Introduction
A small percentage of patients presenting with
breast cancer are found to have metastatic disease at the point of
presentation. Breast cancer, with distant metastases beyond the
regional lymph basin, remains a therapeutic challenge. The
mainstays of therapy in such advanced disease are systemic
therapies, including chemotherapy or endocrine therapy, or
palliative loco-regional strategies, including targeted
radiotherapy or surgery to metastases (1). Current recommendations for treatment of
such advanced stage IV disease include no curative resection, as
evidence of a survival benefit for primary resection in such cases
is lacking (2).
However, recent previous studies suggest that
primary tumour resection may be an independent factor in improving
survival and in addition, control of local symptoms (3,4). It has
been suggested that resection of the primary tumour in stage IV
disease aids survival by reducing the tumour burden, specifically
the number of circulating tumour cells (5). Additionally, recent evidence have
postulated a model in which metastases can ‘self-seed’ and
circulate back to the primary tumour, accelerating growth and
angiogenesis through cytokine action (6). Furthermore, the role of the primary
tumour in advanced disease is a central theme of currently debated
disease models centred around the postulated role of cancer stem
cells (7).
However, a consensus regarding a curative role for
primary resection in stage IV disease remains to be determined, as
the relevant evidence is far from unanimous. It is suggested that
the beneficial effect observed in other previous studies may be the
result of a selection bias.
In order to better examine this issue, the present
study performed a systematic review of the literature and a
meta-analysis in order to calculate the survival benefit of primary
resections in stage IV breast cancer.
Materials and methods
Data sources and searches
A comprehensive search of the PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Ovid SP
(http://ovidsp.uk.ovid.com/sp-3.18.0b/ovidweb.cgi)
databases was performed to identify relevant published literature
prior to February 25th 2015. The search keywords used
were as follows: ‘Stage IV breast cancer’ and ‘surgical excision’
or ‘surgery’ or ‘local treatment’.
The authors as per pre-specified inclusion and
exclusion criteria assessed the articles identified.
Inclusion and exclusion criteria
Prospective clinical trials and retrospective case
series regarding female adult patients with reported outcomes as a
function of surgical resection of primary breast cancer in the
presence of histologically confirmed distant metastases were
included. Conservative and extended resections were included, with
no stipulations regarding systemic therapies and the use of
radiation or surgery in the regional lymph basin.
The exclusion criteria were as follows: i) Studies
reporting no hazard ratios (HRs) for overall survival of adult
female patients, according to multivariate analysis; ii) studies
that failed to report 95% confidence intervals (CIs) for the HRs;
iii) unavailability of full text for data extraction; iv) reviews,
case reports, letters or commentaries.
Data extraction and management
Data was extracted by the authors independently
using characteristics of included studies, the baseline
characteristics of included patients and the aforementioned
outcomes. The recorded data included author, publication date,
study design, participants, interventions, median age, oestrogen
receptor (ER) status, human epidermal growth factor receptor 2
status and metastatic sites.
Measures of treatment effect and
statistical analysis
HRs and CIs for overall survival as a function of
surgery of primary breast cancer with or without other treatment
modalities were retrieved for each study. An HR<1 meant a
reduced risk of mortality for surgically treated patients compared
with those who did not undergo primary tumour resection.
A meta-analysis of HRs was performed with both fixed
effect and random effect models considered. Statistical
heterogeneity among the included studies was assessed using
Cochran's Q test, and a χ2 test and I2
statistic was used to quantify the inconsistency: A value of 0–100%
indicated increasing heterogeneity. The assumption of homogeneity
was considered invalid for P<0.1. Summary estimates were
reported from the random-effects models.
Potential publication biases were evaluated with
Begg's funnel plots for overall survival and subsequently with both
Begg's and Egger's tests. Duval and Tweedie's trim and fill method
was also performed.
Results of the meta-analyses were reported as a
classical forest plot. Statistical analyses were performed using
Review Manager 5.1 and Comprehensive Meta-Analysis version 3.0.
Results
Literature search results and the
characteristics of the included studies
A total of 1,628 studies were retrieved, of which 19
initially met the inclusion criteria. Of these studies, two were
excluded at the end of the selection phase due to a lack of HRs for
overall survival (OS) in the multivariate analysis. One additional
study was excluded as it failed to report a 95% CI for HR, thus
precluding calculation of the standard error for meta-analysis.
