Introduction
Mantle cell lymphoma (MCL) is an aggressive subtype
of B-cell non-Hodgkin's lymphoma (NHL) that usually involves lymph
nodes and extranodal organs, such as the bone marrow, spleen and
gastrointestinal tract. MCL is characterized by a subset of
CD5−, CD20−, CD79a− and cyclin
D1+ tumor cells (1). Skin
involvement is very rare in MCL; in these cases, cutaneous lesions
may represent the first symptom of the disease (2). In the present report, a rare case of MCL
with cutaneous involvement as the first manifestation of the
disease is presented, along with a detailed 4-year follow up, and
the previously reported cases of MCL with cutaneous manifestations
are reviewed.
Case report
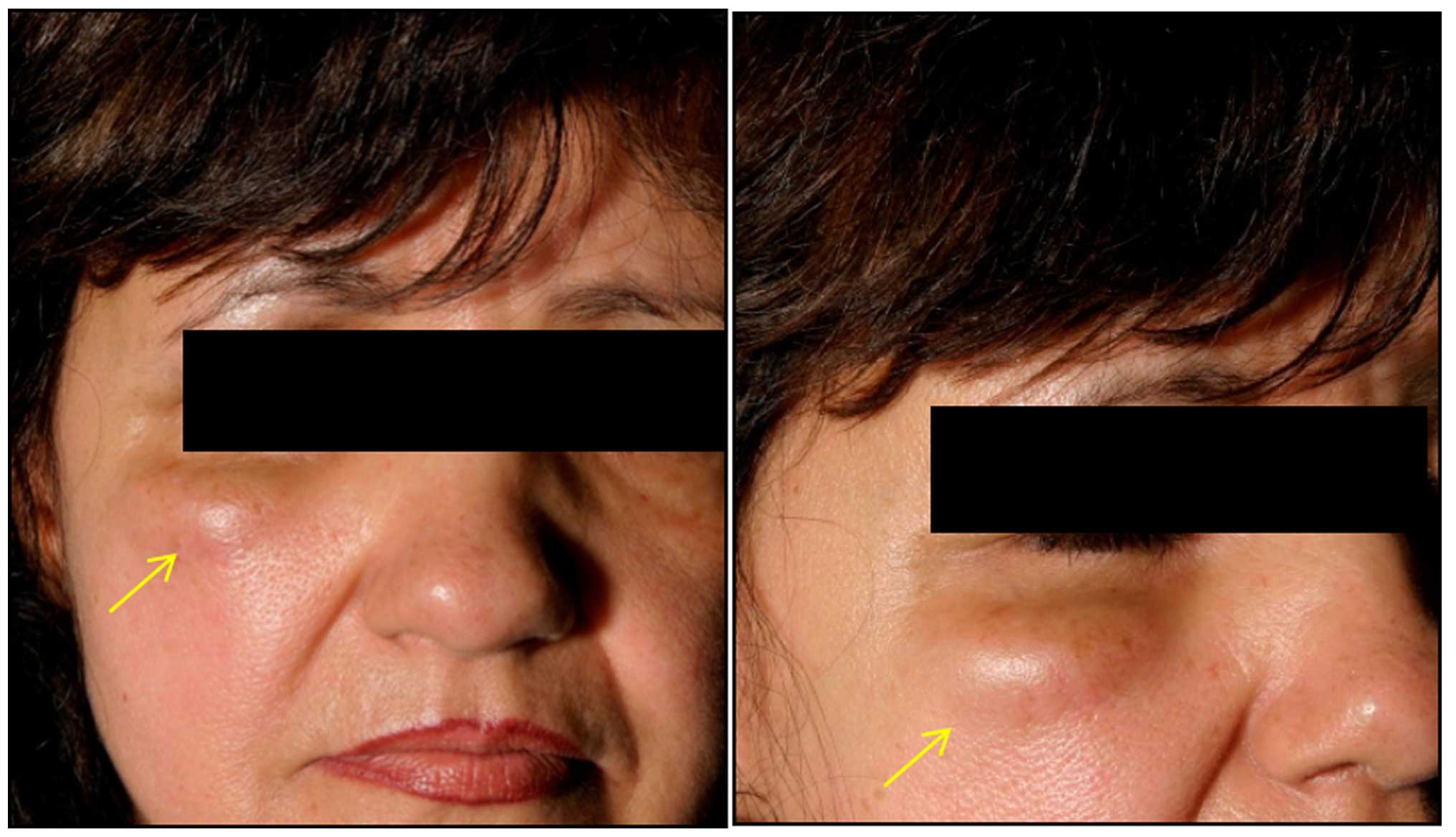
A 55-year-old Caucasian woman was referred to our
clinic in July, 2008 for investigation of a large subcutaneous
nodule (~4 cm) in the right infraorbital region. The patient
reported that the tumor had increased in size over the previous 12
months (Fig. 1). The patient also
described experiencing other symptoms, including fever, night
sweats and weight loss. A physical examination revealed no other
pathological findings and hypothyroidism was noted as a
pre-existing condition.
