonzalez JL and Vilches J: Loss
of mitochondrial membrane potential is inhibited by bombesin in
etoposide-induced apoptosis in PC-3 prostate carcinoma cells. Mol
Cancer Ther. 6:1292–1299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mizukami S, Kikuchi K, Higuchi T, Urano Y,
Mashima T, Tsuruo T and Nagano T: Imaging of caspase-3 activation
in HeLa cells stimulated with etoposide using a novel fluorescent
probe. FEBS Lett. 453:356–360. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Matsushima Y, Kanzawa F, Miyazawa N,
Sasaki Y and Saijo N: In vitro antitumor activity of teniposide
against carcinoma of the lung in human tumor clonogenic assay.
Anticancer Res. 6:921–924. 1986.PubMed/NCBI
|
|
124
|
Sánchez-Alcázar JA, Khodjakov A and
Schneider E: Anticancer drugs induce increased mitochondrial
cytochrome c expression that precedes cell death. Cancer
Res. 61:1038–1044. 2001.PubMed/NCBI
|
|
125
|
Uyar D, Takigawa N, Mekhail T, Grabowski
D, Markman M, Lee F, Canetta R, Peck R, Bukowski R and Ganapathi R:
Apoptotic pathways of epothilone BMS 310705. Gynecol Oncol.
91:173–178. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Thomson AER and Robinson MA: Cytocidal
action of colchicine in vitro on lymphocytes in chronic lymphocytic
leukaemia. Lancet. 2:868–870. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chen XM, Liu J, Wang T and Shang J:
Colchicine-induced apoptosis in human normal liver L-02 cells by
mitochondrial mediated pathways. Toxicol In Vitro. 26:649–655.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Jelínek M, Balušíková K, Schmiedlová M,
Němcová-Fürstová V, Šrámek J, Stančíková J, Zanardi I, Ojima I and
Kovář J: The role of individual caspases in cell death induction by
taxanes in breast cancer cells. Cancer Cell Int. 15:82015.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
André N, Braguer D, Brasseur G, Gonçalves
A, Lemesle-Meunier D, Guise S, Jordan MA and Briand C: Paclitaxel
induces release of cytochrome c from mitochondria isolated
from human neuroblastoma cells'. Cancer Res. 60:5349–5353.
2000.PubMed/NCBI
|
|
130
|
Khawaja NR, Carré M, Kovacic H, Estève MA
and Braguer D: Patupilone-induced apoptosis is mediated by
mitochondrial reactive oxygen species through Bim relocalization to
mitochondria. Mol Pharmacol. 74:1072–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Salim AA, Xiao X, Cho KJ, Piggott AM,
Lacey E, Hancock JF and Capon RJ: Rare Streptomyces sp.
polyketides as modulators of K-Ras localisation. Org Biomol Chem.
12:4872–4878. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Pusceddu S, Indini A and Procopio G:
Everolimus treatment in advanced solid tumors: a personal view.
Future Science. 2015.OA March 20, (Epub ahead of print)
doi:10.4155/fso.14.1. View Article : Google Scholar
|
|
133
|
Chambraud B, Belabes H, Fontaine-Lenoir V,
Fellous A and Baulieu EE: The immunophilin FKBP52 specifically
binds to tubulin and prevents microtubule formation. FASEB J.
21:2787–2797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Shirane M and Nakayama KI: Inherent
calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and
inhibits apoptosis. Nat Cell Biol. 5:28–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tanaka K, Fujita N, Higashi Y and Ogawa N:
Neuroprotective and antioxidant properties of FKBP-binding
immunophilin ligands are independent on the FKBP12 pathway in human
cells. Neurosci Lett. 330:147–150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Simon N, Morin C, Urien S, Tillement JP
and Bruguerolle B: Tacrolimus and sirolimus decrease oxidative
phosphorylation of isolated rat kidney mitochondria. Br J
Pharmacol. 138:369–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zini R, Simon N, Morin C, Thiault L and
Tillement JP: Tacrolimus decreases in vitro oxidative
phosphorylation of mitochondria from rat forebrain. Life Sci.