Therefore, 16 studies met the full inclusion criteria for this
meta-analysis (Table I) (2–4,8–20). All
previous studies were retrospective case studies.
 | Table I.Characteristics of the included
previous retrospective case studies. |
Table I.
Characteristics of the included
previous retrospective case studies.
| Author, year | No. participants/No.
surgically treated/No. not surgically treated | Follow-up time
(months) | Age (years) | HR (95% CI)
P-value | Use of systemic
therapy/radiotherapy (%) | Factors associated
with increased overall survival | (Refs.) |
|---|
| Akay et al,
2014 | 172/79/93 | 33 | 51 (mean) | 0.9 (0.2–1.6)
P=0.0001 | 45 (57%) | Local control
significantly associated with surgery | 3 |
| Babiera et al,
2006 | 306/224/82 | 32.1 (median) | 52 (22–88) | 0.5 (0.21–1.19)
P=0.12 | 98%/NR | Number of metastatic
sites Her2neu status | 4 |
| Bafford et al,
2009 | 147/61/86 | NR | 49.2 (28.5–79.7) | 0.47 P=0.003 | 87 (CT) 57
(HT)/38 | Positive ER and
Her2neu status | 8 |
| Blanchard et
al, 2008 | 395/242/153 | NR | 63.30 | 0.71 (0.556–0.906)
P=0.006 | NR | ER and PR positive
status. Reduced number of mets. Surgery with negative margins
(HR=0.5) | 9 |
| Dominici et
al, 2011 | 290/54/236 | NR | 53.4 (mean) | 0.94 (0.84–1.05)
P=0.27 | 39 (74%)/7 (13%) | ER+, fewer met sites
and use of ET associated with longer survival | 10 |
| Fields et al,
2007 | 406/187/222 | 142 (123–157) | 55.9 (mean) | 0.53 (0.42–0.67)
P<0.0001 | NR/NR | Presence of bny mets
vs visceral mets, lower age | 11 |
| Gnerlich et
al, 2007 |
9,734/4,575/5,159 | NR | 62 | 0.63 (0.60–0.66)
P<0.001 | NR/34 | NR | 12 |
| Hazard et al,
2008 | 11/47/64 | 26.9 (2.5–138) | 52.7 (mean) | 0.798 (0.40–1.60)
P=0.520 | 100/67 | Median survival times
noted | 13 |
| Khan et al,
2002 |
1,6023/9,162/6,861 | NR | 62.3 (mean) | 0.61 (0.58–0.61)
negative surgical margins 0.751 (0.71–0.793) positive surgical
margins P<0.0001 | 77.5/NR | CT, HT negative
surgical margins, reduced number of met sites and soft tissue vs
visceral mets indicated a higher rate of overall survival | 14 |
| Neuman et al,
2010 | 186/69/117 | 52 | 53 | 0.71 (0.47–1.06)
P=0.1 | NR/NR | ER+, PR+ and HER2+
associated with longer survival | 16 |
| Pathy et al,
2011 | 375/139/236 | NR | 49 | 0.58 (0.48–0.69)
P=NR | CT 75 (54%) HT 92
(66.2%)/93 (66.9%) | Age under 65
benefited most as surgery as did negative surgical margins | 17 |
| Pérez-Fidalgo et
al, 2011 | 208/123/85 | 29.86 | 55.9 (mean) | 0.52 (0.35–0.77)
P<0.001 | CT 103 (83.8%) HT 19
(15.4%)/57 (46.3%) | Benefits seen mainly
in those with visceral disease | 18 |
| Rapiti et al,
2006 | 300/127/173 | NR | 61.8 | 0.6 (0.4–1.0) P=0.049
overall negative surgical margins 0.5 (0.3–0.7) P=0.0003 positive
surgical margins 0.8 (0.5–1.1) | 53 (CT) 43
(HT)/21 | Effect particularly
evident for women with only bony metastases | 19 |
| Ruiterkamp et
al, 2010 | 728/288/440 | NR | 60.2 (mean) | 0.62 (0.51–0.76)
P<0.0001 | 89/34 | Age, number of
metastatic sites, use of systemic therapy | 2 |
| Rashaan et al,
2012 | 171/59/112 | NR | NR | 0.9 (0.6–1.4)
P=0.5 | NR/NR | Age <50 found to
be associated with a better outcomeas was a small tumour and no
comorbidity | 20 |
| Shien et al,
2009 | 344/160/184 | 33 (29.2–38.0) | 54 | 0.89 P-value not
reported as insignificant) | 100/NR | Age <50 | 23 |
Meta-analysis for OS
The present study first tested the overall null
hypothesis, which stated that all treatment effects equalled zero.
