Introduction
Colorectal cancer (CRC) remains one of the most
common causes of cancer-associated mortality (1). Over the past decades, novel therapeutic
options have been introduced as treatments for metastatic CRC
(mCRC). A combination of chemotherapy with targeted therapy has
been regarded as a standard first-line treatment plan (2). Cetuximab, a monoclonal antibody, targets
the extracellular domain of the epidermal growth factor receptor
(EGFR), exerting an important role in the treatment of patients
with mCRC. However, studies concerning the effects of
cetuximab-based chemotherapy as a first-line mCRC treatment have
demonstrated divergent results (2–4). RAS
family proteins (including KRAS and NRAS) exert important roles in
EGFR-mediated intracellular signaling cascades. Mutations in RAS
genes (occurring at loci in exons 2, 3 and 4) are often identified
in mCRC, and the most common of these is KRAS exon 2 (codon 12/13).
In several previous studies, including CRYSTAL phase III (2) and OPUS phase II (3) randomized clinical trials (RCTs), the use
of cetuximab was limited to treatment for mCRC patients with the
wild-type KRAS gene. However, as revealed in the COIN (4) and NORDIC-VII (5) trials, cetuximab is not always effective
in KRAS wild-type patients. The previous studies evaluating
anti-EGFR monoclonal antibodies in mCRC were amended to focus on
KRAS exon 2-selected populations, either retrospectively evaluating
outcomes in KRAS exon 2 wild-type patients or prospectively
enrolling KRAS exon 2 wild-type patients (6–8). Mutations
of KRAS predominantly lie in codons 12 and 13 of exon 2. However,
additionally, there are ~5% of CRC patients who have mutations in
KRAS exons 3 or 4, usually at codons 61 or 146, and a further ~5%
of patients with CRC with mutations in NRAS exons 2, 3 or 4.
Furthermore, almost 10% of patients with CRC have mutations in BRAF
(9). It was suggested that other
mutations, including ones in RAS (in exons 3 and 4 of KRAS and
exons 2, 3 and 4 of NRAS) and BRAF (KRAS exon 2), in patients with
wild-type mCRC patients may exert similar negative effects on the
efficacy of EGFR-targeted therapy (2,3,10). Previously, data from certain
retrospective analyses of several phase III trials indicated that
all RAS mutations were regarded as a negative predictive factor of
anti-EGFR therapy (11). Similar
analyses performed for patients with BRAF mutations, mutually
exclusive of RAS mutations, demonstrated a consistently poor
prognosis, regardless of the treatment strategy employed (11,12).
Therefore, the meta-analysis in the present study
was performed to assess the efficacy of adding cetuximab to
chemotherapies in the first-line treatment of mCRC, and to
investigate the prognosis and outcomes for cetuximab-based
chemotherapy in populations of differing RAS and BRAF mutation
status.
Materials and methods
Search strategy
Two independent investigators (L. Lin and L.L. Chen)
searched electronic databases, including PubMed, Embase, the
Cochrane library, the American Society of Clinical Oncology and the
European Society For Medical Oncology, up to August 2015. The
following search items were used: ‘Colorectal Neoplasms’ AND
‘cetuximab’ AND ‘Clinical Trial’ AND ‘Ras’, and relevant Medical
Subject Heading (‘MeSH’) terms were utilized. References cited in
the publications were searched to identify additional relevant
studies. Additional articles that were missed from the search
strategy were also searched after.
Eligibility criteria
Eligible studies had to meet the following criteria:
i) the study was an RCT; ii) the patients had pathologically
confirmed mCRC; iii) cetuximab-based chemotherapy was compared with
chemotherapy ± other targeted agents (e.g. bevacizumab) as the
first-line treatment; iv) the outcomes of interest were survival
according to the RAS and BRAF gene mutation status; v) the study
either provided, or allowed for, the calculation of hazard ratios
(HRs) with corresponding 95% confidence intervals (CIs); and vi)
only studies with full text were included. If duplicated or
overlapped data were identified in multiple reports, the one with
most comprehensive information was included. Studies with a
single-arm design, or RCTs with arms all containing cetuximab, were
excluded. Finally, studies not published in English were
excluded.
Quality assessment
Two investigators (L. Lin and L.L. Chen)
independently rated the quality of the retrieved studies. The risk
of bias items recommended by The Cochrane Handbook for Systematic
Reviews of Interventions (13) was
selected.
Data extraction
Two investigators (L. Lin and L.L. Chen)
independently extracted the following information from each study.
