Introduction
Healthy individuals are equipped with defense
mechanisms against bacterial overgrowth and the small intestine is
relatively sterile (1). When one of
those mechanisms is impaired, small intestinal bacterial overgrowth
(SIBO) may occur. SIBO is usually caused by abnormal intestinal
structure, intestinal dysfunction and impaired intestinal mucosal
function (2). SIBO is a serious
condition associated with a number of diseases, which may severely
affect further treatment of the patients and compromise their
quality of life.
Colorectal cancer (CRC) is one of the most common
gastrointestinal malignant tumors. With the improvement of living
standards and changes in dietary habits, the incidence and
mortality of CRC have been continuously increasing in recent years
(3). Moreover, with the advances in
endoscopy and medical technology, the resection rate of CRC
patients has increased. Postoperative CRC patients are commonly
affected by symptoms such as unformed stools, constipation,
bloating and abdominalgia. However, remission may be achieved with
anti-SIBO treatments, such as bifid triple viable capsule and
compound lactobacillus acidophilus, which are administered to
regulate the intestinal flora based on the original treatment.
CRC and SIBO may exhibit overlapping symptoms.
Moreover, the postoperative changes in the anatomical structure of
the intestinal tract provides SIBO with breeding grounds in
postoperative CRC patients. Therefore, it is reasonable to
hypothesize that SIBO is likely to occur in such patients. On the
basis of this hypothesis, we investigated whether treatment for
SIBO may improve the digestive symptoms of postoperative CRC
patients.
Patients and methods
Patients
A total of 43 postoperative CRC patients, aged 38–83
years (26 men and 17 women), who were hospitalized at the
departments of Gastroenterology, General Surgery, Hepatobiliary
Surgery and Oncology of Qingdao Municipal Hospital between January,
2012 and January, 2014, were selected. The patients were diagnosed
with CRC through imaging examinations such as gastroscopy,
colonoscopy and barium meal for the entire digestive system, and
other accessory examinations, such as measurement of tumor markers.
The diagnoses were confirmed with histopathological examinations
following surgical removal of the tumors. All the patients signed
written informed consent forms for the examinations. Furthermore,
30 healthy individuals (16 men and 14 women), aged 24–62 years,
were recruited from the outpatient clinic and schools for the
control group.
All the investigated subjects were required to meet
the following criteria: i) No other conditions, such as diabetes,
thyroid diseases, intestinal pseudo-obstruction, irritable bowel
syndrome (IBS), or any other disease that may lead to poor
gastrointestinal motility, and no lactose intolerance; ii) no use
of antibiotics, antacids or probiotics over the last month; iii) no
history of treatment with hormones, antidepressants and opioids; no
long-term history of heavy smoking; iv) no colonoscopy or enema
within the last 4 weeks; v) no history of renal insufficiency or
infection.
All the procedures were performed under consensus
agreements and in accordance with the Ethics Review Committee of
Qingdao Municipal Hospital.
Glucose hydrogen breath test
(GHBT)
All the investigated subjects underwent GHBT (model:
HHBT-01; Shenzhen CNNC Headway Biotechnology Co., Ltd.). i)
Sedative hypnotics, smoking, and the consumption of cooked
wheat-containing foods, vegetables, fruits, bean products, dairy
products and other foods rich in cellulose, were not allowed one
day before the test; ii) rice was allowed as staple food, with meat
and eggs as non-staple foods on the day before the test, avoiding
overeating; iii) consumption of solid or liquid foods was not
allowed after 8:00 pm, to ensure a comparatively low value of
fasting breath hydrogen (FBH) on the morning of the test (12-h
fasting); iv) smoking was prohibited one day before and throughout
the test. Eating and drinking were also not allowed during the
test, as were strenuous exercise and sleeping. All the investigated
subjects were advised to brush their teeth carefully before the
test and were kept in a sitting position.
