Introduction
Following introduction of the prostate-specific
antigen (PSA) screening test, the majority of prostate cancer
patients are diagnosed in the early stages of the disease and
undergo definitive local treatment, such as radical prostatectomy
(RP) and radiotherapy. However, a subset of patients experience
biochemical recurrence (BCR) or succumb to prostate cancer
(1,2).
Thus, prevention of recurrence and progression to lethal prostate
cancer represents a major public health challenge.
The established risk factors for prostate cancer are
age, race and family history, which are all non-modifiable
(1). To date, several researchers
have investigated the association between modifiable lifestyle
factors, such as obesity, smoking and a high-fat diet, and the risk
and prognosis of prostate cancer (1,3). The
identification of modifiable factors that affect the clinical
course of prostate cancer may be useful for preventing recurrence
and progression after definitive local treatment. Recent studies
have demonstrated a decreased risk of high-grade prostate cancer in
men with lower circulating total cholesterol (TC) levels (4–6). In
addition, several studies reported that statin, a
cholesterol-lowering drug, may protect against high-stage or
high-grade prostate cancer (7).
Furthermore, it has been demonstrated that statins decrease the
risk of BCR or prostate cancer-specific mortality (8,9). However,
to date, only a limited number of studies have investigated the
association between pretreatment serum TC level and the prognosis
of prostate cancer, particularly in the PSA era. The aim of this
study was to investigate the clinical implications of preoperative
serum TC level in Japanese prostate cancer patients who underwent
RP.
Patients and methods
Patients
This retrospective study was conducted according to
the ethical guidelines for clinical studies of the Ministry of
Health, Labor and Welfare of Japan, and was approved by the ethics
committee of our institution (no. 1621). Clinical data from 719
Japanese patients who underwent RP and pelvic lymph node dissection
for clinical T1-3N0M0 prostate cancer at our institution from 2000
to 2010 were collected by reviewing the patients' medical charts.
Patients who received neoadjuvant hormonal therapy (n=112) or
high-intensity focused ultrasonography (n=1) and patients with
unavailable serum TC level data (n=44) were excluded from the
study. Finally, a total of 562 patients were included in our study.
These patients were treated by open retropubic (n=316) or
robot-assisted (n=246) RP, and only lymph node sampling was
performed. Clinical stages were assigned according to the 2002 TNM
staging system (10), and pathology
outcomes, such as pathological stage, status of surgical margins,
lymphovascular invasion (LVI), perineural invasion (PNI) and the
Gleason score (GS) of the RP specimens, were obtained from the
official pathology reports. Peripheral blood samples were primarily
obtained from all the patients at the time of hospitalization. Data
on statin use was extracted from the patients' records at the time
of RP. Body mass index (BMI) was calculated as weight (kg) divided
by the square of the height (m2) and expressed as
kg/m2.
Data analyses
The data were expressed as mean ± standard deviation
(SD). The primary outcome measure in this study was BCR. The time
of BCR was defined as the earliest date that the postoperative
serum PSA levels increased to ≥0.2 ng/ml, and BCR was confirmed by
a second PSA examination result that was equal to or higher than
the initially recorded PSA levels. The day of surgery was reported
as the PSA recurrence day if postoperative serum PSA levels did not
decrease to ≤0.1 ng/ml. Factors analyzed included age at RP,
preoperative serum PSA levels, BMI, preoperative serum TC level,
statin use, and all relevant pathological factors [GS,
extracapsular extension (ECE), seminal vesicle invasion (SVI),
surgical margin status, LVI, PNI and lymph node metastasis] of the
RP specimen. Age at RP, preoperative serum PSA levels, BMI and
preoperative TC level were included as continuous variables. BMI
was also analyzed as a categorical variable in the Cox regression
analyses. Patients were classified using the World Health
Organization criteria as follows: BMI <25 kg/m2
(normal weight), BMI 25–30 kg/m2 (overweight) and BMI
≥30 kg/m2 (obesity) (11).