Disagreements were revolved by consensus. From each of the eligible
studies, the following information was collected: The first
author's family name, publication year, treatment regimens, sample
size, blind trial type, type of controls, HRs with corresponding
95% CIs or relevant data for HR, and the 95% CI calculation for OS
and/or PFS. The data were extracted separately according to the RAS
and BRAF mutation status.
Statistical analysis
The efficacy of adding cetuximab to the chemotherapy
regimen in the treatment of mCRC, based on the data from RCTs, was
assessed. The endpoints of interest in the pooled analysis were OS
and PFS, and they were thus expressed by HRs with 95% CIs for each
study. The association between the efficacy of adding cetuximab to
the chemotherapy in the treatment of mCRC, and OS or PFS, was
considered as a weighted average of the individual estimate of the
HR in every included study, using the inverse variance method. The
natural logarithms of the HRs (lnHRs) were considered to obey a
normal distribution. If the HRs and the corresponding 95% CIs were
reported, lnHRs and the corresponding natural logarithms of the
upper and lower limbs of the distribution were used as data points
in the pooling analysis. A sensitivity analysis was also performed
to examine the impact on the overall results (PFS and OS),
depending on the heterogeneity between the included studies. Prior
to the synthesis of the original data, I2 statistics
were used to assess the homogeneity (13). Studies with an I2 of 25–50,
50–75 or >75% were considered to have low, moderate or high
heterogeneity, respectively (14).
The pooled HRs were first calculated using the fixed-effects model.
If there was high heterogeneity among the studies, the
randomized-effects model was used. Subgroup analysis was performed
according to the KRAS and BRAF gene type, with the aim of exploring