Experimental methods
Using the disposable gas mouth blowpipe, the
investigated subject exhaled slowly for as long as possible, with
the flow rate being controlled at 250 ml/min. When the first
exhalation was completed, the patients continued exhaling after
taking a breath, until the figures on the liquid-crystal display
screen stopped rising, which required ~70 sec. First, FBH was
measured. Subsequently, 50–80 g glucose plus 200–250 ml warm boiled
water was used as the substrate (the patients gargled after having
the substrate in order to minimize the effect of oral bacteria on
the test). The expiratory hydrogen concentration was measured every
20 min after taking the substrate for 2 h in total.
SIBO diagnostic criteria
SIBO was considered to be positive if the expiratory
hydrogen concentration had increased by >12 ppm after taking the
glucose substrate, and negative otherwise.
Treatment
Patients diagnosed as SIBO-positive were
continuously treated with oral rifaximin (1,200 mg/day) for 10
days. GHBT was performed again at the end of the treatment, to
investigate and analyze the changes in expiratory hydrogen
concentration and gastrointestinal symptoms score (GISS).
GISS
The visual analogue scale (VAS) was applied to all
patients to evaluate the symptoms of diarrhea, constipation,
bloating, abdominalgia, poor appetite and fever. The total score of
each item was defined as the overall GISS. The symptom scores were
compared separately between SIBO-positive and SIBO-negative
patients, SIBO-positive patients before and after treatment, and
