Introduction
Urachal carcinoma is a rare non-urothelial
carcinoma, accounting for 0.01% of all malignancies and for
0.17–0.34% of all bladder tumors (1,2). Urachal
carcinoma is more common among men, and the majority of the
patients are aged >50 years (3).
Although hematuria is the most common symptom, the disease is
usually advanced when this symptom appears (4). As urachal carcinoma frequently invades
the bladder at the dome or elsewhere along its midline, which is
not easily detected during the early stages, it is often discovered
late (5). Furthermore, there is
currently no effective treatment for urachal carcinoma, leading to
a poor prognosis. We herein report the cases of two patients with
urachal carcinoma and urachal mucinous carcinoma. The relevant
literature was also reviewed in order to help improve the diagnosis
and treatment of this rare disease.
Case reports
Case 1
The patient was a 32-year-old man who noted painless
hematuria with blood clots for 1 month. There were no obvious
precipitating or alleviating factors. On physical examination,
there were no positive physical findings and the patient
experienced no other discomfort. The results of the laboratory and
imaging examinations (hemogram, urinalysis, prothrombin time,
activated partial thromboplastin time, liver and kidney function
tests and chest X-ray) were normal. However, urinary tract
ultrasonography revealed an abnormal mixed signal at the bottom of
the bladder. On further cystoscopy, only a small blood clot was
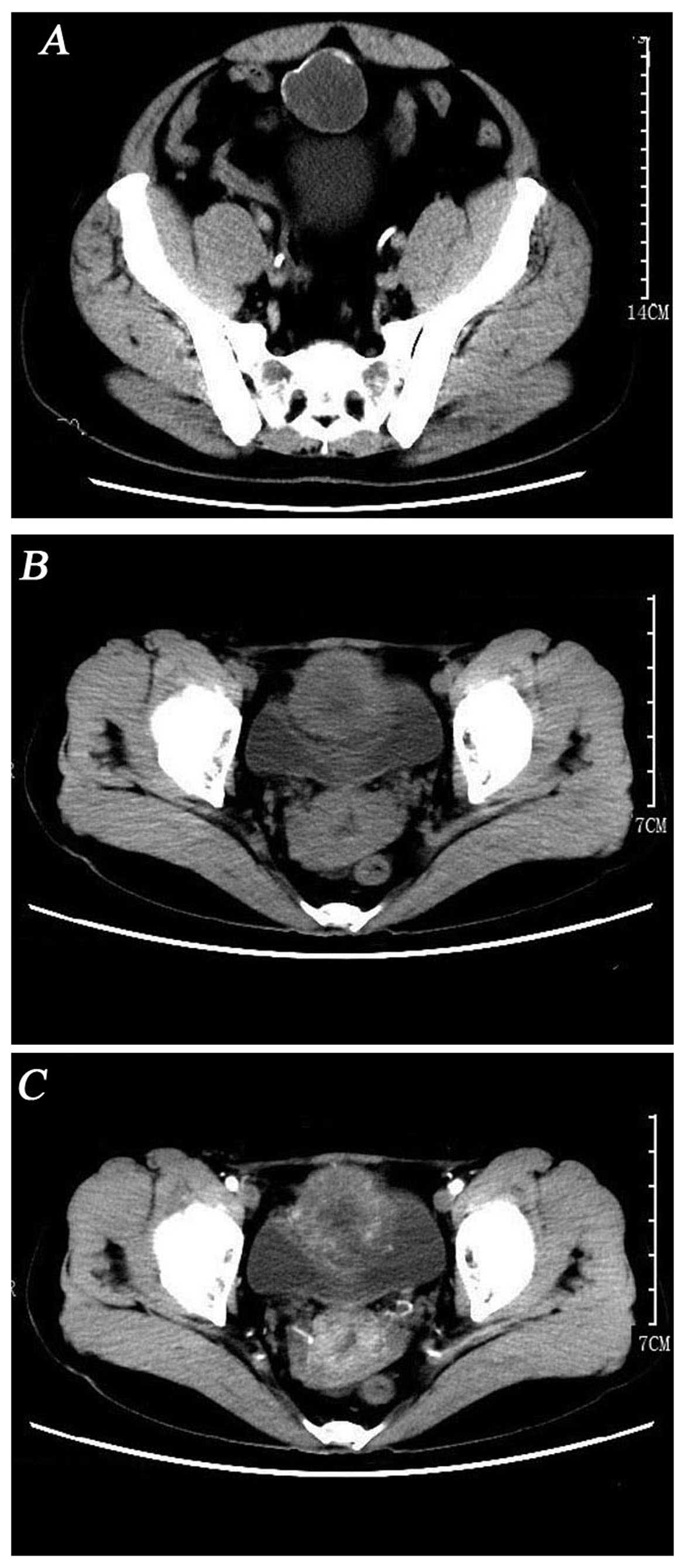
detected. However, computed tomography (CT) revealed a circular
hypodense mass located at the top and anterior part of the bladder,
sized ~44×43 mm, without enlarged lymph nodes or other sign of
metastatic disease (Fig. 1A).