The variables of the different groups were compared using the
Pearson's chi-square test, the Mann-Whitney U test, or analysis of
variance. Univariate and multivariate Cox regression analyses were
performed to assess the association between BCR and the
clinicopathological variables. Multivariate Cox regression analyses
were performed using a forward stepwise variable selection
procedure, and survival curves were constructed using the
Kaplan-Meier method with log-rank tests. The patients were divided
into two groups by the cut-off identified by the root node in a
recursive partitioning analysis according to the previous report by
de Martino et al (12). All
P-values were two-tailed, and P-values <0.05 were considered to
indicate statistically significant differences. All statistical
analyses were performed using Stata software, version 11
(StataCorp, College Station, TX, USA) and JMP software, version 9
(SAS Institute, Cary, NC, USA).
Results
Serum TC level and clinicopathological
factors
The patients' characteristics are summarized in
Table I. The mean ± SD serum TC level
of all patients was 209±32.1 mg/dl (median, 209 mg/dl;
interquartile range, 190–230 mg/dl). The serum TC level was found
to be significantly correlated with age (Spearman's r = −0.101,
P=0.016), but not with PSA level (P=0.737) or BMI (P=0.274) (data
not shown). In addition, low TC level was found to be significantly
associated with statin use and presence of LVI (P=0.003 and
P=0.014, respectively; Table
II).
 | Table I.Patient characteristics (n=562). |
Table I.
Patient characteristics (n=562).
| Characteristics | Valuesa |
|---|
| Age, years | 65.9±6.4 |
| Psa, ng/ml | 10.6±10.1 |
| Body mass index,
kg/m2 | 23.7±2.9 |
|
<25 | 377 (67.1) |
|
25–30 | 170 (30.2) |
| ≥30 | 15 (2.7) |
| Total serum
cholesterol, mg/dl | 209±32.1 |
| Statin use |
|
| No | 493 (87.7) |
| Yes | 69 (12.3) |
| Clinical stage |
|
| T1c | 417 (71.2) |
| T2 | 136 (27.2) |
| T3 | 9 (1.6) |
| Pathological
factors |
|
| RP Gleason score |
|
| ≤6 | 100 (17.8) |
| 7 | 338 (60.1) |
| ≥8 | 124 (22.1) |
| Extracapsular
extension |
|
| (−) | 381 (67.8) |
| (+) | 181 (32.2) |
| Seminal vesicle
invasion |
|
| (−) | 511 (90.9) |
| (+) | 51 (9.1) |