Initial blood tests revealed a moderate decrease in
the white blood cell count (3.86/nl), with an inverted cellular
type (neutrophils: 74.4%; and lymphocytes: 14.6%), mildly elevated
lactate dehydrogenase (233 U/l) and β2-microglobulin (2.9 mg/l)
levels, with a normal hemoglobin concentration (12.6 g/dl) and
platelet count (182/nl). The hepatic and renal functions were
normal.
Excision of the nodular lesion was performed.
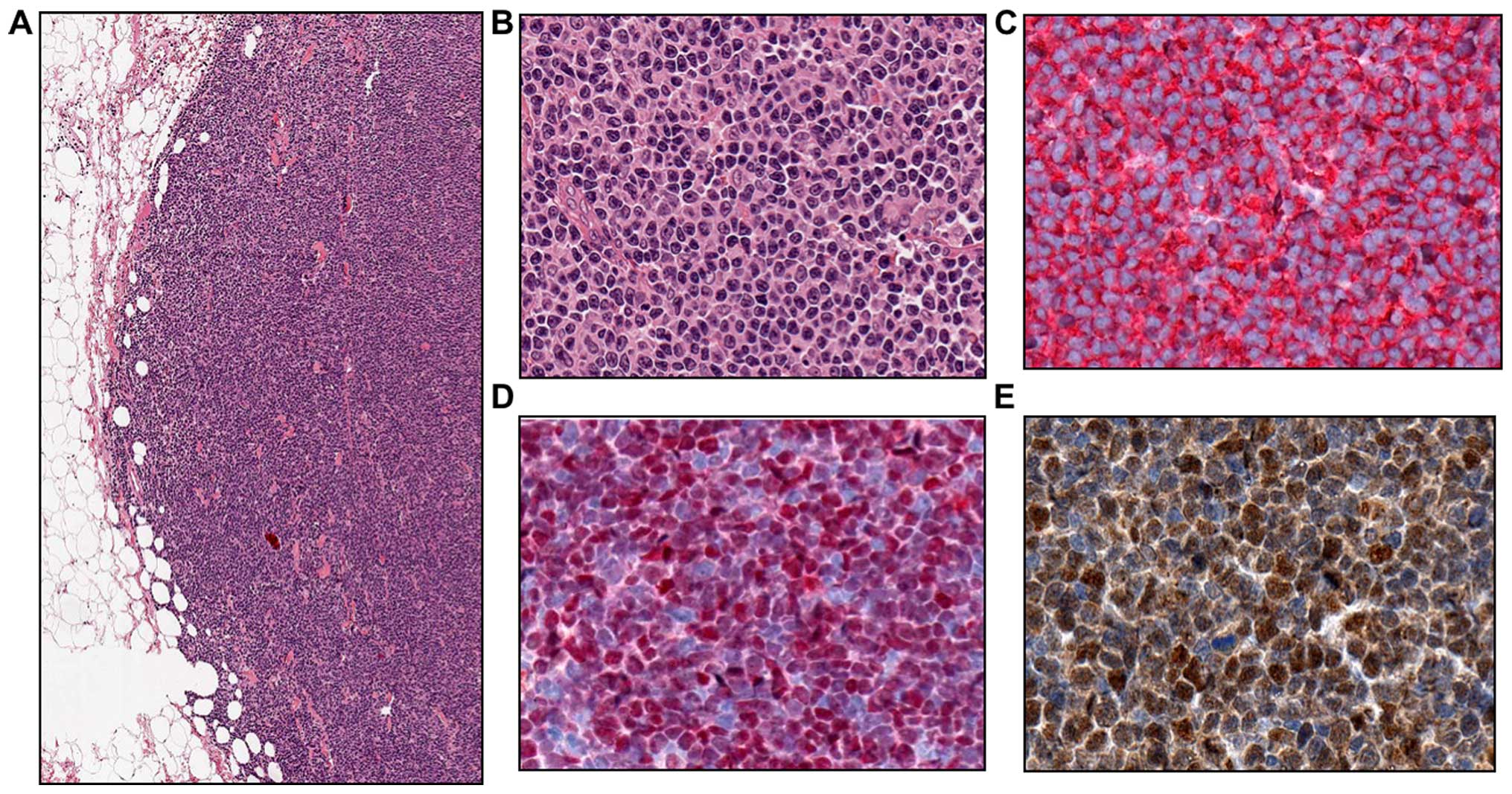
Histological examination revealed a deep subcutaneous infiltration
by a nodular mass of medium-sized lymphoid cells with irregular
nuclei. Immunohistochemical analysis revealed a population of