Following the doctors' recommendation, the patient
consented to radical resection of the urachal tumor and partial
cystectomy in August, 2015. We found that the lower part of the
tumor was connected to the anterior wall of the bladder, whereas
the upper part of the tumor was connected with the umbilical
region. The tumor was sized ~7×8 cm. On gross pathological
examination, the resected specimen was grey-yellow and gray-red,
with a diameter of 6 cm. A small area covered by mucosa was
identified on the surface, sized ~2.6×1.8 cm. On cross-section, the
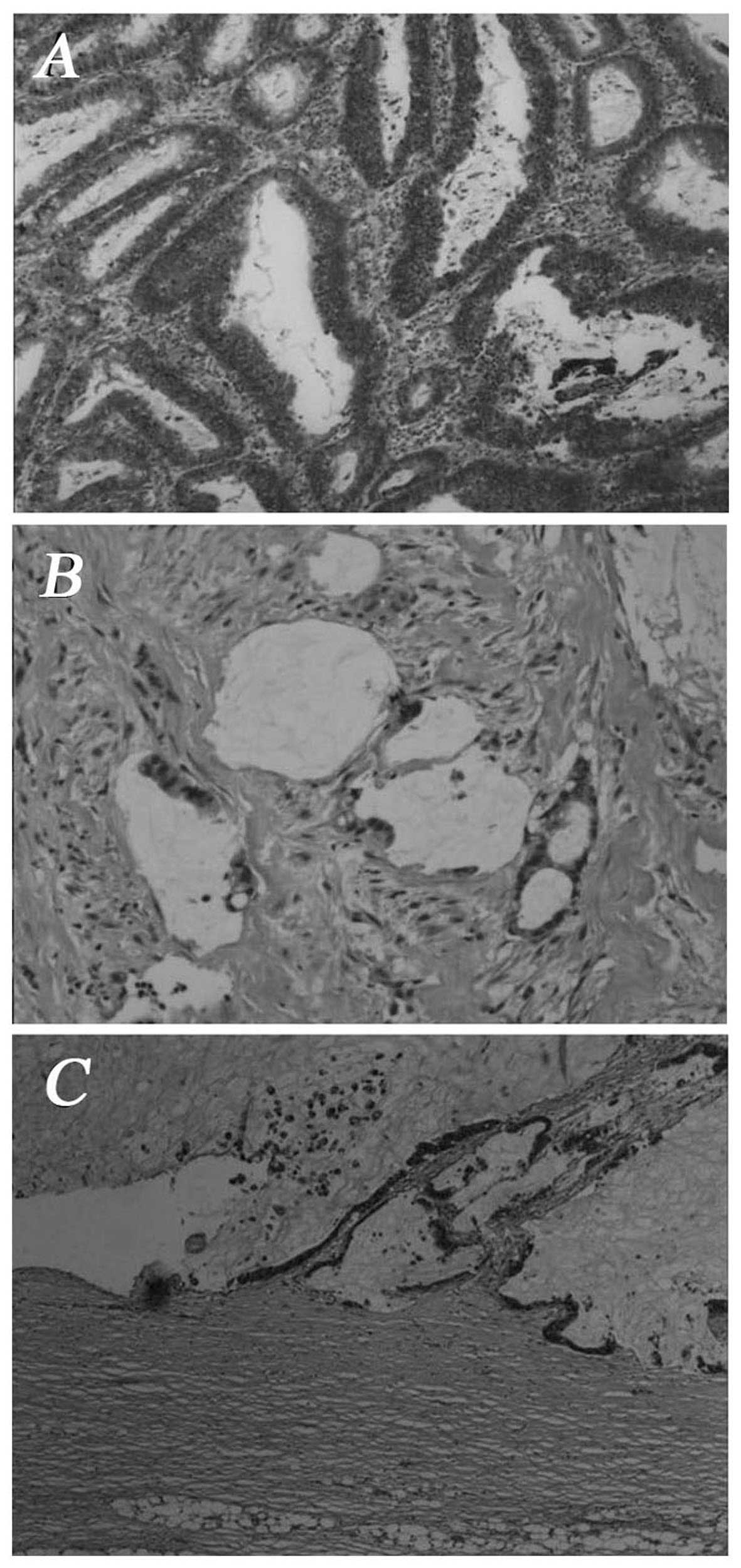
tumor included two jelly-like nodules; on microscopic examination,
the nodules were cystides containing mucus; goblet cells were
visible. Adenoid tumor cell formations were observed invading the
smooth muscle of the bladder wall. The carcinoma tissue displayed
tubular and alveolar cell structures (Fig. 2A and B). The pathological diagnosis
was mixed-type urachal adenocarcinoma. Postoperatively, the patient
recovered well. After 10 months of follow-up, the patient has no
evidence of recurrence on laboratory and imaging examinations.
Informed consent was obtained from the patient.
Case 2
The patient was a 50-year-old woman who presented
with gross hematuria with mild dysuria, urgency and frequent
urination for 1 year. There was no other discomfort or obvious
change. The patient had not received any treatment. The urinary
tract ultrasonography revealed an abnormal echo in the bladder and
routine urinalysis was positive for occult blood. Further magnetic
resonance imaging revealed extensive thickening of the bladder wall
and the possibility of malignant tumor of the bladder was
considered. On cystoscopy, an irregular mass with eroded surface,
sized ~6×5 cm, was identified at the top and anterior wall of the
bladder. Following admission, blood routine examination