| Surgical margin |
|
| (−) | 306 (54.4) |
| (+) | 256 (45.6) |
| Lymphovascular
invasion |
|
| (−) | 414 (73.7) |
| (+) | 148 (26.3) |
| Perineural
invasion |
|
| (−) | 242 (43.1) |
| (+) | 320 (56.9) |
| Lymph node
metastases |
|
| (−) | 555 (98.8) |
| (+) | 7 (1.2) |
 | Table II.Association between serum TC level and
clinicopathological factors. |
Table II.
Association between serum TC level and
clinicopathological factors.
|
| Serum TC level |
|
|---|
|
|
|
|
|---|
| Factors | Mean | SD | P-value |
|---|
| Statin use |
|
| 0.003 |
| No | 211 | 31.7 |
|
| Yes | 199 | 33.0 |
|
| Clinical T stage |
|
| 0.292 |
| T1c | 209 | 32.6 |
|
| T2a | 217 | 32.1 |
|
| T2b | 211 | 28.2 |
|
| T2c | 204 | 8.3 |
|
| T3 | 213 | 10.7 |
|
| Gleason score |
|
| 0.280 |
| ≤7 | 211 | 32.0 |
|
| ≥8 | 207 | 32.2 |
|
| Extracapsular
extension |
|
| 0.867 |
| (−) | 210 | 30.4 |
|
| (+) | 210 | 35.5 |
|
| Seminal vesicle
invasion |
|
| 0.229 |
|
(−) | 210 | 32.2 |
|
|
(+) | 204 | 30.5 |
|
| Surgical
margin |
|
| 0.232 |
|
(−) | 208 | 32.0 |
|
|
(+) | 212 | 32.2 |
|
| Lymphovascular
invasion |
|
| 0.014 |
|
(−) | 211 | 31.9 |
|
|
(+) | 204 | 32.1 |
|
| Perineural
invasion |
|
| 0.663 |
|
(−) | 209 | 32.1 |
|
|
(+) | 210 | 32.1 |
|
| lymph node
metastases |
|
| 0.834 |
|
(−) | 210 | 32.2 |
|
|
(+) | 212 | 22.5 |
|
Serum TC level and prognosis
During follow-up (mean, 54.0 months), 168 patients
(168/562, 29.9%) experienced BCR, with a 5-year BCR-free rate of
67.2%. In the univariate analysis, a higher serum PSA level at
diagnosis, advanced clinical tumor stage, ECE, positive surgical
margin, SVI, LVI, PNI, higher GS (GS ≥8) based on the RP specimen,
increased BMI, and a lower preoperative serum TC level, were
significantly associated with BCR (Table III). BCR-free survival curves
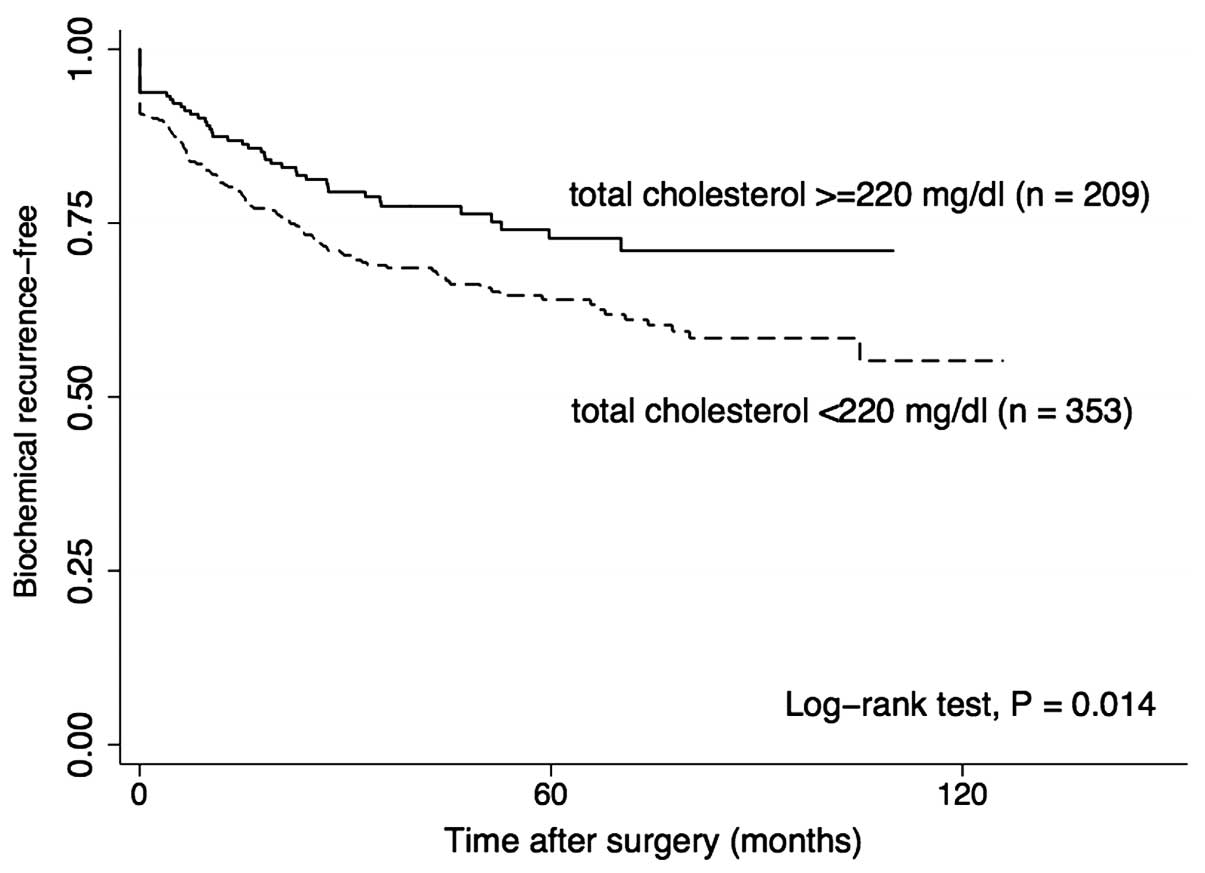
according to the TC level are presented in Fig. 1. The cut-off TC level was defined by
recursive partitioning analysis. The 5-year BCR-free rate in
patients with high TC level (TC ≥220 mg/dl) was higher compared
with that in patients with low TC levels (TC <220 mg/dl) (72.8
vs. 64.0%, respectively; P=0.014).
 | Table III.Results of univariate and
multivariate analyses. |
Table III.