lymphoblasts that were strongly positive for CD5, CD20, CD79a,
cyclin D1, sex-determining region Y-box 11 (SOX11) and Ki67
(Fig. 2), but negative for CD10 and
CD23.
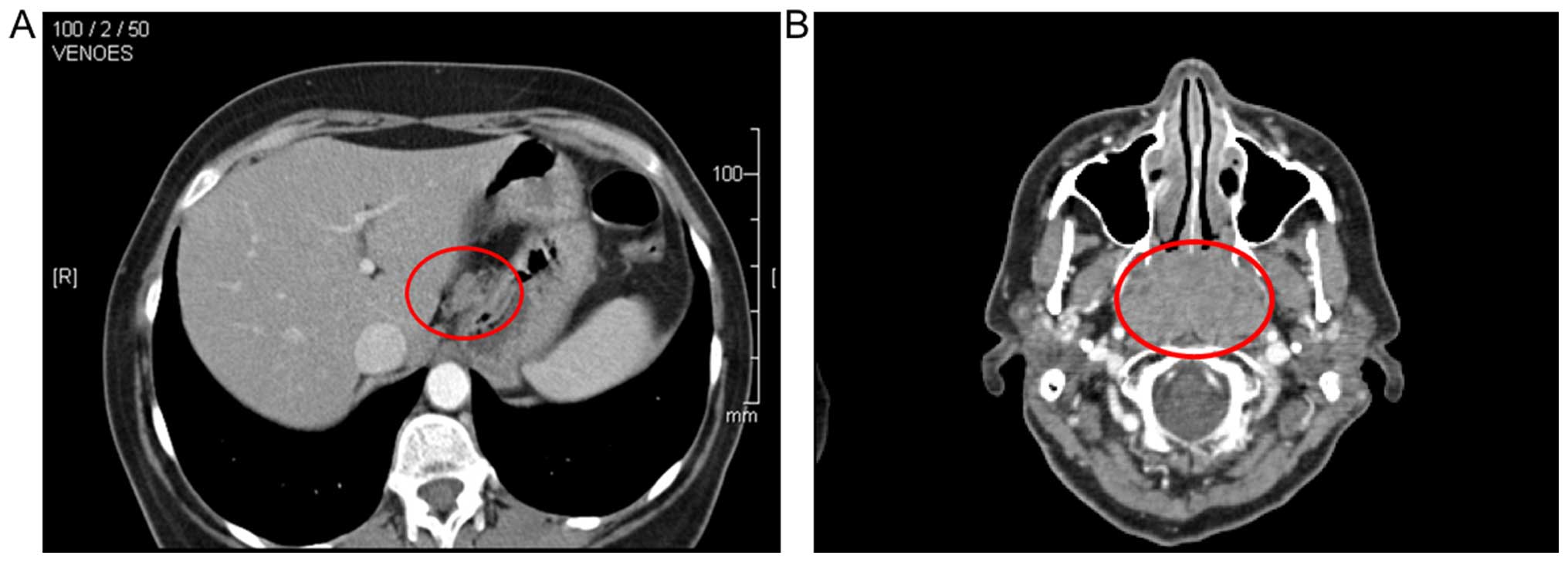
Contrast-enhanced computed tomography (CT) of the
neck and thorax revealed a 3.2×5.5×3.6-cm hyperdense enhancing mass
in the epipharynx, involving the left side of the tonsillar
region/bed. Several enlarged cervical and axillary lymph nodes were
enhanced bilaterally. Enlarged lymph nodes were also identified in
the lesser curvature of the stomach, in the paraaortic and coeliac
regions, and in the area between the inferior vena cava and the
head of the pancreas (Fig. 3).
