Introduction
Gastric cancer displays multifactorial etiology and
its genetic and immunological background remains to be fully
elucidated. It is a malignancy characterized by a highly invasive
and metastatic nature, properties which require interaction of the
gastric cancer cells with the extracellular matrix (ECM) through
cell adhesion, migration, proliferation and metastasis.
Fibronectin is one of the major structural
components of the basement membrane (1–8).
Fibronectin serves a prominent role in cell adhesion, growth,
migration and differentiation, and it is important for processes,
including wound healing and embryonic development via integrins and
other cell surface receptors (1–8). Altered
expression of fibronectin, degradation and organization have been
associated with a number of pathologies, including cancer (1–8). Several
of the morphological changes observed in tumor types and
tumor-derived cell lines have been attributed to decreased
expression of fibronectin, increased fibronectin degradation,
and/or decreased expression of fibronectin-binding receptors,
including α5β1 integrins (1–8). Fibronectin is also a candidate biomarker
for several solid tumor types, including ovarian, prostate and
breast cancer (1,2). In addition to its implication in cancer
development, fibronectin also acts as a potential biomarker for
treatment-associated resistance (5).
However, a clinical association between the
expression of fibronectin and carcinogenesis in gastric cancer was
observed in only a few trials (6–8). Increased
expression of fibronectin was also detected in gastric cancer cell
lines with increased gastric cancer cell proliferation and
metastatic potential (6,7). Therefore, an unsatisfactory
understanding of the molecular function of fibronectin exists and
the possible clinical significance of fibronectin has remained
unclear in patients with gastric cancer.
Although all available findings were provided from
pre-clinical tissue or cell-based trials, so far, no clinical study
has investigated the clinical significance of this ECM marker in
the plasma and/or serum in patients with gastric cancer. Therefore,
the significance of the serological levels of fibronectin in
gastric cancer patients remains unknown. The present study
evaluated the soluble serum levels of fibronectin in gastric cancer
patients and assessed its associations with the prognosis, known
various clinical variables and response to chemotherapy, in order
to examine whether fibronectin is a potential novel biomarker for
use in the treatment of gastric cancer.
Materials and methods
Patients and therapy
The present study included 63 consecutive patients
admitted to Institute of Oncology, Istanbul University (Istanbul,
Turkey). All patients had histologically confirmed gastric cancer
and had received no chemotherapy or chemoradiation in the last 6
months. The staging was determined, according to the American Joint
Committee on Cancer and International Union against Cancer staging
systems. The pre-treatment evaluation included assessment of
detailed clinical history and physical examination, with a series
of biochemistry tests, including lactate dehydrogenase (LDH),
complete blood cell counts, including thrombocytes (PLTs),
leukocytes (WBCs), hemoglobin (Hb) and serum tumor markers,
carcinoembryonic antigen (CEA) and cancer antigen (CA) 19.9. Those
with Eastern Cooperative Oncology Group performance status of ≤2,
and appropriate blood chemistry tests received chemotherapy on an
outpatient basis, which included different combinations of
fluorouracil, folinic acid, capecitabine, docetaxel, cisplatin,
epirubicin, with/without radiotherapy depending on the stage of
disease. Follow-up programs included clinical, laboratory and
radiological assessments performed at 8-week intervals during
chemotherapy or every 12 weeks for no anticancer treatment. The
response to treatment was determined according to the revised
RECIST criteria version 1.1 (9).
For comparison of serum levels of fibronectin, 30
age- and sex-matched healthy controls were included in the
analysis. Informed consent was obtained from all patients and the
study was reviewed and approved by the local ethical committee.
Measurement of serum fibronectin
levels
Serum samples were obtained on first admission,
prior to administration of any adjuvant and metastatic treatment,
or follow-up patients. Blood samples were obtained from patients
with gastric cancer and healthy controls by venipuncture and
clotted at room temperature. The sera were collected following
centrifugation for 10 min at 4,000 rpm at room temperature and
frozen immediately at −20°C until analysis.