Results of univariate and
multivariate analyses.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | P-value | HR | 95% CI | P-value |
|---|
| Age at diagnosis,
years |
|
|
Continuous | 1.022 | 0.087 |
|
| 0.896 |
| PSA, ng/ml |
|
|
Continuous | 1.034 | <0.001 | 1.018 | 1.007–1.029 | 0.002 |
| Statin use |
|
| No vs.
yes | 1.211 | 0.442 |
|
| 0.120 |
| Clinical T
stage |
|
| 1 | 1 |
|
|
|
|
| 2 | 1.593 | 0.006 |
|
| 0.522 |
| 3 | 10.681 | <0.001 |
|
| 0.021 |
| Gleason score |
|
| ≤7 vs.
≥8 | 2.816 | <0.001 | 1.596 | 1.107–2.302 | 0.012 |
| Extracapsular
extension |
|
| (−) vs.
(+) | 3.910 | <0.001 | 1.687 | 1.136–2.503 | 0.009 |
| Seminal vesicle
invasion |
|
| (−) vs.
(+) | 4.160 | <0.001 |
|
| 0.192 |
| Surgical
margin |
|
| (−) vs.
(+) | 4.191 | <0.001 | 2.564 | 1.701–3.864 | <0.001 |
| Lymphovascular
invasion |
|
| (−) vs.
(+) | 2.279 | <0.001 |
|
| 0.184 |
| Perineural
invasion |
|
| (−) vs.
(+) | 1.913 | <0.001 |
|
| 0.799 |
| Lymph node
metastases |
|
| (−) vs.
(+) | 4.144 | 0.002 |
|
| 0.184 |
| Body mass index,
kg/m2 |
|
|
Continuous | 1.064 | 0.018 |
|
| 0.240 |
| Body mass index,
kg/m2 |
|
|
<25 | 1 |
| – | – | – |
|
25–30 | 1.202 | 0.266 |
|
|
|
|
≥30 | 2.716 | 0.007 |
|
|
|
| Serum total
cholesterol (10 mg/dl) |
|
|
Continuous | 0.995 | 0.030 | 0.925 | 0.879–0.97 | 0.003 |
In the multivariate analysis, the serum TC level was
an independent predictor of BCR [hazard ratio (HR) = 0.925 per 10
mg/dl; 95% confidence interval (CI): 0.879–0.973; P=0.003), as was
the serum PSA level, ECE, positive surgical margin and GS (Table III).
In subpopulation analyses by statin use or surgical
margin status, univariate analyses demonstrated that lower
preoperative serum TC level was significantly associated with BCR
in the statin non-user group (HR=0.941 per 10 mg/dl; 95% CI:
0.983–0.991; P=0.021) and in the negative surgical margin group
(HR=0.883 per 10 mg/dl; 95% CI: 0.799–0.975; P=0.014).
Discussion
In this study, we demonstrated that low preoperative
TC levels were significantly associated with an increased risk of
BCR following RP. Previous epidemiological studies demonstrated
that lower serum TC level was associated with lower risk of
high-grade prostate cancer and advanced disease (4–6). It was
also reported that hypercholesterolemia accelerates the growth of
prostatic tumors in vivo (13). Therefore, we hypothesized that an
elevated preoperative serum TC level may be associated with
increased risk of BCR following RP. Contrary to our expectations,
there was no significant association between preoperative serum TC
level and GS and clinical stage in the present study. A Cox
proportional hazard model demonstrated a dose-dependent inverse
association between preoperative TC level and the risk of BCR.
To date, only a limited number of studies have
described the association between serum TC level and prostate
cancer prognosis. Eichholzer et al reported that low plasma
TC level (<199 mg/dl) was significantly associated with an
increased risk of prostate cancer mortality in individuals aged
>60 years (14). Batty et
al reported that elevated blood TC level was associated with
death from prostate cancer (15).