Magnetic resonance imaging of the head revealed a 4.0×4.1×3.0-cm
homogeneously-enhanced epipharyngeal mass growing through the
choanae into the nasal cavity, with enlarged parapharyngeal lymph
nodes bilaterally. There were no cerebral pathological findings. A
bone marrow biopsy revealed diffuse infiltration by neoplastic
lymphoma cells.
Based on the clinicopathological and
immunohistochemical findings, the diagnosis of a cutaneous variant
of MCL at stage IVEB was established. Furthermore, chronic
hepatitis B of unknown origin was newly diagnosed. Antiviral
therapy with entecavir was initiated. The patient received six
cycles of adjuvant chemotherapy with rituximab, cyclophosphamide,
doxorubicin, vincristine, and prednisone (R-CHOP-14), administered
once a day for 2 weeks, along with three cycles of intrathecal
methotrexate as central nervous system (CNS) prophylaxis, with no
associated side effects. After the initial chemotherapy treatments,
stem cells were collected from the patient's bone marrow, in case
of the need for relapse therapy with a peripheral blood stem cell
transplant (PBSC).
After 10 weeks, the patient was restaged and found
to have a complete remission (CR); she remained in CR until
November, 2009, when she presented with a discrete axillary,
inguinal and mesenteric nodal recurrence and bone marrow
infiltration. The patient was treated with two cycles of rituximab,
cisplatin, cytosine arabinoside and dexamethasone (R-DHAP) and one
cycle of intrathecal methotrexate as CNS prophylaxis. Restaging by
CT and bone marrow biopsy confirmed partial remission.
Subsequently, the patient was consolidated with PBSC using
etoposide, cytosine arabinoside and melphalane (BEAM conditioning
regimen), which was associated with moderate to severe toxicity in
the form of fatigue, neutropenic fever and Clostridium
difficile-positive diarrhea. Following induction of an
intensive antibiotic and infusion treatment regimen, the patient's
symptoms and signs of infection regressed. At present, 2 years
after PBSC and 4 years after the appearance of the skin lesion, the
patient is well, with no evidence of relapse.
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images. A copy of the written consent is available for review by
the Editor of this journal.
Discussion
MCL is a B-cell lymphoma that affects older
patients, with a median age of incidence at 68 years. MCL
represents ~6% of all NHL subtypes (3) with a male-to-female incidence ratio of
3:1 (4,5). The prevalence is estimated to be ~2–3
cases per 100,000 patients per year (6). The overall median survival (OS) of MCL
is ~3–5 years. Adjuvant chemotherapy with the R-CHOP regimen in
patients with untreated MCL achieved an overall response rate of
96% (7,8).
MCL occurs primarily in lymph nodes and extranodal
organs, including the bone marrow, spleen and gastrointestinal
tract. Skin involvement occurs in 2% of MCL cases and is associated
with progressive disease (4,9). Immunophenotypically, MCL consist of
CD5−, CD19−, CD20−,
CD22−, CD45− and CD79a+ tumor
cells that are usually negative for CD10, CD23 and BCL-6. The
majority of MCLs are characterized by overexpression of cyclin D1,
as a result of the t(11;14) (q13;q32)/CCND1-IGH translocation
(1).
SOX11 is a transcription factor that is involved in
embryogenic development. Recent studies have demonstrated
overexpression of SOX11 in MCL (10),
but its prognostic significance remains controversial. Nygren et
al found that the OS of SOX11-negative cases was lower compared
with that of SOX11-positive cases (median OS: 1.5 vs. 3.2 years,
respectively; P=0.014) (11).