SIBO eradicated and not eradicated groups.
Statistical analysis
Statistical analysis of the results was conducted
using SPSS v19.0 software (Armonk, NY, USA). Qualitative data were
evaluated using the χ2 test and P<0.05 was considered
to indicate statistically significant differences. Two-sample
t-tests for a difference in mean and the Mann-Whitney U test of
rank sum test were performed to check the normality and homogeneity
of variance. P<0.05 was considered to to indicate statistically
significant differences.
Results
Prevalence of SIBO in postoperative
CRC patients
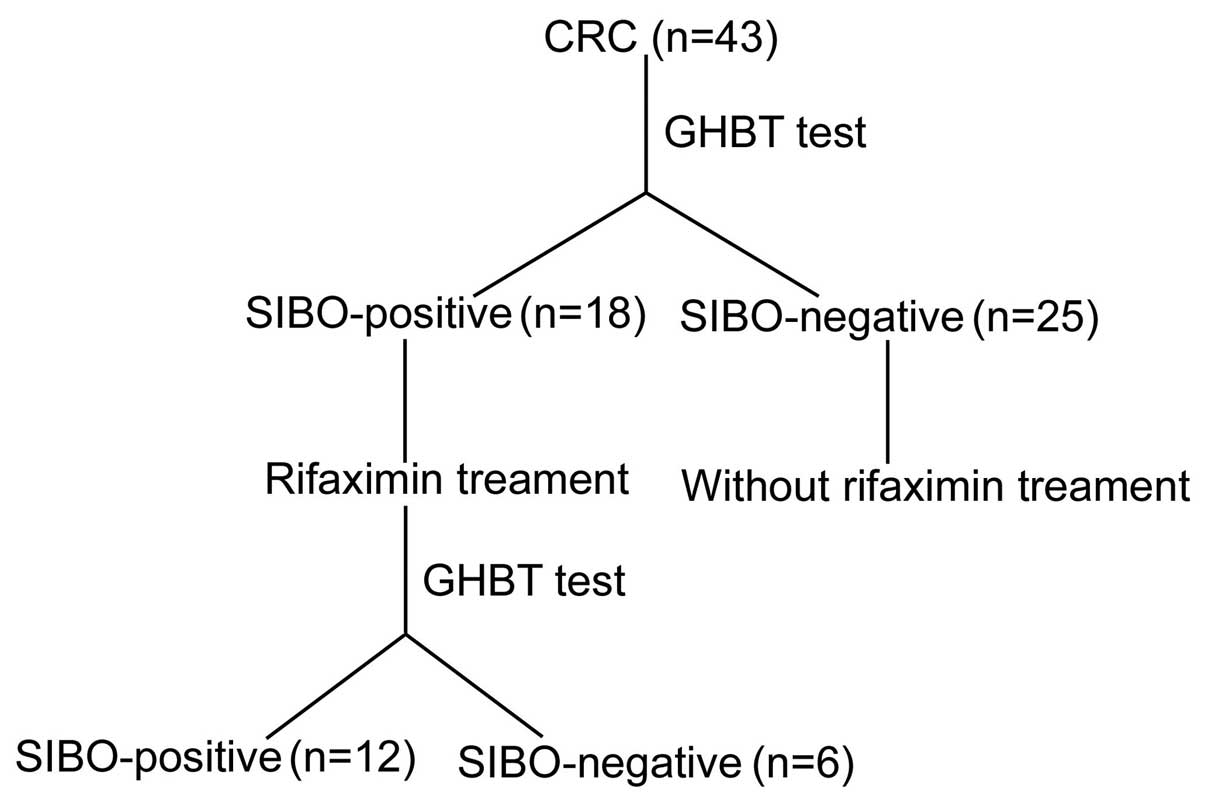
Among the 43 postoperative patients with CRC, 18
were diagnosed as SIBO-positive. However, among the 30 healthy
individuals in the control group, only 2 were found to be
SIBO-positive (41.86 vs. 6.67%; P<0.05). Comparing the CRC group
with the control group, the age difference of the investigated
subjects was statistically significant (P<0.05), while gender
was not (P>0.05) (Table I;
Fig. 1).
 | Table I.Characteristics of study subjects and
prevalence of SIBO. |
Table I.
Characteristics of study subjects and
prevalence of SIBO.
|
| Groups |
| Prevalence |
|
|---|
|
|
|
|
|
|
|---|
| Characteristics | CRC (n=43) | Control (n=30) | P-value | SIBO-positive
(n=18) | SIBO-negative
(n=25) | P-value |
|---|
| SIBO prevalence,
% | 41.89 | 6.67 | 0.001 | – | – | – |
| Age, years ± SD | 63.67±11.09 | 41.30±13.84 | 0.000 | 68.72±10.15 | 60.04±10.45 | 0.010 |
| Male/female | 26/17 | 16/15 | 0.544 | 11/7 | 15/10 | 0.941 |