The Fibronectin enzyme-linked immunosorbent assay
(ELISA; eBioscience, Vienna, Austria) uses a double-antibody
sandwich ELISA to determine the level of human fibronectin in the
samples. The serum samples, standards and biotin-conjugate were
added to the wells, which were pre-coated with human fibronectin
monoclonal antibody and allowed to incubate for 2 h at 23°C.
Unbound material was washed away. Fibronectin combined with
streptavidin-horseradish peroxidase (eBioscience, Vienna, Austria)
were added to form an immune complex and were subsequently allowed
to incubate for 1 h at 23°C. Any unbound material was washed away.
Chromogen solution (eBioscience) was added and incubated for ~10
min in the dark for the conversion of the colorless solution to a
blue solution, the intensity of which was proportional to the
quantity of fibronectin in the sample. Following the addition of
the 100 µl acidic stop solution (eBioscience), the color turned
yellow. The intensity of the colored reaction product was measured
using an automated ELISA reader (RT-1904C Chemistry Analyzer;
Rayto, Atlanta, GA, USA) at 450 nm. The results were expressed as
ng/ml.
Statistical analysis
Continuous variables were categorized using median
values as cut-off point. Assessment of associations, comparisons
between various clinical/laboratory parameters and serum levels of
fibronectin assay were accomplished using Mann-Whitney U test.
Survival was calculated from the date of first admission to the
hospital until mortality resulting from any cause or until the last
contact with the patient or any family member. The Kaplan-Meier
method was used for estimation of survival of the patient and
differences in survival were assessed using the log-rank statistic.
P≤0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA).
Results
A total of 63 patients diagnosed with gastric cancer
were enrolled in the present study. The baseline histopathological
characteristics and the demographic characteristics of the patients
are listed in Table I. The median age
at diagnosis was 62 years (range, 28–82 years).
 | Table I.Patient and disease
characteristics. |
Table I.
Patient and disease
characteristics.
| Variable | n (%) |
|---|
| No. of patients | 63 (100) |
| Age, years |
|
| ≥60 | 35 (56) |
|
<60 | 28 (44) |
| Gender |
|
| Male | 25 (40) |
|
Female | 38 (60) |
| Site of tumor |
|
|
Cardia | 21 (33) |
|
Antrum | 27 (43) |
|
Undetermined | 15 (24) |
| Histology |
|
|
Adenocarcinoma | 42 (67) |
|
Signet-ring cell | 21 (33) |
| Grade |
|
| I–II | 10 (16) |
| III | 44 (70) |
|
Undetermined | 9 (14) |
| Tumor stage |
|
| 1–3 | 14 (22) |
| 4 | 22 (35) |
|
Unknown | 27 (43) |
| No. of lymph node
involvement |
|
| 0–2 | 12 (19) |
| ≥3 | 13 (21) |
|
Unknown | 38 (60) |
| Stage of disease |
|
|
Non-metastatic | 32 (51) |
|
Metastatic | 31 (49) |
| Liver
metastasisa |
|
| Yes | 14 (45) |
| No | 17 (55) |
| Curative
surgeryb |
|
| Yes | 17 (53) |
| No | 9 (28) |
|
Unknown | 6 (19) |
| Serum hemoglobin
level |
|
| Low
(<12g/dl) | 35 (56) |
| Normal
(≥12g/dl) | 28 (44) |
| Serum leukocyte
count |
|
| Normal
(<10,000) | 52 (83) |
| Elevated
(≥10,000) | 11 (17) |
| Serum platelet
count |
|
| Normal
(<350,000) | 54 (86) |
| Elevated
(>350,000) | 9 (14) |
| Serum lactate
dehydrogenase level |
|
| Normal
(<450U/l) | 43 (68) |
| Elevated
(≥450U/l) | 10 (16) |
|
Unknown | 10 (16) |
| Erythrocyte
sedimentation rate,/h |
|
| Elevated
(≥50) | 16 (25) |
| Normal
(<50) | 10 (16) |
|
Unknown | 37 (59) |
| Serum
carcinoembryonic antigen level |
|
| Normal
(<10 ng/ml) | 44 (70) |
| Elevated
(≥10 ng/ml) | 13 (21) |
|
Unknown | 6 (9) |
| Serum cancer antigen
19.9 level |
|
| Normal
(<40 IU/ml) | 32 (51) |
| Elevated
(≥40 IU/ml) | 25 (40) |
|
Unknown | 6 (9) |
| Response to
chemotherapya |
|
|
Responsive | 13 (43) |
|
Non-responsive | 17 (57) |
| Last status |
|
|
Alive | 28 (44) |
|
Deceased | 35 (56) |
The baseline serum fibronectin levels of the
patients with gastric cancer were significantly higher compared
with those in the control group (median values, 606, vs. 193 ng/ml;
P<0.001; Table II). The known
clinical variables, including the age of the patient, gender, site
of lesion, histology, histological grade, stage of disease and
serum levels of LDH, CEA and CA 19.9 were not correlated with serum
fibronectin concentrations (P>0.05; Table III). Additionally, no association
was observed between serum fibronectin level and the response to
chemotherapy (P=0.12).