Conversely, Fernandez et al found that patients with
SOX11-negative tumors developed a predominantly extranodal type of
MCL and were characterized by longer survival compared with
SOX11-positive cases (5-year OS rate, 78 vs. 36%, respectively;
P=0.001) (12).
Skin manifestations of MCL are rare. Thus far,
detailed reports in the English literature have described only 22
patients with MCL exhibiting skin lesions. The median age at the
time of diagnosis is 63.6 years (range, 22–89 years). The most
common cutaneous lesion has been described as a nodule (occurring
in 56.5% of all patients), with a male-to-female ratio of 17:6. The
majority of the patients (73.9%) with this lesion also present with
extracutaneous involvement, such as of the bone marrow, lymph nodes
and spleen. Of the 22 MCL patients with cutaneous manifestations
identified, 12 succumbed to the disease (2,13–23). In those cases, the mean survival time
from the diagnosis of cutaneous manifestations to death was 11.2
months (range, 5 days-36 months). The published cases are
summarized in Table I.
 | Table I.Reported cases of mantle cell lymphoma
with cutaneous manifestations (modified and completed from Canpolat
et al). |
Table I.
Reported cases of mantle cell lymphoma
with cutaneous manifestations (modified and completed from Canpolat
et al).
| First Author
(Refs.) | Gender/age (yrs) | Skin lesion/site | Extracutaneous
manifestations | IHC | Follow-up |
|---|
| Bertero (2) | M/51 | Subcutaneous
nodule/breast | LN, liver,
spleen | CD5, CD19, CD21,
CD22, CD23, CD24, CD74 | A (17 yrs after
onset) |
|
| F/78 | Nodules/breast,
back | None | CD5, CD19, CD21,
CD22, CD24, CD38, CD74 | D (3 yrs after
diagnosis) |
|
| M/43 | Infiltrated
plaques/back, face, arm | LN, liver,
spleen | CD5, CD19, CD20,
CD21, CD23, CD24, CD25, CD45RA, CD74 | A |
|
| M/22 | Nodules/breast | None | CD5, CD19, CD20,
CD21, CD23, CD24, CD25, CD45RA, CD74 | A (5 yrs after
diagnosis) |
| Geerts (13) | F/65 | Nodules,
forehead | LN, BM | CD19, CD20, CD22 | D (11 mo after
diagnosis) |
|
| F/77 | Tumoral plaques/back,
breast, arm | Bronchus wall | CD5, CD22 | D (1.5 yrs after
diagnosis) |
| Martí (14) | F/61 | Tumoral
plaque/abdomen | LN, BM, tonsils | CD5, CD19, CD20,
CD45RA, cyclin D1, CD74 | D (15 mo after
diagnosis) |
| Moody (15) | M/47 | Nodules/ear | LN, BM | CD5, CD19, CD20,
cyclin D1 | A (3 yrs after
onset) |
| Sen (16) | M/85 | Macular rash/leg | LN, BM, buccal
mucosa | CD5, CD20, cyclin
D1 | D (20 mo after
diagnosis) |
|
| M/76 | Nodule/thigh | None | CD5, CD20, cyclin
D1 | A (30 mo after
diagnosis) |
|
| M/56 | Nodules/chest | BM, GI | CD20, cyclin D1 | A (21 mo after
diagnosis) |
|
| M/57 | Maculopapular
rash/legs | LN, BM, PB | CD5, CD20, cyclin
D1 | D (9 mo after
diagnosis) |
|
| M/61 | Plaques/flank, back,
thigh | LN, BM, PB,
leptomeninges | CD5, CD20, cyclin
D1 | D (4 mo after
onset) |
| Dubus (17) | M/56 | Papules/breast,
back | LN, BM, PB | CD5, CD20, CD43 | D (1 yr after
onset) |
|
| M/89 | Infiltrated
papules/breast, back, abdomen | LN, BM, PB | CD5, CD20, CD43 | D (5 days after
onset) |
|
| M/72 | Subcutaneous
nodules/arm, axillary region | LN, BM | CD20, CD43, cyclin
D1 | A (1 yr after
onset) |
| Motegi (18) | M/62 | Nodules,