(hemoglobin, 83g/l), urine routine examination (white blood cell
count, 665.3/µl; red blood cell count, 4,524.8/µl) and measurement
of tumor marker levels (carbohydrate antigen 19-9, 67.05 U/l;
carcinoembryonic antigen, 7.46 ng/ml; pepsinogen I, 91.6 ng/ml)
were performed. The findings on 3D-computed tomographyurography
indicated that the cystic mass of the bladder wall was a malignant
tumor originating in the bladder or urachus (Fig. 1B and C).
Following correction of the anemia, the patient
consented to tumor resection and pelvic lymph node dissection in
January, 2016. Intraoperatively, the tumor was located at the top
of the bladder, was sized ~6×6 cm and had a cauliflower-like
appearance. Grossly, the resected specimen was sized ~8.9×8.5×5.8
cm and included a mass 7.5×6.5×1.9 cm, solid, poorly circumscribed,
gray-red and jelly-like, surrounded by grayish mucosa. On
microscopic examination, the tumor was composed of neoplastic
cells, single or arranged in tubular and alveolar formations. The
neoplastic cells exhibited heteromorphism and invasive growth
(Fig. 2C). The pathological
diagnosis was urachal mucinous adenocarcinoma. Postoperatively, the
patient recovered well. After 5 months of follow-up, the patient
has no evidence of recurrence on laboratory and imaging
examinations. Informed consent was obtained from the patient.
Discussion
The urachus is a canal between the allantois and the
early fetal bladder. With the development of the fetus, the urachal
lumen progressively disappears, but there remains a small
fibromuscular cord connecting the dome of the bladder to the
umbilicus, referred to as the median umbilical ligament. There are
three distinct layers, an outer smooth muscle layer, an
intermediate submucosal connective tissue layer and an inner
luminal layer (6). The cells of
these three layers, particularly the epithelial cells, may give
rise to urachal carcinoma (3).