These results are inconsistent. Although it has been reported that
a high TC level accelerates proliferation of prostate cancer cells,
the serum TC level decreases by effect of cancer metabolism as the
disease progresses (16). Thus, it
may be important to consider the time interval between serum TC
measurement and prostate cancer diagnosis, and the extent of the
disease when investigating the association between serum TC level
and prostate cancer prognosis. Solomon et al suggested that
a relatively low TC level within 1 year of a prostate cancer
diagnosis raises the risk of prostate cancer-related death; a
relatively low TC level may indicate more advanced disease. On the
other hand, a relatively low TC level >6 years prior to prostate
cancer diagnosis reduces the risk of prostate cancer-related death;
this may be associated with lower risk of high-grade cancer or
aggressive disease (16). Although
serum TC level in the present study was measured within 3 months
after prostate cancer diagnosis, serum TC level in previous
epidemiological studies by Platz et al and Mondull et
al, which demonstrated that a lower serum TC level was
associated with lower risk of high-grade prostate cancer and
advanced disease, was measured at least 2 years prior to prostate
cancer diagnosis (4–6). Thus, there may have been a significant
difference in the clinical implications of the serum TC level in
these studies.
The association between a relatively low serum TC
level and BCR remains to be elucidated. The present study included
only clinically localized disease. Thus, it was unlikely that serum
TC levels were affected by prostate cancer cells. One potential
explanation for the observed association between serum TC level and
BCR is an association between serum TC and immune system function.
It has been reported that men with hypocholesterolemia had
significantly fewer circulating lymphocytes, fewer total T cells,
and fewer CD8+ cells compared with th ose with hypercholesterolemia
(17). Thus, patients with relatively
high TC levels may have a better antitumor immune status compared
with patients with relatively low TC levels.
Although this study highlights the important
clinical implications of preoperative serum TC level in prostate
cancer patients who underwent RP, it has some limitations. First,
this is a retrospective analysis of data collected from a single
institution; thus, the number of cases was relatively small.
Second, there may be differences in patient characteristics between
studies on BCR and prostate cancer in Western countries and those
in Asian countries. In Western studies, over half the study
population is overweight or obese (BMI ≥25 kg/m2)
(18–20), whereas <50% of the study
populations in Asian studies have a BMI of ≥25 kg/m2
(21,22). However, serum TC levels appear to be
similar in Asian and Western studies (4,5,14,23).
Third, we only analyzed the association of the TC level with BCR,
since our routine preoperative laboratory examination did not
include assessment of LDL and HDL cholesterol levels. We may need
to investigate the association between LDL and HDL cholesterol
levels and BCR in the future. Despite these limitations, however,
this is the first study to demonstrate an inverse association
between preoperative serum TC level and BCR following RP. Our
findings suggest that the preoperative serum TC level may provide
important clinical information that may prove be useful in patient
counseling. However, further large cohort studies, including
different countries and regions, are warranted to validate the
clinical value of preoperative serum TC level in prostate cancer
patients.
In conclusion, low preoperative serum TC levels were
significantly associated with an increased risk of BCR among
prostate cancer patients who underwent RP. However, our findings
require validation by future studies.
Acknowledgements
The present study was partially supported by the
MEXT-Supported Program for the Strategic Foundation at Private
Universities, 2013–2017.
References
|
1
|
Heidenreich A, Bellmunt J, Bolla M, Joniau
S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel
T, et al: EAU guidelines on prostate cancer Part 1: Screening,
diagnosis, and treatment of clinically localised disease. Eur Urol.
59:61–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mottet N, Bellmunt J, Bolla M, Joniau S,
Mason M, Matveev V, Schmid HP, van der Kwast T, Wiegel T, Zattoni F
and Heidenreich A: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing, and castration-resistant prostate
cancer. Eur Urol. 59:572–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilson KM, Giovannucci EL and Mucci LA:
Lifestyle and dietary factors in the prevention of lethal prostate
cancer. Asian J Androl. 14:365–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Platz EA, Clinton SK and Giovannucci E:
Association between plasma cholesterol and prostate cancer in the
PSA era. Int J Cancer. 123:1693–1698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Platz EA, Till C, Goodman PJ, Parnes HL,
Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IM Jr and
Kristal AR: Men with low serum cholesterol have a lower risk of
high-grade prostate cancer in the placebo arm of the prostate
cancer prevention trial. Cancer Epidemiol Biomarkers Prev.
18:2807–2813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mondul AM, Clipp SL, Helzlsouer KJ and
Platz EA: Association between plasma total cholesterol
concentration and incident prostate cancer in the CLUE II cohort.