ulceras/back, upper extermities, chest, penis shaft | LN, tonsils, spleen,
GI tract | CD5, CD20, CD43,
cyclin D1 | A (4 mo after
onset) |
| Estrozi (19) | M/72 | Nodule/face | None | CD5, CD20, CD45,
cyclin D1 | A (6 mo after
diagnosis) |
| Ishibashi (20) | M/68 | Nodules/thigh,
arm | LN, BM, PB | CD20, cyclin D1 | D (3 mo after
diagnosis) |
| Canpolat (21) | F/49 | Papules/face,
shoulders, back, chest | LN, BM, spleen | CD5, CD20, CD43,
CD79a, cyclin D1 | D (4 mo after
onset) |
| Zattra (22) | M/77 | Nodules,
plaques/entire body | None | CD5, CD20, CD22,
CD79a, cyclin D1 | A (28 mo after
diagnosis) |
| Lynch (23) | M/83 | Nodules/thigh | Could not be entirely
excluded | CD5, CD20, CD79a,
cyclin D1 | D (2 mo after
diagnosis) |
| Our case | F/55 | Subcutaneous
nodule/face | LN, BM,
tonsils | CD5, CD20, CD79a,
cyclin D1, SOX11 | A (4 yrs after
diagnosis) |
We herein described a rare case of MCL with
involvement of the skin in the right infraorbital region, with a
detailed 4-year follow-up and review of the available literature.
The patient's skin biopsy revealed that the neoplastic tumor cells
exhibited the characteristic cytology of MCL and were positive for
CD5, CD20, CD79a, cyclin D1 and SOX11. To the best of our
knowledge, this is the first report of a case of MCL with
SOX11-positive tumor cells in a skin lesion. Our patient remains
alive and well, with no evidence of disease relapse. This study
also highlights the need for further investigation, in order to
elucidate the role of SOX11 in the pathogenesis and prognosis of
MCL with cutaneous manifestations.
Acknowledgements
The authors wish to acknowledge the valuable
contribution of Dr Thomas Koculak for the high-quality images.
Glossary
Abbreviations
Abbreviations:
|
MCL
|
mantle cell lymphoma
|
|
NHL
|
non-Hodgkin's lymphoma
|
|
PBSC
|
peripheral blood stem cell
transplant
|
|
CR
|
complete remission
|
|
OS
|
overall survival
|
|
CNS
|
central nervous system
|
References
|
1
|
Leonard JP, Williams ME, Goy A, Grant S,
Pfreundschuh M, Rosen ST and Sweetenham JW: Mantle cell lymphoma:
Biological insights and treatment advances. Clin Lymphoma Myeloma.
9:267–277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertero M, Novelli M, Fierro MT and
Bernengo MG: Mantle zone lymphoma: An immunohistologic study of
skin lesions. J Am Acad Dermatol. 30:23–30. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weigert O, Unterhalt M, Hiddemann W and
Dreyling M: Mantle cell lymphoma: State-of-the-art management and
future perspective. Leuk Lymphoma. 50:1937–1950. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samaha H, Dumontet C, Ketterer N, Moullet
I, Thieblemont C, Bouafia F, Callet-Bauchu E, Felman P, Berger F,
Salles G and Coiffier B: Mantle cell lymphoma: A retrospective
study of 121 cases. Leukemia. 12:1281–1287. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oinonen R, Franssila K, Teerenhovi L,
Lappalainen K and Elonen E: Mantle cell lymphoma: Clinical
features, treatment and prognosis of 94 patients. Eur J Cancer.
34:329–336. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meusers P, Hense J and Brittinger G:
Mantle cell lymphoma: Diagnostic criteria, clinical aspects and
therapeutic problems. Leukemia. 11(Suppl 2): S60–S64.