This is equivalent to testing whether all HRs in all studies are
equal to 1, indicating no effect from surgery. Both non-directional
and directional tests rejected the null hypothesis.
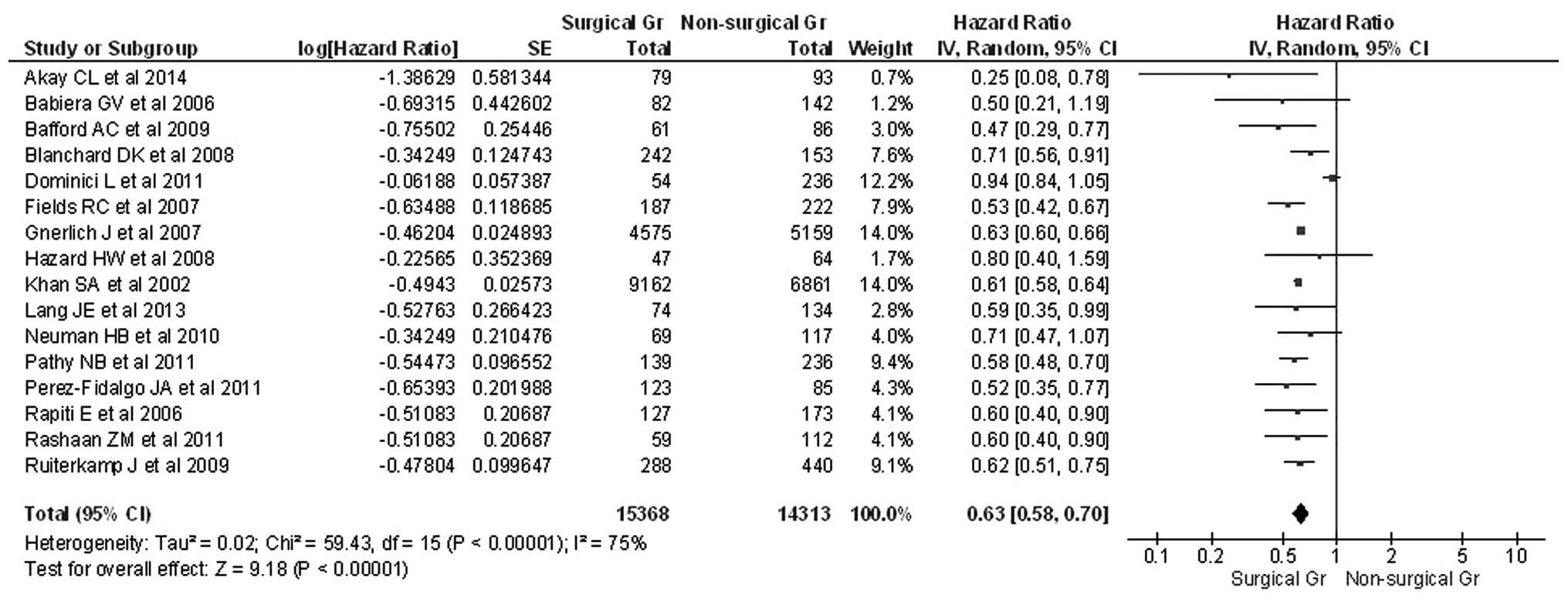
The HRs for OS and standard errors for the estimated
HRs were reported or extrapolated for all included studies.
Significant heterogeneity was observed by calculating the
χ2 test for heterogeneity (P<0.0001) and the
I2 test demonstrated an index of 75%, indicating
considerable inconsistency between the selected studies. Therefore,
the present study assumed a random effects model that takes into
account variability within and between studies. The pooled HR for
OS was 0.63 with a 95% CI of 0.58–0.70 (Table I), confirming the suggestion that
surgery is beneficial in terms of reducing the risk of mortality by
37%. These results all illustrated in the forest plot in Fig. 1.