63:357–368. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zoli W, Ulivi P, Tesei A, Fabbri F,
Rosetti M, Maltoni R, Giunchi DC, Ricotti L, Brigliadori G, Vannini
I, et al: Addition of 5-fluorouracil to doxorubicin-paclitaxel
sequence increases caspase-dependent apoptosis in breast cancer
cell lines. Breast Cancer Res. 7:R681–R689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yang Y, Niu X, Zhang Q, Hao L, Ding Y and
Xu H: The efficacy of abraxane on osteosarcoma xenografts in nude
mice and expression of secreted protein, acidic and rich in
cysteine. Am J Med Sci. 344:199–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Saif MW: U.S. Food and Drug Administration
approves paclitaxel protein-bound particles (Abraxane®) in
combination with gemcitabine as first-line treatment of patients
with metastatic pancreatic cancer. JOP. 14:686–688. 2013.PubMed/NCBI
|
|
141
|
Coward P, Lee D, Hull MV and Lehmann JM:
4-Hydroxytamoxifen binds to and deactivates the estrogen-related
receptor gamma. Proc Natl Acad Sci USA. 98:8880–8884. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Richards J, Lim AC, Hay CW, Taylor AE,
Wingate A, Nowakowska K, Pezaro C, Carreira S, Goodall J, Arlt W,
et al: Interactions of abiraterone, eplerenone, and prednisolone
with wild-type and mutant androgen receptor: A rationale for
increasing abiraterone exposure or combining with MDV3100. Cancer
Res. 72:2176–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Darbre PD and King RJB: Differential
effects of steroid hormones on parameters of cell growth. Cancer
Res. 47:2937–2944. 1987.PubMed/NCBI
|
|
144
|
Yates J and King RJB: Correlation of
growth properties and morphology with hormone responsiveness of
mammary tumor cells in culture. Cancer Res. 41:258–262.
1981.PubMed/NCBI
|
|
145
|
Talwar GP, Raina K, Gupta JC, Ray R,
Wadhwa S and Ali MM: A recombinant luteinising-hormone-releasing-
hormone immunogen bioeffective in causing prostatic atrophy.
Vaccine. 22:3713–3721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Brandes AA, Ermani M, Turazzi S, Scelzi E,
Berti F, Amistà P, Rotilio A, Licata C and Fiorentino MV:
Procarbazine and high-dose tamoxifen as a second-line regimen in
recurrent high-grade gliomas, A phase II study. J Clin Oncol.
17:645–650. 1999.PubMed/NCBI
|
|
147
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: COU-AA-301 Investigators: Abiraterone and increased survival
in metastatic prostate cancer. N Engl J Med. 364:1995–2005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kosaka T, Miyajima A, Yasumizu Y, Miyazaki
Y, Kikuchi E and Oya M: Limited in vitro efficacy of CYP17A1
inhibition on human castration resistant prostate cancer. Steroids.
92:39–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Louderbough JMV, Lopez JI and Schroeder
JA: Matrix hyaluronan alters epidermal growth factor
receptor-dependent cell morphology. Cell Adhes Migr. 4:26–31. 2010.
View Article : Google Scholar
|
|
150
|
Hara F, Aoe M, Doihara H, Taira N, Shien
T, Takahashi H, Yoshitomi S, Tsukuda K, Toyooka S, Ohta T, et al:
Antitumor effect of gefitinib ('Iressa') on esophageal squamous
cell carcinoma cell lines in vitro and in vivo. Cancer Lett.
226:37–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Stegmaier K, Corsello SM, Ross KN, Wong
JS, Deangelo DJ and Golub TR: Gefitinib induces myeloid
differentiation of acute myeloid leukemia. Blood. 106:2841–2848.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Augustin A, Lamerz J, Meistermann H,
Golling S, Scheiblich S, Hermann JC, Duchateau-Nguyen G, Tzouros M,
Avila DW, Langen H, et al: Quantitative chemical proteomics
profiling differentiates erlotinib from gefitinib in EGFR wild-type
non-small cell lung carcinoma cell lines. Mol Cancer Ther.