1997.PubMed/NCBI
|
|
7
|
Howard OM, Gribben JG, Neuberg DS,
Grossbard M, Poor C, Janicek MJ and Shipp MA: Rituximab and CHOP
induction therapy for newly diagnosed mantle-cell lymphoma:
Molecular complete responses are not predictive of progression-free
survival. J Clin Oncol. 20:1288–1294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lenz G, Dreyling M, Hoster E, Wörmann B,
Dührsen U, Metzner B, Eimermacher H, Neubauer A, Wandt H,
Steinhauer H, et al: Immunochemotherapy with rituximab and
cyclophosphamide, doxorubicin, vincristine and prednisone
significantly improves response and time to treatment failure, but
not long-term outcome in patients with previously untreated mantle
cell lymphoma: Results of a prospective randomized trial of the
German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol.
23:1984–1992. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Argatoff LH, Connors JM, Klasa RJ, Horsman
DE and Gascoyne RD: Mantle cell lymphoma: A clinicopathologic study
of 80 cases. Blood. 89:2067–2078. 1997.PubMed/NCBI
|
|
10
|
Xu W and Li JY: SOX11 expression in mantle
cell lymphoma. Leuk Lymphoma. 51:1962–1967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nygren L, Wennerholm Baumgartner S,
Klimkowska M, Christensson B, Kimby E and Sander B: Prognostic role
of SOX11 in a population-based cohort of mantle cell lymphoma.
Blood. 119:4215–4223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernàndez V, Salamero O, Espinet B, Solé
F, Royo C, Navarro A, Camacho F, Beà S, Hartmann E, Amador V, et
al: Genomic and gene expression profiling defines indolent forms of
mantle cell lymphoma. Cancer Res. 70:1408–1418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geerts ML and Busschots AM: Mantle-cell
lymphomas of the skin. Dermatol Clin. 12:409–417. 1994.PubMed/NCBI
|
|
14
|
Martí RM, Campo E, Bosch F, Palou J and
Estrach T: Cutaneous lymphocyte-associated antigen (CLA) expression
in a lymphoblastoid mantle cell lymphoma presenting with skin
lesions. Comparison with other clinicopathologic presentations of
mantle cell lymphoma. J Cutan Pathol. 28:256–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moody BR, Bartlett NL, George DW, Price
CR, Breer WA, Rothschild Y and Kraus MD: Cyclin D1 as an aid in the
diagnosis of mantle cell lymphoma in skin biopsies: A case report.
Am J Dermatopathol. 23:470–476. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sen F, Medeiros LJ, Lu D, Jones D, Lai R,
Katz R and Abruzzo LV: Mantle cell lymphoma involving skin:
Cutaneous lesions may be the first manifestation of disease and
tumors often have blastoid cytologic features. Am J Surg Pathol.
26:1312–1318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dubus P, Young P, Beylot-Barry M,
Belaud-Rotureau MA, Courville P, Vergier B, Parrens M, Lenormand B,
Joly P and Merlio JP: Value of interphase FISH for the diagnosis of
t(11:14)(q13;q32) on skin lesions of mantle cell lymphoma. Am J
Clin Pathol. 118:832–841. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motegi S, Okada E, Nagai Y, Tamura A and
Ishikawa O: Skin manifestation of mantle cell lymphoma. Eur J
Dermatol. 16:435–478. 2006.PubMed/NCBI
|
|
19
|
Estrozi B, Sanches JA Jr, Varela PC and
Bacchi CE: Primary cutaneous blastoid mantle cell lymphoma-case
report. Am J Dermatopathol. 31:398–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishibashi M, Yamamoto K, Kudo S and Chen
KR: Mantle cell lymphoma with skin invasion characterized by the
common variant in the subcutis and blastoid transformation in the
overlying dermis. Am J Dermatopathol. 32:180–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Canpolat F, Taş E, Albayrak Sönmez A,
Oktay M, Eskioğlu F and Alper M: Cutaneous presentation of mantle
cell lymphoma. Acta Derm Venerol. 90:548–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zattra E, Zambello R, Marino F, Bordignon
M and Alaibac M: Primary cutaneous mantle cell lymphoma. Acta Derm
Venerol. 91:474–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lynch DW, Verma R, Larson E, Geis MC and
Jassim AD: Primary cutaneous mantle cell lymphoma with blastic
features: report of a rare case with special reference to staging
and effectiveness of chemotherapy. J Cutan Pathol. 39:449–453.
2012. View Article : Google Scholar : PubMed/NCBI
|