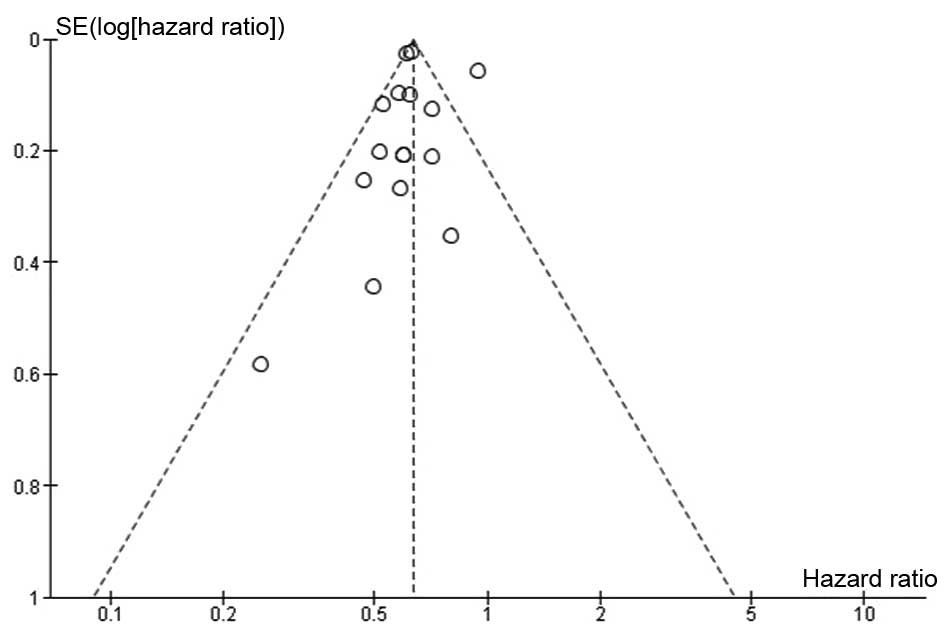
The funnel plot for risk of bias in OS (Fig. 2) revealed that all studies, with the
exception of Dominici et al (10), fall within the 95% CI, and they are
relatively symmetrically distributed. Therefore, it would be
reasonable to surmise that there are significant systematic
differences between the individual studies.
Evidence of publication bias was not revealed in the
present analysis, despite the use of multiple tests for this
purpose (Egger's test, P=0.40785; Begg test, P=0.50 Mazumdar's rank
correlation test, P=0.50).
According to Duval and Tweedie's ‘trim and fill’
method under the random-effect model (point estimate=0.64674; 95%
CI=0.58774–0.71167), the imputed point estimate for OS remained
unchanged.
Discussion
The present meta-analysis confirmed the hypothesis
that resection of the primary tumour in a patient with concomitant
metastatic disease is beneficial in terms of survival, with a 37%
reduction in mortality. These results reiterated the benefits of
surgical resection of the primary tumour in metastatic disease, not
just for advanced breast cancer, but also potentially for other
advanced cancer types.
A number of hypotheses can be postulated regarding
the mechanisms underlying the beneficial effects on prognosis of
primary resection in metastatic breast cancer. Aside from the
self-evident role of reducing the overall tumour burden, removal of
the primary tumour has been shown to reduce the number of
circulating tumour cells, which has been be associated with
improved disease outcomes (5).
Furthermore, recent previous studies describe a
disease model termed ‘tumour self-seeding’, in which the primary
tumour may release cells into the circulation to seed metastases,
which in turn seed the primary tumour, leading to more virulent
disease (6).
Additionally, some of the suggested effects of
primary resections may be explicable under the currently topical
cancer stem cell model, in which metastatic disease is postulated
as a systemic disorder orchestrated by a more finite number of stem
cells within the primary tumour, which recruit further cells by
maintaining an oncogenic microenvironment (7,21).
Furthermore, primary resection of tumours may assist in restoring
an immunocompetent status by reactivating autoimmunity, therefore
increasing the efficacy of any concomitant medication despite the
presence of metastatic disease (22).
Whist this model remains highly contentious, the findings of the
present study are highly suggestive of an active role of the
primary tumour in metastatic disease, which suggests a role for
surgery in this context in addition to palliation and local symptom
control.