12:520–529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Huang HL, Chen YC, Huang YC, Yang KC, Pan
H, Shih SP and Chen YJ: Lapatinib induces autophagy, apoptosis and
megakaryocytic differentiation in chronic myelogenous leukemia K562
cells. PLoS One. 6:e290142011. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Diaz R, Nguewa PA, Parrondo R,
Perez-Stable C, Manrique I, Redrado M, Catena R, Collantes M,
Peñuelas I, Díaz-González JA, et al: Antitumor and antiangiogenic
effect of the dual EGFR and HER-2 tyrosine kinase inhibitor
lapatinib in a lung cancer model. BMC Cancer. 10:1882010.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Chen G, Noor A, Kronenberger P, Teugels E,
Umelo IA and De Grève J: Synergistic effect of afatinib with
su11274 in non-small cell lung cancer cells resistant to gefitinib
or erlotinib. PLoS One. 8:e597082013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Solca F, Dahl G, Zoephel A, Bader G,
Sanderson M, Klein C, Kraemer O, Himmelsbach F, Haaksma E and Adolf
GR: Target binding properties and cellular activity of afatinib
(BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp
Ther. 343:342–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Stoica GE, Kuo A, Powers C, Bowden ET,
Sale EB, Riegel AT and Wellstein A: Midkine binds to anaplastic
lymphoma kinase (ALK) and acts as a growth factor for different
cell types. J Biol Chem. 277:35990–35998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Sumi Y, Muramatsu H, Hata K, Ueda M and
Muramatsu T: Midkine enhances early stages of collagen gel
contraction. J Biochem. 127:247–251. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Kadomatsu K and Muramatsu T: Midkine and
pleiotrophin in neural development and cancer. Cancer Lett.
204:127–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Friboulet L, Li N, Katayama R, Lee CC,
Gainor JF, Crystal AS, Michellys PY, Awad MM, Yanagitani N, Kim S,
et al: The ALK inhibitor ceritinib overcomes crizotinib resistance
in non-small cell lung cancer. Cancer Discov. 4:662–673. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lisignoli G, Toneguzzi S, Piacentini A,
Cristino S, Grassi F, Cavallo C and Facchini A: CXCL12 (SDF-1) and
CXCL13 (BCA-1) chemokines significantly induce proliferation and
collagen type I expression in osteoblasts from osteoarthritis
patients. J Cell Physiol. 206:78–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Kalinina OV, Pfeifer N and Lengauer T:
Modelling binding between CCR5 and CXCR4 receptors and their
ligands suggests the surface electrostatic potential of the
co-receptor to be a key player in the HIV-1 tropism. Retrovirology.
10:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Massarelli I, Chiellini F, Chiellini E and
Bianucci AM: Three-dimensional models of the oligomeric human
asialoglycoprotein receptor (ASGP-R). Int J Mol Sci. 11:3867–3884.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Fallon RJ and Danaher M: The effect of
staurosporine a protein kinase inhibitor, on asialoglycoprotein
receptor endocytosis. Exp Cell Res. 203:420–426. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Trerè D, Fiume L, De Giorgi LB, Di Stefano
G, Migaldi M and Derenzini M: The asialoglycoprotein receptor in
human hepatocellular carcinomas: Its expression on proliferating
cells. Br J Cancer. 81:404–408. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Becherer U, Guatimosim C and Betz W:
Effects of staurosporine on exocytosis and endocytosis at frog
motor nerve terminals. J Neurosci. 21:782–787. 2001.PubMed/NCBI
|
|
167
|
Belmokhtar CA, Hillion J and
Ségal-Bendirdjian E: Staurosporine induces apoptosis through both
caspase-dependent and caspase-independent mechanisms. Oncogene.
20:3354–3362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zhang XD, Gillespie SK and Hersey P:
Staurosporine induces apoptosis of melanoma by both
caspase-dependent and -independent apoptotic pathways. Mol Cancer
Ther. 3:187–197. 2004.PubMed/NCBI
|
|
169
|
Dunai ZA, Imre G, Barna G, Korcsmaros T,
Petak I, Bauer PI and Mihalik R: Staurosporine induces necroptotic
cell death under caspase-compromised conditions in U937 cells. PLoS
One. 7:e419452012. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Chan KC, Knox WF, Gee JM, Morris J,
Nicholson RI, Potten CS and Bundred NJ: Effect of epidermal growth
factor receptor tyrosine kinase inhibition on epithelial
proliferation in normal and premalignant breast. Cancer Res.
62:122–128. 2002.PubMed/NCBI
|
|
171
|
Maity A, Pore N, Lee J, Solomon D and
O'Rourke DM: Epidermal growth factor receptor transcriptionally
up-regulates vascular endothelial growth factor expression in human
glioblastoma cells via a pathway involving phosphatidylinositol
3-kinase and distinct from that induced by hypoxia. Cancer Res.
60:5879–5886. 2000.PubMed/NCBI
|
|
172
|
Ouchi T, Monteiro ANA, August A, Aaronson
SA and Hanafusa H: BRCA1 regulates p53-dependent gene expression.