important clinical differences among trials that could be expected
to affect the magnitude of the treatment effect. P<0.05 was
considered to indicate a statistically significant difference. All
the statistical tests in this meta-analysis were performed with
Review Manager version 5.3 software (Revman; The Cochrane
collaboration Oxford, United Kingdom). The findings of the present
meta-analysis are shown in forest plots.
Results
Overview of the literature search and
study characteristics
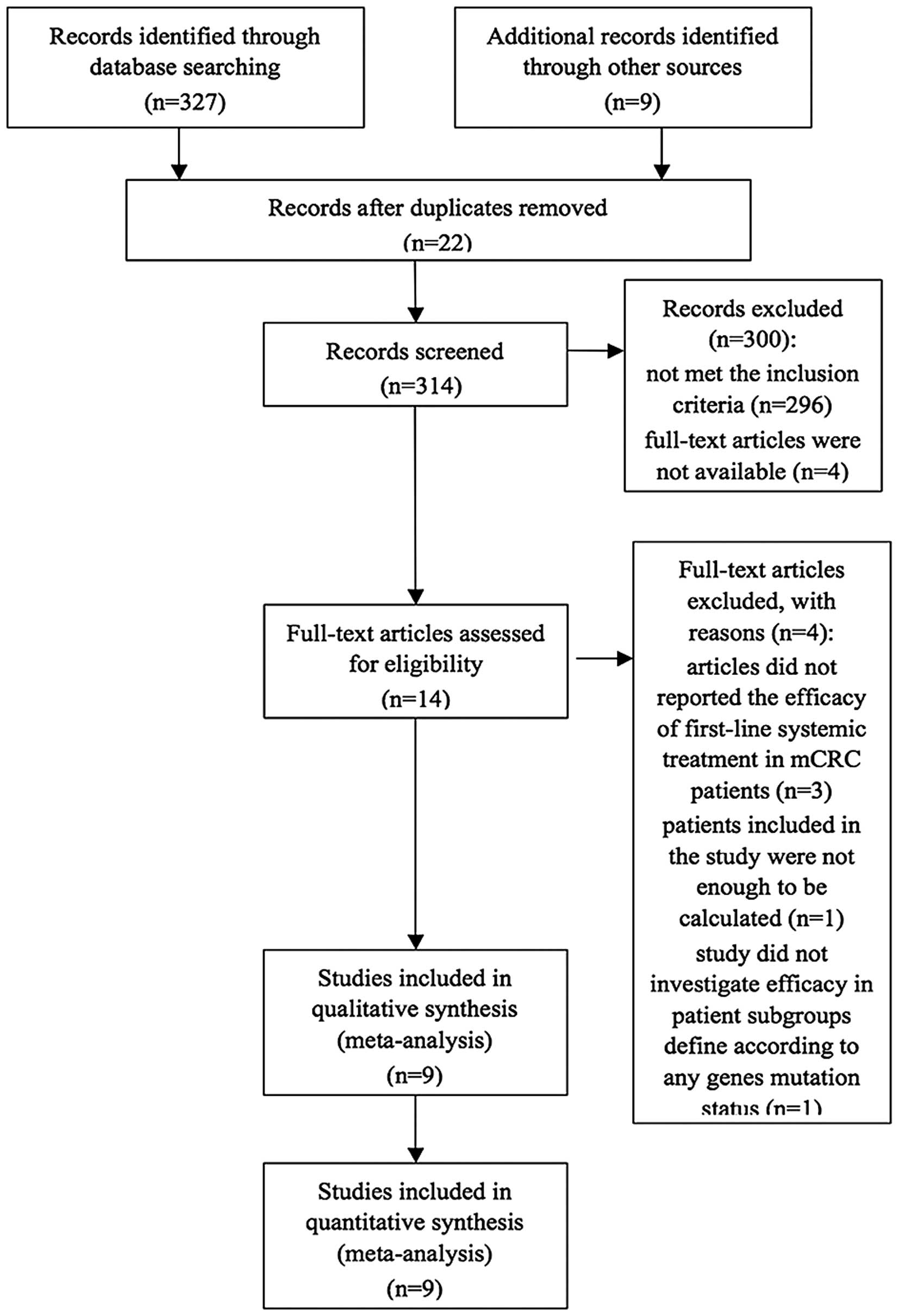
An overall total of 336 studies were retrieved
initially after searching the databases and hand-searched articles.
Of these articles, 14 full-text articles were evaluated in more
detail, and a total of nine articles were included in the
meta-analysis (Fig. 1). All studies
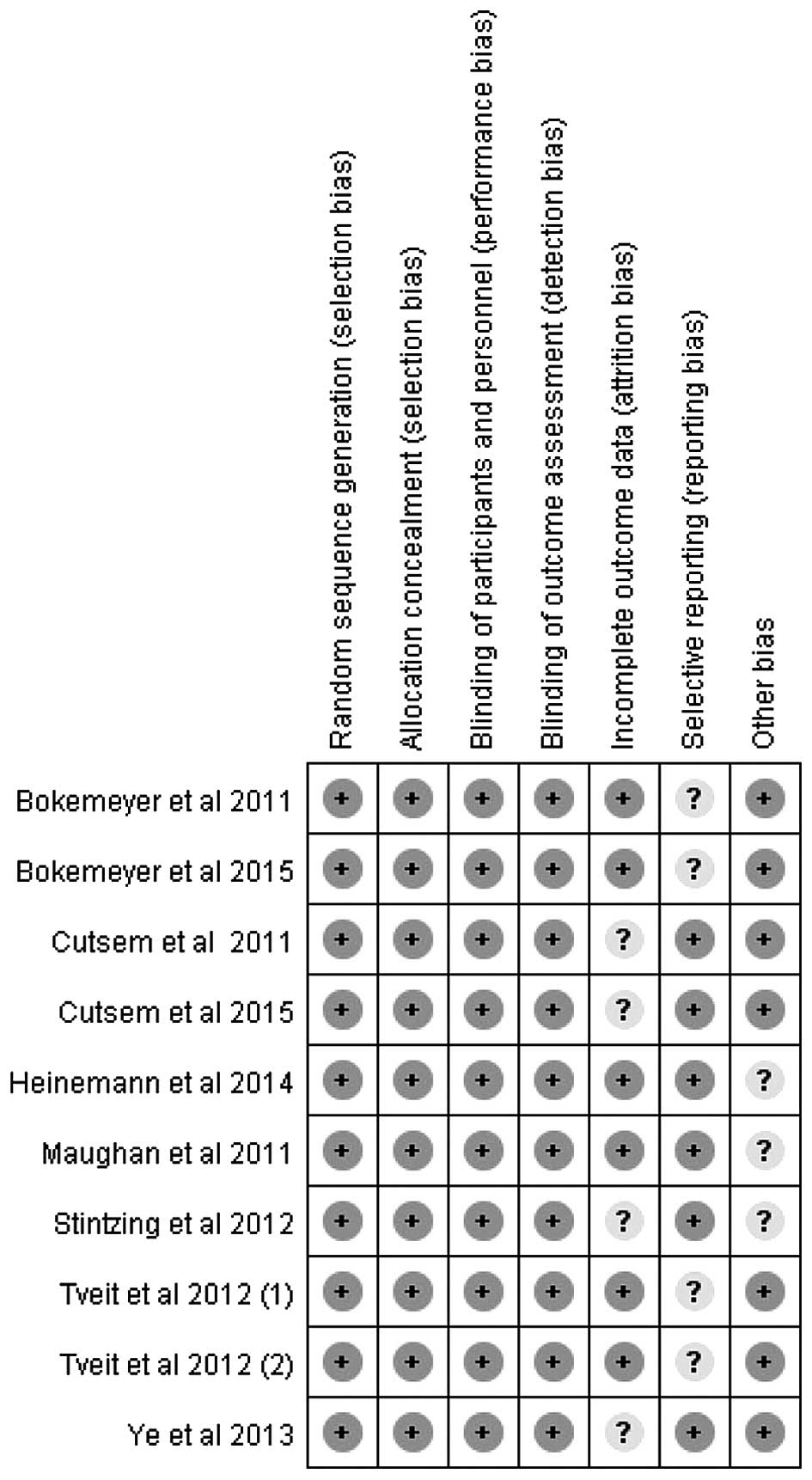
included in the present study were considered to be of moderate
quality, at least. Table I shows the
primary characteristics of the nine studies, and the risk of bias
items is shown in Fig. 2. In the
present review, cetuximab-based chemotherapy was administered as a
first-line treatment in patients with mCRC. Seven of the studies
were open-label, randomized, multicenter phase III trials, and two
of them were updated analyses of the CRYSTAL trial (15), wherein the authors reported on an
updated analysis of the larger cohort, as well as novel data on the
impact of tumor BRAF and RAS mutations, respectively, among
KRAS-wild type tumors on the clinical outcome (2,16). The AIO
KRK-0306 trial (17) and FIRE-3
(18) compared the treatment efficacy
of irinotecan/5-fluorouracil/leucovorin (FOLFIRI) combined with
bevacizumab or cetuximab, which is a first-line treatment for mCRC.
The NORDIC-VII (5) multicenter phase
III trial investigated the efficacy of cetuximab when added to
bolus fluorouracil/folinic acid and oxaliplatin (‘Nordic FLOX’),
administered continuously or intermittently. Patients were randomly