Primary urachal adenocarcinoma is a rare tumor,
first described by Hue and Jacquin in 1863 (7). Approximately 70% of urachal
adenocarcinomas are mucin-producing tumors and exhibit
calcifications (4). Although
hematuria is the most common symptom, the disease is usually
advanced when this symptom appears. The common metastatic sites
include the lymph nodes, peritoneum and lung. The urachal remnant
from the bladder apex tumor towards the umbilicus is not always
identified, but it is a crucial finding establishing the diagnosis.
Thali-Schwab et al analyzed the results of 25 CT
examinations of urachal adenocarcinomas and reported that
calcification is the characteristic sign of urachal adenocarcinoma,
particularly urachal mucinous adenocarcinoma (8). In the cases reported herein,
calcifications were also present.
Urachal carcinomas were divided into five
histological subtypes by Grignon et al (9) in 1991 as follows: Intestinal, mucinous,
signet ring cell and mixed types (Table
I). Molina et al conducted a retrospective study on
urachal carcinoma including 49 patients and found that 89% were
adenocarcinomas, whereas sarcomas and transitional cell carcinomas
represented ~4%. Of the adenocarcinomas, 63.6% may produce mucin
(10). The result is similar to
those of Paner et al (11).
Furthermore, Paner et al also reported that 85% of urachal
adenocarcinomas express CDK2 and 50% express cytokeratin 7. In
addition, they hypothesized that the expression of the Reg IV
protein is associated with the production of mucin, as it is often
observed in the mucinous and signet ring cell subtypes, as well as
focally in the enteric subtype (11). At present, there are two comparative
authoritative theories in diagnosis and staging, namely the Sheldon
staging system (Table II) (12) and the Mayo staging system (Table III) (13). The Mayo Clinic conducted a
retrospective study of 66 patients and, in cases with the same
prediction of cancer-specific mortality, the new Mayo staging
system was found to be simpler compared with the Sheldon system
(13).
 | Table I.Urachal cancer histological subtypes
as defined by Grignon et al (9). |
Table I.
Urachal cancer histological subtypes
as defined by Grignon et al (9).
| Subtype | Definition |
|---|
| Intestinal | Architecture
reminiscent of colon adenocarcinoma |
| Mucinous | Characterized by a
cell or group of cells in a matrix of extracellular mucin |
| Signet ring | Carcinoma cells
spread diffusely through the tissue |
| Unspecified
adenocarcinoma | Pattern does not fit
into any of the abovementioned categories |
| Mixed | Carcinoma exhibiting
≥2 of any of these patterns and none of those represents >75% of
the material examined |
 | Table II.Urachal cancer staging system as
defined by Sheldon et al (12). |
Table II.
Urachal cancer staging system as
defined by Sheldon et al (12).
| Stage | Definition |
|---|
| I | Urachal cancer
confined to urachal mucosa |
| II | Urachal cancer with
invasion confined to urachus itself |
| IIIA | Local urachal cancer
extension to the bladder |
| IIIB | Local urachal cancer
extension to the abdominal wall |
| IIIC | Local urachal cancer
extension to the peritoneum |
| IIID | Local urachal cancer
extension to viscera other than the bladder |
| IVA | Metastatic urachal
cancer to the lymph nodes |
| IVB | Metastatic urachal
cancer to distant sites |
 | Table III.Urachal cancer staging system as
defined by the Mayo Clinic (13). |
Table III.
Urachal cancer staging system as
defined by the Mayo Clinic (13).
| Stage | Definition |
|---|
| I | Tumors confined to
the urachus and or bladder |
| II | Tumors extending
beyond the muscular layer of the urachus and/or the bladder |
| III | Tumors infiltrating
the regional lymph nodes |
| IV | Tumors infiltrating
non regional lymph nodes or other distant sites |
There is currently no effective treatment for this
rare disease, and surgery is the main therapeutic option. In order
to compare the prognosis of surgical and non-surgical treatment,
Pinthus et al conducted a retrospective study including 40
patients with urachal adenocarcinoma and discovered that surgical
treatment was associated with higher survival rates (14). There are currently two main surgical
treatments, namely partial and radical cystectomy. When comparing
partial with radical cystectomy, Bruins et al observed no
significant differences in survival (15). However, the recurrence rate following
partial cystectomy is higher compared with that for radical
cystectomy (3). Thus, extensive
tumor resection may be curative in the majority of non-metastatic
urachal cancers (16). Furthermore,
there is currently no definitive evidence regarding the curative
effect of chemotherapy and radiotherapy.