Proc Natl Acad Sci USA. 95:2302–2306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Arizti P, Fang L, Park I, Yin Y, Solomon
E, Ouchi T, Aaronson SA and Lee SW: Tumor suppressor p53 is
required to modulate BRCA1 expression. Mol Cell Biol. 20:7450–7459.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Andreassen A, Øyjord T, Hovig E, Holm R,
Flørenes VA, Nesland JM, Myklebost O, Høie J, Bruland OS, Børresen
AL, et al: p53 abnormalities in different subtypes of human
sarcomas. Cancer Res. 53:468–471. 1993.PubMed/NCBI
|
|
175
|
O'Hare T, Pollock R, Stoffregen EP, Keats
JA, Abdullah OM, Moseson EM, Rivera VM, Tang H, Metcalf CA III,
Bohacek RS, et al: Inhibition of wild-type and mutant Bcr-Abl by
AP23464, a potent ATP-based oncogenic protein kinase inhibitor:
Implications for CML. Blood. 104:2532–2539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Grisolano JL, O'Neal J, Cain J and
Tomasson MH: An activated receptor tyrosine kinase, TEL/PDGFbetaR,
cooperates with AML1/ETO to induce acute myeloid leukemia in mice.
Proc Natl Acad Sci USA. 100:9506–9511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Aoki M, Nabeshima K, Koga K, Hamasaki M,
Suzumiya J, Tamura K and Iwasaki H: Imatinib mesylate inhibits cell
invasion of malignant peripheral nerve sheath tumor induced by
platelet-derived growth factor-BB. Lab Invest. 87:767–779. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Hercus TR, Thomas D, Guthridge MA, Ekert
PG, King-Scott J, Parker MW and Lopez AF: The
granulocyte-macrophage colony-stimulating factor receptor, linking
its structure to cell signaling and its role in disease. Blood.
114:1289–1298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Ding W, Shanafelt TD, Lesnick CE,
Erlichman C, Leis JF, Secreto C, Sassoon TR, Call TG, Bowen DA,
Conte M, et al: Akt inhibitor MK2206 selectively targets CLL B-cell
receptor induced cytokines, mobilizes lymphocytes and synergizes
with bendamustine to induce CLL apoptosis. Br J Haematol.
164:146–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Zhao Y-Y, Tian Y, Zhang J, Xu F, Yang YP,
Huang Y, Zhao HY, Zhang JW, Xue C, Lam MH, et al: Effects of an
oral allosteric AKT inhibitor (MK-2206) on human nasopharyngeal
cancer in vitro and in vivo. Drug Des Devel Ther. 8:1827–1837.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Agarwal E, Chaudhuri A, Leiphrakpam PD,
Haferbier KL, Brattain MG and Chowdhury S: Akt inhibitor MK-2206
promotes anti-tumor activity and cell death by modulation of AIF
and Ezrin in colorectal cancer. BMC Cancer. 14:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Fu YR, Yi ZJ, Yan YR and Qiu ZY:
Hydroxycamptothecin-induced apoptosis in hepatoma SMMC-7721 cells
and the role of mitochondrial pathway. Mitochondrion. 6:211–217.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Uckun FM, Stewart CF, Reaman G, Chelstrom
LM, Jin J, Chandan-Langlie M, Waddick KG, White J and Evans WE: In
vitro and in vivo activity of topotecan against human B-lineage
acute lymphoblastic leukemia cells. Blood. 85:2817–2828.
1995.PubMed/NCBI
|
|
184
|
Caserini C, Pratesi G, Tortoreto M,
Bedogné B, Carenini N, Supino R, Perego P, Righetti SC and Zunino
F: Apoptosis as a determinant of tumor sensitivity to topotecan in
human ovarian tumors, preclinical in vitro/in vivo studies. Clin
Cancer Res. 3:955–961. 1997.PubMed/NCBI
|
|
185
|
Kim MK, James J and Annunziata CM:
Topotecan synergizes with CHEK1 (CHK1) inhibitor to induce
apoptosis in ovarian cancer cells. BMC Cancer. 15:1962015.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Tolis C, Peters GJ, Ferreira CG, Pinedo HM
and Giaccone G: Cell cycle disturbances and apoptosis induced by
topotecan and gemcitabine on human lung cancer cell lines. Eur J
Cancer. 35:796–807. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Szalay K, Rázga Z and Duda E: TNF inhibits
myogenesis and downregulates the expression of myogenic regulatory
factors myoD and myogenin. Eur J Cell Biol. 74:391–398.