A number of the previous studies included here
highlighted additional positive prognostic factors in terms of OS
in the course of univariate analysis. The most common were: A
reduced number of metastatic sites (‘oligometastatic state’);
positive ER status; a younger age; a smaller primary tumour
(2,4,8–11,16,17,20,23).
Additionally, Pathy et al (19) observed that patients with positive
margins received no benefit from resection in terms of OS (17). Rapiti et al (19) reported similar findings in the course
of their study (19). This may have
important implications regarding the surgical treatment that would
be beneficial in this patient group. Furthermore, it raises the
question whether a mastectomy would be a more appropriate
intervention compared with tumour resection. Finally, the timing of
the surgery in relation to adjuvant and neo-adjuvant therapies
remains an area of uncertainty. These questions fall out of the
remit of this meta-analysis and are worthy issues for exploration
in course of future prospective studies.
A major limitation of this meta-analysis is that the
paucity of prospective data in the available literature.
Furthermore, despite adjusting for heterogeneity through use of
random-effect modeling, a high level of inconsistency remains.
Another limitation that must be acknowledged was the lack
specificity regarding the non-surgical treatment administered
(i.e., whether the patients underwent chemotherapy, radiotherapy,
endocrine therapy or a combination). Finally, patient populations
that underwent surgery were predominantly younger, therefore
precluding comparison with other patient groups.
Prospective data would be required to confirm or
refute the present findings. One ongoing trial may answer some of
these questions. It is randomised cohort trial comparing immediate
resection of the primary tumour, followed by systemic therapy and
systemic therapy, followed by delayed surgical resection (24). It is prudent to revise this
meta-analysis when the results of this and other similar trials
become available.
Whilst acknowledging the limitations of this study,
the present findings are highly suggestive of a significant benefit
for resection of the primary tumour in advanced metastatic breast
cancer, and would support a discussion regarding the inclusion of
primary resection in the treatment options offered to the
patient.
Acknowledgements
The present study was funded by grants from the
Breast Cancer Hope Foundation (London, UK).
References
|
1
|
Petrelli F and Barni S: Surgery of primary
tumors in stage IV breast cancer: An updated meta-analysis of
published studies with meta-regression. Med Oncol. 29:3282–3290.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruiterkamp J, Voogd AC, Bosscha K,
Tjan-Heijnen VC and Ernst MF: Impact of breast surgery on survival
in patients with distant metastases at initial presentation: A
systematic review of the literature. Breast Cancer Res Treat.
120:9–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akay CL, Ueno NT, Chisholm GB, Hortobagyi
GN, Woodward WA, Alvarez RH, Bedrosian I, Kuerer HM, Hunt KK, Huo L
and Babiera GV: Primary tumor resection as a component of
multimodality treatment may improve local control and survival in
patients with stage IV inflammatory breast cancer. Cancer.
120:1319–1328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Babiera GV, Rao R, Feng L, Meric-Bernstam
F, Kuerer HM, Singletary SE, Hunt KK, Ross MI, Gwyn KM, Feig BW, et
al: Effect of primary tumor extirpation in breast cancer patients
who present with stage IV disease and an intact primary tumor. Ann
Surg Oncol. 13:776–782. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Budd GT, Cristofanilli M, Ellis MJ,
Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV,
Terstappen LW and Hayes DF: Circulating tumor cells versus
imaging-predicting overall survival in metastatic breast cancer.
Clin Cancer Res. 12:6403–6409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MY, Oskarsson T, Acharyya S, Nguyen
DX, Zhang XH, Norton L and Massagué J: Tumor self-seeding by
circulating cancer cells. Cell. 139:1315–1326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiotaki R, Polioudaki H and
Theodoropoulos PA: Cancer stem cells in solid and liquid tissues of
breast cancer patients: Characterization and therapeutic
perspectives. Curr Cancer Drug Targets. 15:256–269. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bafford AC, Burstein HJ, Barkley CR, Smith
BL, Lipsitz S, Iglehart JD, Winer EP and Golshan M: Breast surgery
in stage IV breast cancer: Impact of staging and patient selection
on overall survival. Breast Cancer Res Treat. 115:7–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blanchard DK, Shetty PB, Hilsenbeck SG and
Elledge RM: Association of surgery with improved survival in stage
IV breast cancer patients. Ann Surg. 247:732–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dominici L, Najita J, Hughes M, Niland J,
Marcom P, Wong YN, Carter B, Javid S, Edge S, Burstein H and
Golshan M: Surgery of the primary tumor does not improve survival
in stage IV breast cancer. Breast Cancer Res Treat. 129:459–465.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fields RC, Jeffe DB, Trinkaus K, Zhang Q,
Arthur C, Aft R, Dietz JR, Eberlein TJ, Gillanders WE and
Margenthaler JA: Surgical resection of the primary tumor is
associated with increased long-term survival in patients with stage
IV breast cancer after controlling for site of metastasis. Ann Surg
Oncol. 14:3345–3351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gnerlich J, Jeffe DB, Deshpande AD, Beers
C, Zander C and Margenthaler JA: Surgical removal of the primary
tumor increases overall survival in patients with metastatic breast
cancer: Analysis of the 1988–2003 SEER data. Ann Surg Oncol.