 | Table II.Serum fibronectin levels in gastric
cancer patients and healthy controls. |
Table II.
Serum fibronectin levels in gastric
cancer patients and healthy controls.
|
| Patients (n=63) | Controls (n=30) |
|
|---|
|
|
|
|
|
|---|
| Marker | Median | Range | Median | Range | P-value |
|---|
| Fibronectin
(ng/ml) | 606 | 44–950 | 193 | 58–614 | <0.001 |
 | Table III.Distribution and survival comparisons
of serum fibronectin levels on various clinical parameters in
patients with gastric cancer. |
Table III.
Distribution and survival comparisons
of serum fibronectin levels on various clinical parameters in
patients with gastric cancer.
| Parameter | Distribution
P-value | Survival P-value |
|---|
| Age of patient |
|
|
|
<60/≥60 years | 0.26 | 0.61 |
| Gender |
|
|
|
Male/female | 0.84 | 0.56 |
| Site of tumor |
|
|
|
Cardia/antrum | 0.36 | 0.04 |
| Histology |
|
|
|
Adenocarcinoma/signet
ring | 0.12 | 0.22 |
| Grade |
|
|
|
I–II/III | 0.61 | 0.10 |
| Tumor stage |
|
|
|
1–3/4 | 0.53 | 0.06 |
| No. of lymph node
involvement |
|
|
|
0–2/≥3 | 0.38 | 0.21 |
| Curative surgery |
|
|
|
Yes/no | 0.24 | 0.36 |
| Metastasis |
|
|
|
Yes/no | 0.53 | 0.03 |
| Liver metastasis |
|
|
|
Yes/no | 0.65 | 0.11 |
| Serum hemoglobin
level |
|
|
|
Low/normal | 0.50 | 0.34 |
| Serum leukocyte
count |
|
|
|
High/normal | 0.72 | 0.30 |
| Serum platelet
count |
|
|
|
High/normal | 0.48 | 0.51 |
| Erythrocyte
sedimentation rate |
|
|
|
High/normal | 0.59 | 0.02 |
| Serum lactate
dehydrogenase level |
|
|
|
High/normal | 0.95 | 0.11 |
| Serum
carcinoembryonic antigen level |
|
|
|
High/normal | 0.82 | 0.01 |
| Serum cancer
antigen 19.9 level |
|
|
|
High/normal | 0.85 | 0.04 |
| Response to
chemotherapy |
|
|
|
Yes/no | 0.12 | 0.05 |
| Serum fibronectin
level |
|
|
| </≥
median | – | 0.43 |
The median follow-up time was 25 weeks (range, 1–164
weeks). At the end of the observation period, 35 patients (55.6%)
had succumbed to mortality. The median survival time for all
patients was 42.0±4.2 weeks (95% confidence intervals = 33.8–50.2).