assigned to receive either standard Nordic FLOX (arm A), cetuximab
and FLOX (arm B), or cetuximab combined with intermittent FLOX (arm
C). The predominant comparison was made between arms A and B. Arms
B vs. A, and arms C vs. arms A, were defined as Tveit 1 and Tveit
2, respectively. Of the seven articles, the study by Ye et
al (19) was the only one that
assessed the effects of cetuximab plus chemotherapy as a first-line
treatment, which was restricted to unresectable colorectal liver
metastases. The remaining two studies by Bokemeyer et al
(3,20)
were randomized phase II studies, which reported an updated
analysis based on the OPUS study: These updated retrospective
analyses comparatively investigated efficacy in the patient
subgroups, defined according to the BRAF and RAS mutation
status.
 | Table I.Baseline characteristics of patients
in the trials included in the meta-analysis. |
Table I.
Baseline characteristics of patients
in the trials included in the meta-analysis.
| Authors, year | Clinical trial | Type of study | Treatment
regimen | Subgroup
analysis | Refs. |
|---|
| Maughan et al,
2011 | COIN | Phase III | Oxa+FU vs.
Oxa+FU+cet | KRAS BRAF | (4) |
| Bokemeyer et
al, 2011 | OPUS | Phase II | FOLFOX-4+cet vs.
FOLFOX-4 | ITT KRAS BRAF | (3) |
| Van Cutsem et
al, 2011 | CRYSTAL | Phase III | FOLFIRI vs.
FOLFIRI+cet | ITT KRAS BRAF | (2) |
| Tveita et al, 2012 | NORDIC-VII | Phase III | A; Nordic FLOX B:
FLOX+cet C: intermittent FLOX +cet | ITT KRAS | (5) |
| Stintzing et
al, 2012 | AIO-0306 | Phase III | FOLFIRI+cet vs.
FOLFIRI +bev | ITTb | (17) |
| Ye et al,
2013 |
| Phase III | CT+cet vs. CT | ITTc | (19) |
| Heinemann et
al, 2014 | FIRE-3 | Phase III | FOLFIRI +cet vs.
FOLFORI +bev | ITTc KRAS RAS | (18) |
| Van Cutsem et
al, 2015 | CRYSTAL | Phase III | FOLFIRI vs.
FOLFIRI+cet | KRAS RAS | (16) |
| Bokemeyer et
al, 2015 | OPUS | Phase II | FOLFOX-4+cet vs.