14:2187–2194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hazard HW, Gorla SR, Scholtens D, Kiel K,
Gradishar WJ and Khan SA: Surgical resection of the primary tumor,
chest wall control and survival in women with metastatic breast
cancer. Cancer. 113:2011–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan SA, Stewart AK and Morrow M: Does
aggressive local therapy improve survival in metastatic breast
cancer? Surgery. 132:620–626; discussion 626–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lang JE, Tereffe W, Mitchell MP, Rao R,
Feng L, Meric-Bernstam F, Bedrosian I, Kuerer HM, Hunt KK,
Hortobagyi GN and Babiera GV: Primary tumor extirpation in breast
cancer patients who present with stage IV disease is associated
with improved survival. Ann Surg Oncol. 20:1893–1899. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neuman HB, Morrogh M, Gonen M, Van Zee KJ,
Morrow M and King TA: Stage IV breast cancer in the era of targeted
therapy: Does surgery of the primary tumor matter? Cancer.
116:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pathy NB, Verkooijen HM, Taib NA, Hartman
M and Yip CH: Impact of breast surgery on survival in women
presenting with metastatic breast cancer. Br J Surg. 98:1566–1572.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pérez-Fidalgo JA, Pimentel P, Caballero A,
Bermejo B, Barrera JA, Burgues O, Martinez-Ruiz F, Chirivella I,
Bosch A, Martínez-Agulló A and Lluch A: Removal of primary tumor
improves survival in metastatic breast cancer. Does timing of
surgery influence outcomes? Breast. 20:548–554. 2011.PubMed/NCBI
|
|
19
|
Rapiti E, Verkooijen HM, Vlastos G,
Fioretta G, Neyroud-Caspar I, Sappino AP, Chappuis PO and Bouchardy
C: Complete excision of primary breast tumor improves survival of
patients with metastatic breast cancer at diagnosis. J Clin Oncol.
24:2743–2749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rashaan ZM, Bastiaannet E, Portielje JE,
van de Water W, van der Velde S, Ernst MF, van de Velde CJ and
Liefers GJ: Surgery in metastatic breast cancer: Patients with a
favorable profile seem to have the most benefit from surgery. Eur J
Surg Oncol. 38:52–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kakarala M and Wicha MS: Implications of
the cancer stem-cell hypothesis for breast cancer prevention and
therapy. J Clin Oncol. 26:2813–2820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Danna EA, Sinha P, Gilbert M, Clements VK,
Pulaski BA and Ostrand-Rosenberg S: Surgical removal of primary
tumor reverses tumor-induced immunosuppression despite the presence
of metastatic disease. Cancer Res. 64:2205–2211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shien T, Kinoshita T, Shimizu C, Hojo T,
Taira N, Doihara H and Akashi-Tanaka S: Primary tumor resection
improves the survival of younger patients with metastatic breast
cancer. Oncol Rep. 21:827–832. 2009.PubMed/NCBI
|
|
24
|
Ruiterkamp J, Voogd AC, Tjan-Heijnen VC,
Bosscha K, van der Linden YM, Rutgers EJ, Boven E, van der Sangen
MJ and Ernst MF: Dutch Breast Cancer Trialists' Group (BOOG):
SUBMIT: Systemic therapy with or without up front surgery of the
primary tumor in breast cancer patients with distant metastases at
initial presentation. BMC Surg. 12:52012. View Article : Google Scholar : PubMed/NCBI
|