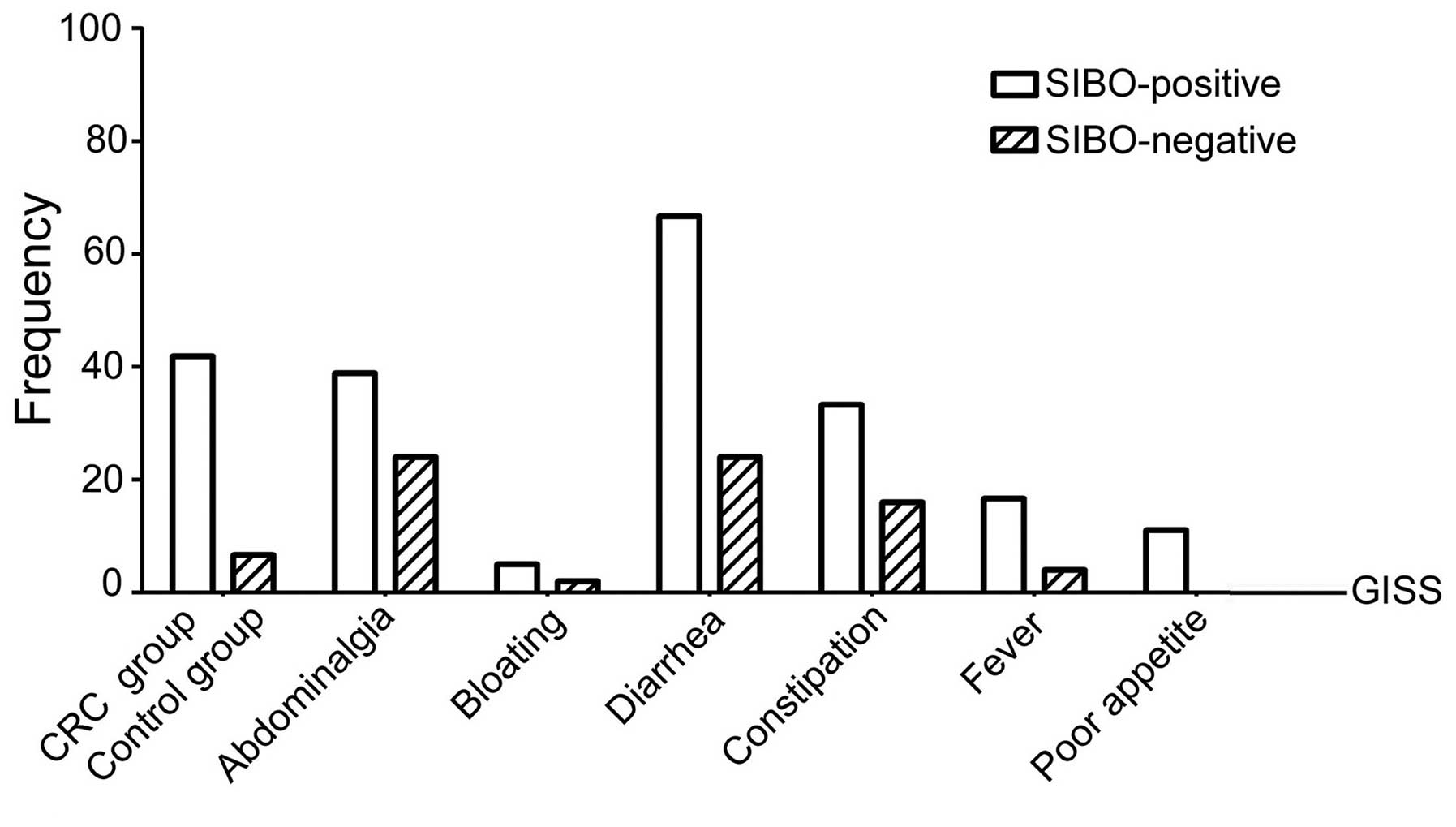
Digestive tract symptoms of SIBO
As regards the incidence of abdominalgia (34.88%),
bloating (32.56%), diarrhea (41.86%), constipation (23.26%), fever
(9.30%) and poor appetite (4.65%) among the 43 patients, the
incidence of diarrhea was the highest, followed by abdominalgia and
bloating (P<0.05) (Table II).
 | Table II.Prevalence of gastrointestinal
symptoms. |
Table II.
Prevalence of gastrointestinal
symptoms.
|
|
| Prevalence, %
(n) |
|
|---|
|
|
|
|
|
|---|
| Gastrointestinal
symptoms | Overall prevalence
(%) | SIBO-positive
(n=18) | SIBO-negative
(n=25) | P-value |
|---|
| Abdominalgia | 34.88 | 38.89 (7) | 32.00 (8) | 0.640 |
| Bloating | 32.56 | 50.00 (9) | 20.00 (5) | 0.038 |
| Diarrhea | 41.86 | 66.67 (12) | 24.00 (6) | 0.005 |
| Constipation | 23.26 | 33.33 (6) | 16.00 (4) | 0.336 |
| Fever | 9.30 | 16.67 (3) | 4.00 (1) | 0.380 |
| Poor appetite | 4.65 | 11.11 (2) | 0.0 (0) | 0.092 |
Among the 18 SIBO-positive patients, the difference
among symptoms was statistically significant (P<0.05). The
incidence of diarrhea was the highest, and it was significantly
higher compared with that among SIBO-negative patients (66.67 vs.
24.0%, respectively; P<0.05). Among the 25 SIBO-negative
patients, the difference among symptoms was statistically
significant (P<0.05) and the incidence of abdominalgia was the
highest, although the difference was not statistically significant
(32.0 vs. 38.89%; P>0.05). The differences among all the other
symptoms were not statistically significant (P>0.05) (Table II; Fig.
2).