The 1-year survival rates were 42.2% (95% confidence intervals =
28.3–56.1). The presence of metastasis (P=0.03), antrum
localization (P=0.04), elevated erythrocyte sedimentation rate
(P=0.02), higher serum CEA levels (P=0.01), elevated serum CA 19.9
concentrations (P=0.04) and unresponsiveness to chemotherapy
(P=0.05) exhibited statistically significant worsened survival
variables (Table III). However,
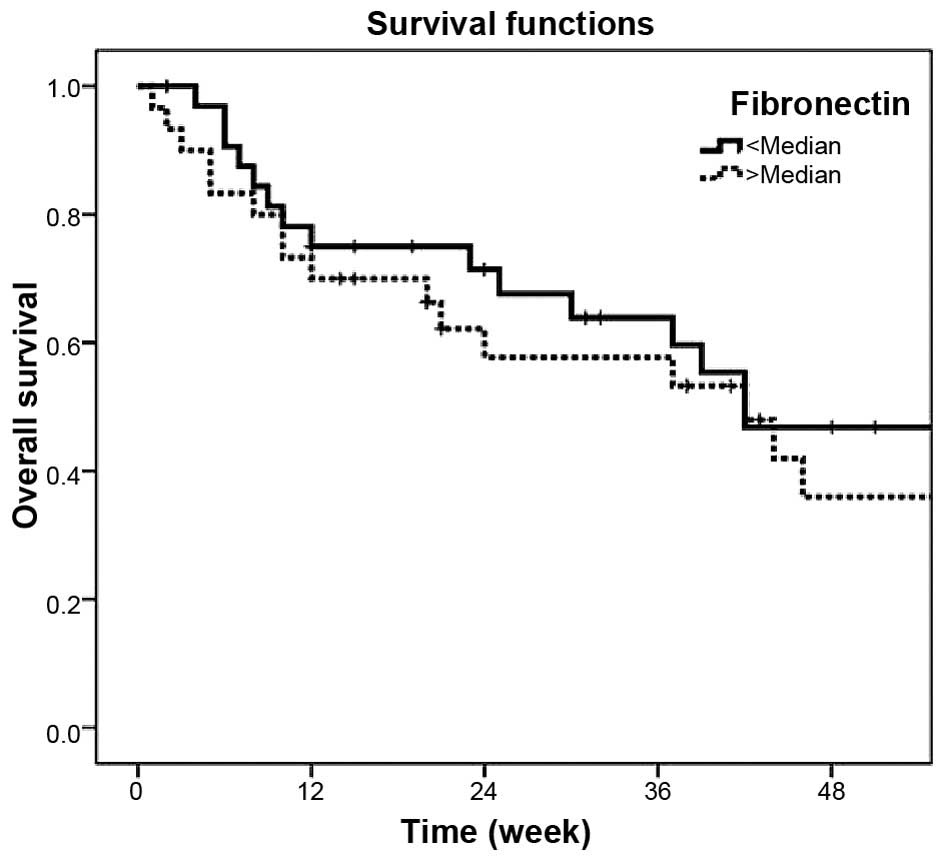
serum fibronectin concentrations was not associated with prognosis
of the outcome (P=0.43; Table III;
Fig. 1).
Discussion
Although overexpression of the fibronectin gene was
observed in human gastric cancer, its possible clinical
significance has remained unclear in patients with gastric cancer.
The possible reason for this unsatisfactory situation is limited
data, as only a few studies have been previously performed
(6–8).
Performing an immunohistochemical study of the
basement membrane antigens, laminin, collagen IV, fibronectin, and
type IV collagenase in a series of 87 gastric cancer types, 90% of
the cancers expressed fibronectin, predominantly in the connective
tissue at the invading edge of the tumor (6). Fibronectin expression was also
significantly associated with the expanding growth pattern of the
neoplasm. However, expression of fibronectin was not significantly
correlated with the DNA content or aneuploidy. Similarly, Grigioni
et al (7) observed a stronger
staining for fibronectin in the connective tissue at the invading
edge of the gastric cancer. These findings may reflect compacting
of fibronectin pre-existing in the host connective tissue, which
may facilitate cancer cell adhesion.