FOLFOX-4 | KRAS RAS | (20) |
A pooled analysis of OS and PFS between
cetuximab-based therapy and chemotherapy ± other targeted agents in
KRAS exon 2 wild-type patients was performed.
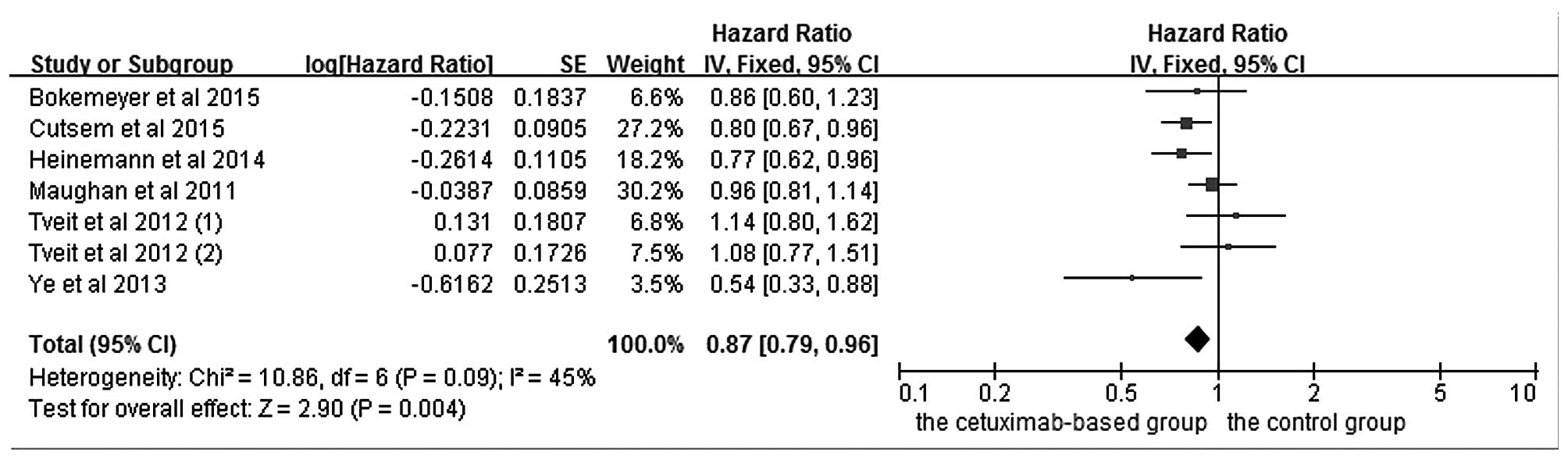
The effects of cetuximab-based treatment on OS are
shown in Fig. 3. OS data were
available in six RCTs (4,5,16,18–20). The
aggregated results suggested that there was a significant OS
benefit from cetuximab-based chemotherapy (HR=0.87, 95%
CI=0.79–0.96, Z=2.91, P=0.004). All six RCTs reported data
concerning PFS. However, even though the pooled analysis of PFS
with an I2 value of 77% was considered to have high
heterogeneity, the randomized-effects model was not available to be
used.