VAS was applied to all the patients to calculate
GISS. The value of SIBO-positive patients was significantly higher
compared with that of SIBO-negative patients (8 vs. 4,
respectively; P<0.05). Among 6 gastrointestinal symptoms, every
score in the SIBO-positive patients was higher compared with that
in SIBO-negative patients, but only the difference in diarrhea was
statistically significant (7 vs. 5, respectively; P<0.05)
(Table III).
 | Table III.GISS of SIBO-positive and
SIBO-negative groups prior to treatment. |
Table III.
GISS of SIBO-positive and
SIBO-negative groups prior to treatment.
|
| Median severity score
(range) |
|
|---|
|
|
|
|
|---|
| Gastrointestinal
symptoms | SIBO-positive
(n=18) | SIBO-negative
(n=25) | P-value |
|---|
| Global GISS | 8 (6–14.25) | 4 (1–7) | 0.002 |
| Abdominalgia | 5 (2–6) | 3 (2–6) | 0.604 |
| Bloating | 6 (3–7.5) | 4 (2.5–5.5) | 0.249 |
| Diarrhea | 7 (4–8) | 5 (3–6.25) | 0.036 |
| Constipation | 5.5 (2.75–6.5) | 6.5 (4.5–7) | 0.386 |
| Fever | 6 (4–6) | 4 (4–4) | 0.317 |
| Poor appetite | 5.5 (3.75–4.5) | 0 (0–0) | 0.102 |
Effect of rifaximin on SIBO
SIBO-positive patients were continuously treated
with rifaximin (1,200 mg/day) for 10 days. GHBT was performed at
the end of the treatment course and GISS was calculated. Following
rifaximin treatment, the overall GISS was lower compared with that
prior to treatment (8 vs. 4, respectively; P<0.05). Only the
difference in diarrhea was statistically significant compared with
the values prior to treatment (7 vs. 3, respectively; P<0.05),
while no statistically significant differences were observed
regarding the rest of the symptoms (Table IV).
 | Table IV.GISS of the SIBO-positive group before
and after treatment. |
Table IV.
GISS of the SIBO-positive group before
and after treatment.
|
| Median severity score
(range) |
|
|---|
|
|
|
|
|---|
| Gastrointestinal
symptoms | Pre-treatment | Post-treatment | P-value |
|---|
| Global GISS | 8 (6–14.25) | 4 (3–9.50) | 0.037 |
| Abdominalgia | 5 (2–6) | 3 (2–6) | 0.561 |
| Bloating | 6 (3–7.50) | 3 (1.50–5.50) | 0.120 |
| Diarrhea | 7 (4–8) | 3 (2–4) | 0.008 |
| Constipation | 5.5 (2.75–6.50) | 4 (2.50–5.80) | 0.450 |
| Fever | 6 (4–6) | 3 (3–3) | 0.102 |
| Poor appetite | 5.5 (3.75–4.50) | 6 (3–6) | 0.655 |
In addition, 6 of the 18 SIBO-positive patients
became SIBO-negative, with a negative conversion ratio of 33.33%.
No symptoms were experienced by the 2 SIBO-positive patients in the
control group; thus, no treatment was administered (Table V).
 | Table V.Comparison between SIBO
turned-negative group and SIBO non-turned-negative group. |
Table V.
Comparison between SIBO
turned-negative group and SIBO non-turned-negative group.
| Global GISS | Pre-treatment | P-value | Post-treatment | P-value |
|---|
| Turned-negative
group | 8 (6–10.80) | 0.742 | 3 (1–4) | 0.023 |
| Non-turned-negative
group | 7.5 (6–26.3) |
| 7.5 (3.3–20.8) |
|
Discussion
In recent years, several studies demonstrated that
SIBO was correlated with several digestive system diseases or
symptoms, such as IBS, cirrhosis and acute severe pancreatitis.
Postoperative CRC patients also exhibited several digestive
problems (4,5). In this study, GHBT was performed in 43
postoperative patients with CRC and 30 healthy individuals. A total
of 41.86% postoperative CRC patients were found to have an abnormal
expiratory hydrogen concentration, while 6.67% of the control group
were found to be SIBO-positive. The overall symptoms of the CRC
patients with SIBO, particularly diarrhea, were improved following
rifaximin treatment, whereas 33.33% of the SIBO-positive patients
became SIBO-negative. Therefore, SIBO detection and treatment were
necessary for postoperative CRC patients.