Numerous studies have investigated
fibronectin/integrin-mediated signal transduction, and the majority
of these have specifically investigated the interaction between
fibronectin and integrin through the attachment of cells to the
fibronectin-containing ECM (8).
However, a large portion of fibronectin is soluble within the body.
Soluble fibronectin exists predominantly in the blood plasma and
reaches each part of the body through the bloodstream. Similar to
tissue, soluble fibronectin also binds integrin and activates
consequent signaling events (8).
All previous findings regarding this ECM marker were
provided by tissue-cell scale trials. With the exception of the
present study, so far, unfortunately, fibronectin was not studied
in sera of gastric cancer patients. The present study aimed to
determine the clinical significance of the serum fibronectin levels
in patients with gastric cancer. Serum levels of fibronectin were
quantitatively analyzed by solid-phase ELISA. The results
demonstrated that the analysis of serum fibronectin was able to
discriminate between the gastric cancer patients and healthy
individuals, indicating that fibronectin is a good serological
diagnostic marker of gastric cancer. However, no significant
associations were observed between the levels of serum fibronectin
and the tumor characteristics, including stage, histology, grade
and serum tumor markers, in the present study. Additionally, it was
also revealed that serum levels of fibronectin were not associated
with survival. Therefore, in the present study, fibronectin levels
in serum were not a useful prognostic marker to predict tumor
prognosis in patients with gastric cancer. Additionally, the link
between serum fibronectin concentrations and chemosensitivity has
raised the possibility of using fibronectin as a predictor of
response to chemotherapy in patients scheduled to undergo various
chemotherapeutic regimens. It was demonstrated in the present study
that serum fibronectin levels may not be a potential predictor of
clinical response to chemotherapy in gastric cancer patients. A
contrasting finding was also observed in patients with other tumor
types (5).
In conclusion, the present study revealed that serum
levels of fibronectin were only a diagnostic marker in gastric
cancer patients. However, its predictive and prognostic values were
not determined in these patients. The small sample size and short
follow-up time of the present study may be considered as
significant limitation and may have influenced these results.
However, the present study contributed to the literature, as it
contained all stages and groups of disease. Further studies in a
larger patient population are necessary to determine the potential
clinical significance of this assay in patients with gastric
cancer.
References
|
1
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hynes RO: Fibronectins (1st). New York,
NY: Springer-Verlag. 1989.
|
|
3
|
Williams CM, Engler AJ, Slone RD, Galante
LL and Schwarzbauer JE: Fibronectin expression modulates mammary
epithelial cell proliferation during acinar differentiation. Cancer
Res. 68:3185–3192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han S, Khuri FR and Roman J: Fibronectin
stimulates non-small cell lung carcinoma cell growth through
activation of Akt/mammalian target of rapamycin/S6 kinase and
inactivation of LKB1/AMP-activated protein kinase signal pathways.
Cancer Res. 66:315–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jerhammar F, Ceder R, Garvin S, Grénman R,
Grafström RC and Roberg K: Fibronectin 1 is a potential biomarker
for radioresistance in head and neck squamous cell carcinoma.
Cancer Biol Ther. 10:1244–1251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
David L, Nesland JM, Holm R and
Sobrinho-Simoes M: Expression of laminin, collagen IV, fibronectin
and type IV collagenase in gastric carcinoma. Cancer. 73:518–527.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grigioni WF, Biagini G, Errico AD, Milani
M, Villanaci V, Garbisa S, Mattioli S, Gozzetti G and Mancini AM:
Behavior of basement membrane antigens in gastric and colorectal
cancer. Immunohistochemical study. Acta Pathol Jpn. 36:173–184.
1986.PubMed/NCBI
|
|
8
|
Li Y, Chen Y, Tao Y, Wang Y, Chen Y and Xu
W: Fibronectin increases RhoA activity through inhibition of PKA in
the human gastric cancer cell line SGC-7901. Mol Med Rep. 4:65–69.
2011.PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D and
Verweij J: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|