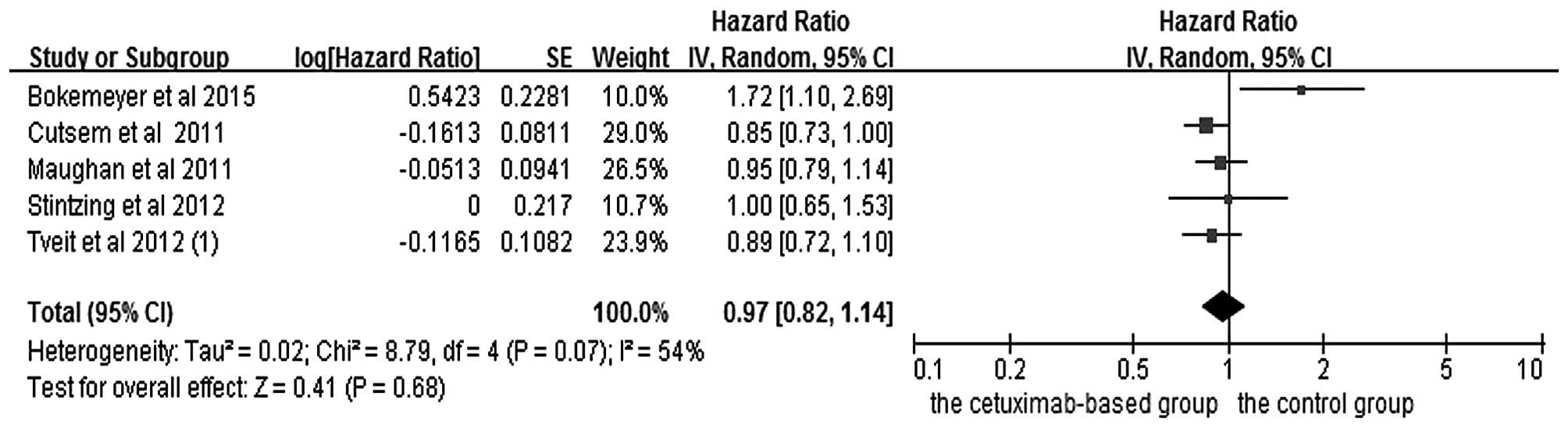
Pooled analysis of OS and PFS between
cetuximab-based therapy and chemotherapy ± other targeted agents in
KRAS exon 2 mutation-type patients
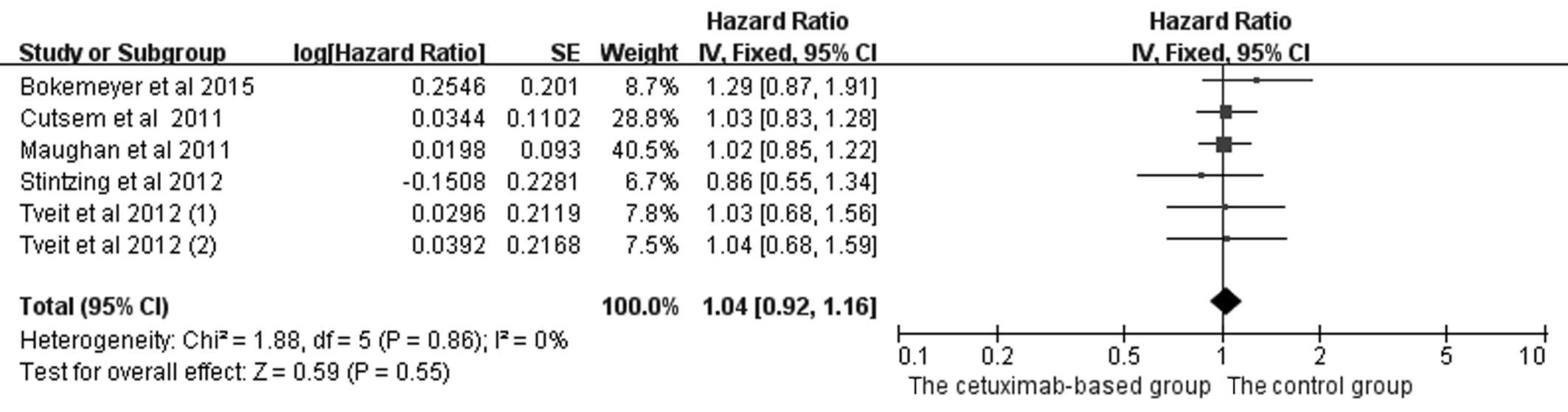
In the analysis of OS and PFS in patients with mCRC
treated with chemotherapy, five studies (2,4,5,17,20) were included, and the data are shown in
Figs. 4 and 5. Since PFS was not an appropriate end point
for the stop-and-go principle in the NORDIC-VII trial (5), comparisons including arm C were of
interest primarily for OS. The OS (HR=1.04, 95% CI=0.92–1.16,
Z=0.59, P=0.55) and PFS (HR=0.97, 95% CI=0.82–1.14, Z=0.41, P=0.68)
benefits were not identified in the combined treatments.