It has been demonstrated that SIBO, as well as
bacteremia, sepsis and intestinal endotoxemia induced by SIBO, are
serious conditions associated with a number of diseases, which may
affect further treatment of the patients and severely compromise
their quality of life. The overgrown bacteria compete with the host
for dietary vitamin B12, interfere with the metabolism of bile
salts and affect the absorption of amino acids, thereby leading to
B12 hypovitaminosis, diarrhea and hypoalbuminemia (6). Furthermore, the overgrown small
intestinal bacteria may greatly increase the number of the bacteria
that adhere to the intestinal wall, subsequently producing a large
number of metabolites and toxins that may damage the structure of
the intestinal mucosa (7,8). Therefore, SIBO adversely affects the
intestinal tract through various factors, thereby aggravating
gastrointestinal tract symptoms.
It was found that the clinical symptoms of the
patients with SIBO were more severe compared with those of patients
without SIBO. In addition, among all SIBO-positive patients, the
incidence of diarrhea was the highest, followed by abdominalgia and
bloating. However, among SIBO-negative patients, the incidence of
abdominalgia was the highest, while that of diarrhea and bloating
was not as high. This difference in diarrhea scores was
statistically significant (P<0.05). The points mentioned above
indicate that patients with severe gastrointestinal symptoms are
more likely to have SIBO, particularly when diarrhea is the main
symptom. SIBO may aggravate the patients' gastrointestinal
symptoms, and treating SIBO improves such symptoms.
The symptoms of IBS may be significantly alleviated
through the application of probiotics or antibiotics to eradicate
SIBO. Previously reported data even demonstrated that the
gastrointestinal symptoms of some patients with IBS were completely
relieved following eradication of SIBO (9–12).
Rifaximin is a semi-synthetic antibiotic designed based on
rifamycin. It is a type of broad-spectrum oral bactericidal that
cannot be absorbed through the intestinal wall. Thus, there are no
known interactions with other drugs and its adverse reactions are
mild (13,14). Recently, an increasing number of
researchers verified the therapeutic effect of rifaximin on
intestinal diseases. A dose of 550 mg rifaximin three times a day
for 14 days has been used to treat patients with IBS, as reported
by Pimentel et al (15); they
found that 40.8% of the symptoms improved, compared with 31.2% of
IBS patients treated with placebo (P<0.05). In another study,
IBS patients treated with rifaximin for 14 days were followed up
and it was found that their symptoms of bloating, diarrhea and
abdominalgia were significantly improved within 3 months (16). The study of Biancone et al
(17) demonstrated that short-course
treatment with rifaximin for patients with Crohn's disease combined
with SIBO was effective. All those studies indicate that rifaximin
is effective against SIBO.
In our study, it was found that, after treatment
with rifaximin for 10 days, the GISS of 18 SIBO-positive patients
decreased (P<0.05), and 33.33% of those were no longer
SIBO-positive. The overall symptoms, particularly diarrhea, were
significantly alleviated following treatment for SIBO, proving that
treatment for SIBO is an effective measure for alleviating the
gastrointestinal symptoms of postoperative patients with CRC.
Therefore, the identification and timely treatment of SIBO are
crucial.
Although treatment with rifaximin for SIBO may
relieve some of the symptoms, it did not relieve all the
gastrointestinal symptoms, and the turned-negative rate was rather
low. Therefore, more effective agents for the eradication of SIBO
are urgently required.
Acknowledgements
The present study was supported in part by a grant
from the Qingdao Science and Technology Bureau (grant no.
13-1-3-14-NSH to Ms. Lin Xu).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
FBH
|
fasting breath hydrogen
|
|
GHBT
|
glucose hydrogen breath test
|
|
GISS
|
gastrointestinal symptoms score
|
|
IBS
|
irritable bowel syndrome
|
|
SIBO
|
small intestinal bacterial
overgrowth
|
|
VAS
|
visual analogue scale
|
References
|
1
|
Bustillo I, Larson H and Saif MW: Small
intestine bacterial overgrowth: An underdiagnosed cause of diarrhea
in patients with pancreatic cancer. JOP. 10:576–578.