In conclusion, urachal carcinoma is a rare type of
cancer that is difficult to diagnose early. We herein present two
cases of urachal carcinoma, with the aim to help further elucidate
this disease and reduce the rate of clinical and pathological
misdiagnosis.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81101922), the
Science and Technology Development Fund Project of Shenzhen (nos.
JCY20130402114702124 and JCY20150403091443329) and funds from the
Guangdong Key Medical Subject.
References
|
1
|
Ravi R, Shrivastava BR, Chandrasekhar GM,
Prahlad S, Balasubramanian KV and Mallikarjuna VS: Adenocarcinoma
of the urachus. J Surg Oncol. 50:201–203. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow YC, Lin WC, Tzen CY, Chow YK and Lo
KY: Squamous cell carcinoma of the urachus. J Urol. 163:903–904.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gopalan A, Sharp DS, Fine SW, Tickoo SK,
Herr HW, Reuter VE and Olgac S: Urachal carcinoma: A
clinicopathologic analysis of 24 cases with outcome correlation. Am
J Surg Pathol. 33:659–668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takeuchi M, Matsuzaki K, Yoshida S,
Nishitani H and Uehara H: Imaging findings of urachal mucinous
cystadenocarcinoma associated with pseudomyxoma peritonei. Acta
Radiol. 45:348–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siefker-Radtke A: Urachal adenocarcinoma:
A clinician's guide for treatment. Semin Oncol. 39:619–624. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nix JT, Menville JG, Albert M and Wendt
DL: Congenital patent urachus. J Urol. 79:264–273. 1958.PubMed/NCBI
|
|
7
|
Hue L and Jacquin M: Colloid carcinoma of
the umbilical and the anterior abdominal wall having invaded the
urinary bladder. Union Med Seine-Inf Rouen. 6:4181863.(In
French).
|
|
8
|
Thali-Schwab CM, Woodward PJ and Wagner
BJ: Computed tomographic appearance of urachal adenocarcinomas:
Review of 25 cases. Eur Radiol. 15:79–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grignon DJ, Ro JY, Ayala AG, Johnson DE
and Ordóñez NG: Primary adenocarcinoma of the urinary bladder. A
clinicopathologic analysis of 72 cases. Cancer. 67:2165–2172. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molina JR, Quevedo JF, Furth AF,
Richardson RL, Zincke H and Burch PA: Predictors of survival from
urachal cancer: A Mayo Clinic study of 49 cases. Cancer.
110:2434–2440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paner GP, McKenney JK, Barkan GA, Yao JL,
Frankel WL, Sebo TJ, Shen SS and Jimenez RE: Immunohistochemical
analysis in a morphologic spectrum of urachal epithelial neoplasms:
Diagnostic implications and pitfalls. Am J Surg Pathol. 35:787–798.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheldon CA, Clayman RV, Gonzalez R,
Williams RD and Fraley EE: Malignant urachal lesions. J Urol.
131:1–8. 1984.PubMed/NCBI
|
|
13
|
Ashley RA, Inman BA, Sebo TJ, Leibovich
BC, Blute ML, Kwon ED and Zincke H: Urachal carcinoma:
Clinicopathologic features and long-term outcomes of an aggressive
malignancy. Cancer. 107:712–720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pinthus JH, Haddad R, Trachtenberg J,
Holowaty E, Bowler J, Herzenberg AM, Jewett M and Fleshner NE:
Population based survival data on urachal tumors. J Urol.
175:2042–2047; discussion 2047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruins HM, Visser O, Ploeg M,
Hulsbergen-van de Kaa CA, Kiemeney LA and Witjes JA: The clinical
epidemiology of urachal carcinoma: Results of a large, population
based study. J Urol. 188:1102–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herr HW, Bochner BH, Sharp D, Dalbagni G
and Reuter VE: Urachal carcinoma: Contemporary surgical outcomes. J
Urol. 178:74–78; discussion 78. 2007. View Article : Google Scholar : PubMed/NCBI
|