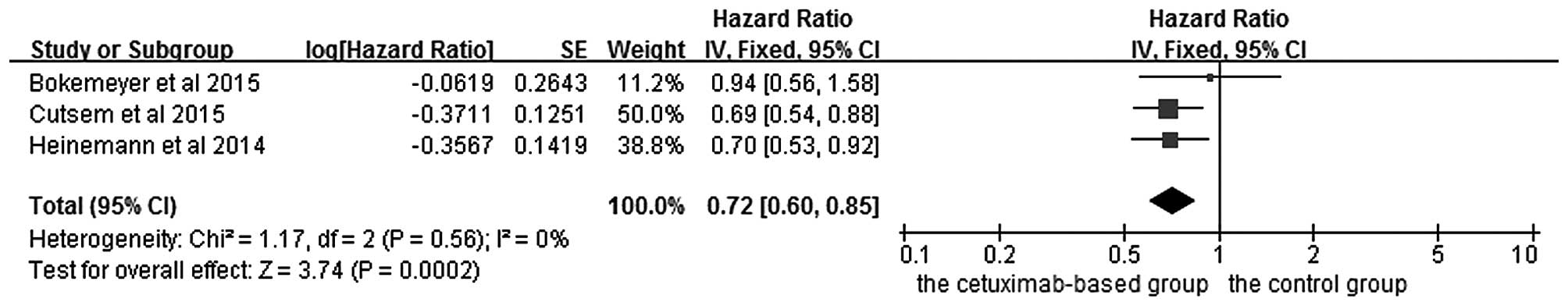
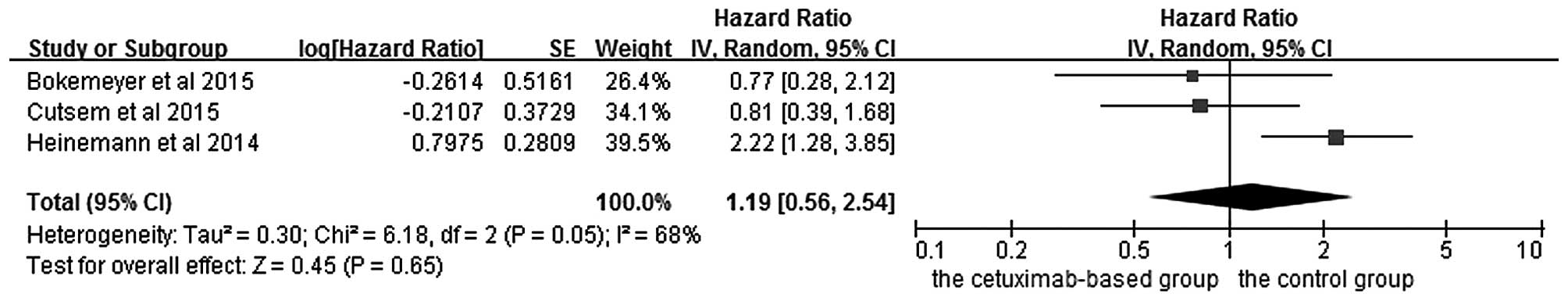
Subgroup analysis of efficacy
according to RAS mutation status in KRAS exon 2 wild-type
patients
The effects of cetuximab-based chemotherapy
treatment on OS and PFS in KRAS exon 2 wild-type and other
RAS-mutant subgroup patients are shown in Figs. 6–9. OS
and PFS data were available for three trials (16,18,20). These
individuals with wild-type KRAS exon 2 were divided into two
subgroups: The ‘all RAS wild-type’ subgroup (no mutations in exons
2, 3 and 4 for either KRAS or NRAS), and the ‘new RAS mutant’
subgroup (wild-type for KRAS exon 2, but with a KRAS mutation in
exons 3 or 4 and/or a NRAS mutation in exons 2, 3 or 4). A
significant OS benefit of cetuximab-based chemotherapy was evident
in patients without any RAS mutations (HR=0.72, 95% CI=0.60–0.85,
Z=3.74, P=0.0002), although not for PFS (HR=0.68, 95% CI=0.45–1.03,
Z=1.82, P=0.07). For the KRAS exon 2 wild-type with other RAS
mutations, KRAS-wild/RAS-mutant tumors, no significant differences
in the cetuximab-based treatment effects were observed in either
PFS (HR=1.19, 95% CI=0.56–2.54, Z=0.45, P=0.65) or OS (HR=1.19, 95%
CI=0.81–1.74, Z=0.88, P=0.38). In other words, adding cetuximab to
the treatment for patients with KRAS exon 2 wild-type/other
RAS-mutants did not improve PFS and OS compared with the control
groups.
Subgroup analysis of efficacy
according to BRAF mutation status in KRAS exon 2 wild-type
patients
Three RCTs reported data of KRAS and BRAF tumor
mutation status. However, not all the studies reported available
data on OS and PFS, so it was therefore not possible to perform
meta-analysis. The CRYSTAL trial (2)
reported PFS (median, 8.0 vs. 5.6 months, HR=0.934, P=0.87) and OS
(median, 14.1 vs. 10.3 months, HR=0.908, P=0.74) in patients with
KRAS exon 2 wild-type/BRAF mutations who were treated with FOLFIRI
plus cetuximab, although no statistical significance was
identified. In addition, no trial was concerned with independent
treatment for patients with BRAF mutations. Thus, with the current
data, the BRAF mutation status cannot be a predictive factor for
treatment outcomes of cetuximab plus FOLFIRI. KRAS exon 2
wild-type/BRAF wild-type patients treated with FOLFOX-4 plus
cetuximab demonstrated marked improvements in PFS.