2009.PubMed/NCBI
|
|
2
|
Bures J, Cyrany J, Kohoutova D, Förstl M,
Rejchrt S, Kvetina J, Vorisek V and Kopacova M: Small intestinal
bacterial overgrowth syndrome. World J Gastroenterol. 16:2978–2990.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Durko L and Malecka-Panas E: Lifestyle
modifications and colorectal cancer. Curr Colorectal Cancer Rep.
10:45–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madrid AM, Hurtado C, Gatica S, Chacón I,
Toyos A and Defilippi C: Endogenous ethanol production in patients
with liver cirrhosis, motor alteration and bacterial overgrowth.
Rev Med Chil. 130:1329–1334. 2002.(In Spanish). PubMed/NCBI
|
|
5
|
Fisher D, Rajon D, Breitz H, Goris M,
Bolch W and Knox S: Dosimetry model for radioactivity localized to
intestinal mucosa. Cancer Biother Radiopharm. 19:293–307. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan X and Sellin JH: Review article: Small
intestinal bacterial overgrowth, bile acid malabsorption and gluten
intolerance as possible causes of chronic watery diarrhoea. Aliment
Pharmacol Ther. 29:1069–1077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Husebye E, Hellström PM, Sundler F, Chen J
and Midtvedt T: Influence of microbial species on small intestinal
myoelectric activity and transit in germ-free rats. Am J Physiol
Gastrointest Liver Physiol. 280:G368–G380. 2001.PubMed/NCBI
|
|
8
|
Passos MC, Serra J, Azpiroz F,
Tremolaterra F and Malagelada JR: Impaired reflex control of
intestinal gas transit in patients with abdominal bloating. Gut.
54:344–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basseri RJ, Weitsman S, Barlow GM and
Pimentel M: Antibiotics for the treatment of irritable bowel
syndrome. Gastroenterol Hepatol (NY). 7:455–493. 2011.
|
|
10
|
Jolley J: High-dose rifaximin treatment
alleviates global symptoms of irritable bowel syndrome. Clin Exp
Gastroenterol. 4:43–48. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marie I, Ducrotté P, Denis P, Menard JF
and Levesque H: Small intestinal bacterial overgrowth in systemic
sclerosis. Rheumatology (Oxford). 48:1314–1319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan AH, Mahadeva S, Thalha AM, Gibson PR,
Kiew CK, Yeat CM, Ng SW, Ang SP, Chow SK, Tan CT, et al: Small
intestinal bacterial overgrowth in Parkinson's disease.
Parkinsonism Relat Disord. 20:535–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saadi M and McCallum RW: Rifaximin in
irritable bowel syndrome: Rationale, evidence and clinical use.
Ther Adv Chronic Dis. 4:71–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karanje RV, Bhavsar YV, Jahagirdar KH and
Bhise KS: Formulation and development of extended-release micro
particulate drug delivery system of solubilized rifaximin. AAPS
PharmSciTech. 14:639–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pimentel M, Morales W, Chua K, Barlow G,
Weitsman S, Kim G, Amichai MM, Pokkunuri V, Rook E, Mathur R and
Marsh Z: Effects of rifaximin treatment and retreatment in
nonconstipated IBS subjects. Dig Dis Sci. 56:2067–2072. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meyrat P, Safroneeva E and Schoepfer AM:
Rifaximin treatment for the irritable bowel syndrome with a
positive lactulose hydrogen breath test improves symptoms for at
least 3 months. Aliment Pharmacol Ther. 36:1084–1093. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biancone L, Vernia P, Agostini D, Ferrieri
A and Pallone F: Effect of rifaximin on intestinal bacterial
overgrowth in Crohn's disease as assessed by the
H2-glucose breath test. Curr Med Res Opin. 16:14–20.
2000. View Article : Google Scholar : PubMed/NCBI
|