1997.PubMed/NCBI
|
|
188
|
Fiers W, Beyaert R, Brouckaert P,
Everaerdt B, Haegeman C, Suffys P, Tavernier J and Vanhaesebroeck
B: TNF Its potential as an antitumour agent. Dev Biol Stand.
69:143–151. 1988.PubMed/NCBI
|
|
189
|
Smith RA and Baglioni C: The active form
of tumor necrosis factor is a trimer. J Biol Chem. 262:6951–6954.
1987.PubMed/NCBI
|
|
190
|
Udagawa N, Takahashi N, Jimi E, Matsuzaki
K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E, et
al: Osteoblasts/stromal cells stimulate osteoclast activation
through expression of osteoclast differentiation factor/RANKL but
not macrophage colony-stimulating factor: Receptor activator of
NF-kappa B ligand. Bone. 25:517–523. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Luan X, Lu Q, Jiang Y, Zhang S, Wang Q,
Yuan H, Zhao W, Wang J and Wang X: Crystal structure of human RANKL
complexed with its decoy receptor osteoprotegerin. J Immunol.
189:245–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Ciusani E, Croci D, Gelati M, Calatozzolo
C, Sciacca F, Fumagalli L, Balzarotti M, Fariselli L, Boiardi A and
Salmaggi A: In vitro effects of topotecan and ionizing radiation on
TRAIL/Apo2L-mediated apoptosis in malignant glioma. J Neurooncol.
71:19–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Chen S, Gomez SP, McCarley D and
Mainwaring MG: Topotecan-induced topoisomerase IIalpha expression
increases the sensitivity of the CML cell line K562 to subsequent
etoposide plus mitoxantrone treatment. Cancer Chemother Pharmacol.
49:347–355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Naldini L, Weidner KM, Vigna E, Gaudino G,
Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R,
Michalopoulos GK, et al: Scatter factor and hepatocyte growth
factor are indistinguishable ligands for the MET receptor. EMBO J.
10:2867–2878. 1991.PubMed/NCBI
|
|
195
|
Stamos J, Lazarus RA, Yao X, Kirchhofer D
and Wiesmann C: Crystal structure of the HGF beta-chain in complex
with the Sema domain of the Met receptor. EMBO J. 23:2325–2335.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Paulson AK, Linklater ES, Berghuis BD, App
CA, Oostendorp LD, Paulson JE, Pettinga JE and Melnik MK: VandeW
oude GF and Graveel CR: MET and ERBB2 are coexpressed in
ERBB2+ breast cancer and contribute to innate
resistance. Mol Cancer Res. 11:1112–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Xie Q, Su Y, Dykema K, Johnson J, Koeman
J, De Giorgi V, Huang A, Schlegel R, Essenburg C, Kang L, et al:
Overexpression of HGF promotes HBV-induced hepatocellular carcinoma
progression and is an effective indicator for Met-targeting
therapy. Genes Cancer. 4:247–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Simonetti S, Molina MA, Queralt C, de
Aguirre I, Mayo C, Bertran-Alamillo J, Sanchez JJ, Gonzalez-Larriba
JL, Jimenez U, Isla D, et al: Detection of EGFR mutations with
mutation-specific antibodies in stage IV non-small-cell lung
cancer. J Transl Med. 8:1352010. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Maseki S, Ijichi K, Tanaka H, Fujii M,
Hasegawa Y, Ogawa T, Murakami S, Kondo E and Nakanishi H:
Acquisition of EMT phenotype in the gefitinib-resistant cells of a
head and neck squamous cell carcinoma cell line through
Akt/GSK-3β/snail signalling pathway. Br J Cancer. 106:1196–1204.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Stahtea XN, Roussidis AE, Kanakis I,
Tzanakakis GN, Chalkiadakis G, Mavroudis D, Kletsas D and Karamanos
NK: Imatinib inhibits colorectal cancer cell growth and suppresses
stromal-induced growth stimulation, MT1-MMP expression and pro-MMP2
activation. Int J Cancer. 121:2808–2814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Hirai H, Sootome H, Nakatsuru Y, Miyama K,
Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, et al:
MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy
by standard chemotherapeutic agents or molecular targeted drugs in
vitro and in vivo. Mol Cancer Ther. 9:1956–1967. 2010. View Article : Google Scholar : PubMed/NCBI
|