Cancer Causes Control. 21:61–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Platz EA, Leitzmann MF, Visvanathan K,
Rimm EB, Stampfer MJ, Willett WC and Giovannucci E: Statin drugs
and risk of advanced prostate cancer. J Natl Cancer Inst.
98:1819–1825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allott EH, Howard LE, Cooperberg MR, Kane
CJ, Aronson WJ, Terris MK, Amling CL and Freedland SJ:
Postoperative statin use and risk of biochemical recurrence
following radical prostatectomy: Results from the Shared Equal
Access Regional Cancer Hospital (SEARCH) database. BJU Int.
114:661–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu O, Eberg M, Benayoun S, Aprikian A,
Batist G, Suissa S and Azoulay L: Use of statins and the risk of
death in patients with prostate cancer. J Clin Oncol. 32:5–11.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH and Wittekind CH: UICC. TNM
Classification of Malignant Tumours (6th). Wiley. (New York).
2002.
|
|
11
|
World Health Organization: Obesity:
Preventing and Managing the Global Epidemic. WHO Technical Report
Series no 894 (WHO, Geneva). 2000.
|
|
12
|
de Martino M, Leitner CV, Seemann C,
Hofbauer SL, Lucca I, Haitel A, Shariat SF and Klatte T:
Preoperative serum cholesterol is an independent prognostic factor
for patients with renal cell carcinoma (RCC). BJU Int. 115:397–404.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhuang L, Kim J, Adam RM, Solomon KR and
Freeman MR: Cholesterol targeting alters lipid raft composition and
cell survival in prostate cancer cells and xenografts. J Clin
Invest. 115:959–968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eichholzer M, Stähelin HB, Gutzwiller F,
Lüdin E and Bernasconi F: Association of low plasma cholesterol
with mortality for cancer at various sites in men: 17-y follow-up
of the prospective Basel study. Am J Clin Nutr. 71:569–572.
2000.PubMed/NCBI
|
|
15
|
Batty GD, Kivimäki M, Clarke R, Smith
Davey G and Shipley MJ: Modifiable risk factors for prostate cancer
mortality in London: Forty years of follow-up in the Whitehall
study. Cancer Causes Control. 22:311–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solomon KR and Freeman MR: The complex
interplay between cholesterol and prostate malignancy. Urol Clin
North Am. 38:243–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muldoon MF, Marsland A, Flory JD, Rabin
BS, Whiteside TL and Manuck SB: Immune system differences in men
with hypo- or hypercholesterolemia. Clin Immunol Immunopathol.
84:145–149. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amling CL, Kane CJ, Riffenburgh RH, Ward
JF, Roberts JL, Lance RS, Friedrichs PA and Moul JW: Relationship
between obesity and race in predicting adverse pathologic variables
in patients undergoing radical prostatectomy. Urology. 58:723–728.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siddiqui SA, Inman BA, Sengupta S, Slezak
JM, Bergstralf EJ, Leibovich BC, Zincke H and Blute ML: Obesity and
survival after radical prostatectomy: A 10-year prospective cohort
study. Cancer. 107:521–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Loeb S, Yu X, Nadler RB, Roehl KA, Han M,
Hawkins SA and Catalona WJ: Does body mass index affect
preoperative prostate specific antigen velocity or pathological
outcomes after radical prostatectomy? J Urol. 177:102–106;
discussion 106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narita S, Mitsuzuka K, Yoneyama T,
Tsuchiya N, Koie T, Kakoi N, Kawamura S, Kaiho Y, Oyama C, Tochigi
T, et al: Impact of body mass index on clinicopathological outcome
and biochemical recurrence after radical prostatectomy. Prostate
Cancer Prostatic Dis. 16:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SE, Lee WK, Jeong MS, Abdullajanov M,
Kim DS, Park HZ, Jeong SJ, Yoon CY, Byun SS, Choe G, et al: Is body
mass index associated with pathological outcomes after radical
prostatectomy in Korean men? BJU Int. 107:1250–1255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitahara CM, de Berrington González A,
Freedman ND, Huxley R, Mok Y, Jee SH and Samet JM: Total
cholesterol and cancer risk in a large prospective study in Korea.
J Clin Oncol. 29:1592–1598. 2011. View Article : Google Scholar : PubMed/NCBI
|