In the OPUS trial (3),
only a small number of patients with mutations in the RAS gene were
identified. For patients with the KRAS exon 2 wild-type/BRAF mutant
(n=11), OS was prolonged in those receiving cetuximab plus FOLFOX-4
compared with those receiving FOLFOX-4 therapy alone (median, 20.7
vs. 4.4 months). This difference should be interpreted cautiously
since, given the small sample size, no definitive conclusions
concerning possible predictive or prognostic utility may be
reached. In the COIN trial (4), the
median OS was shorter in patients with BRAF mutations (n=102, 8.8
months) compared with those with both the BRAF wild-type and KRAS
mutations (n=548, 14.4 months) or NRAS mutations (n=38, 13.8
months). The median PFS ranged from 5.6 months in patients with
BRAF mutations, to 9.0 months for those with the wild type of all
of KRAS, NRAS and BRAF.
Discussion
The predominant purpose of the meta-analysis
performed in the present study was to critically evaluate the
efficacy of adding cetuximab to chemotherapy in patient subgroups
defined according to the RAS and BRAF gene type. In the
meta-analysis, an OS benefit was observed in patients with KRAS
exon 2 wild-type tumors (HR=0.87, 95% CI=0.79–0.96, Z=2.90,
P=0.004) and wild-type KRAS/RAS patients (HR=0.72, 95%
CI=0.60–0.85, Z=3.74, P=0.0002). In contrast, in patients carrying
KRAS mutations, no matter whether in exon 2, exon 3 or 4 and/or a
NRAS mutation in exons 2, 3 or 4, no evidence was identified of a
benefit associated with cetuximab according to PFS and OS. In RAS
subgroups (16,18,20),
patients with the KRAS/RAS-wild-type had a longer median OS time
compared with the KRAS exon 2 wild-type. There are also additional
trials [CALGB/SWOG 80405 (21);
NORDIC VII (22)] that could
potentially be retrospectively analyzed to determine whether there
was a beneficial effect when cetuximab was combined with the KRAS
exon 2 wild-type patients.
A potential direction of future study would be to
evaluate individual RAS mutations in order to understand whether
cetuximab efficacy varies among mutations. Several clinical studies
have demonstrated that mCRC patients with the BRAF V600E mutation
appear to have a poor prognosis. However, the association between
the BRAF V600E mutation and the outcome of cetuximab-based
treatment in the first-line treatment for patients with mCRC
remains controversial (2,3). To date, only two meta-analytical studies
have been performed on the BRAF mutation status of HRs for PFS and
OS, and this offers the explanation as to why meta-analysis of the
BRAF mutation on the survival of patients with mCRC treated with
cetuximab was not performed. For the CRYSTAL (2) and the COIN (4) trials, the advantage of the efficacy of
the cetuximab-based chemotherapy is restricted to KRAS exon 2
wild-type/BRAF wild-type patients. In the OPUS trial (3), the results for the KRAS exon 2
wild-type/BRAF wild-type patients were very similar to those of the
KRAS exon 2 wild-type patients receiving cetuximab plus FOLFOX-4,
and were associated with significant improvements in the overall
response rate and PFS. However, the sample size of patients with
mutations in this gene was too small, analysis of the outcomes may
have been influenced by imbalances in prognostic variables, and no
definitive conclusions concerning possible predictive or prognostic
utility may be reached. Analyses of larger numbers of patients are
required to fully explore the biomarker potential of the BRAF
mutation status for mCRC. Previously, patients with BRAF-mutant CRC
have had an extremely poor prognosis compared with BRAF-wild-type
patients, which has been subsequently confirmed by meta-analysis
(23,24). Bokemeyer et al (12) performed an analysis of the CRYSTAL and
OPUS studies, and the objective of this pooled analysis was to
investigate the efficacy of adding cetuximab to standard first-line
chemotherapy according to KRAS and BRAF mutation status. This
analysis collected data, including OS, PFS and the best overall
response rate in 845 patients with KRAS wild-type receiving
cetuximab plus chemotherapy. The cetuximab-based therapy
significantly prolonged OS (HR=0.81, P=0.0062) and PFS (HR=0.66,
P<0.001). The prognosis was worse in each treatment arm for
patients with BRAF mutations compared with those with the BRAF
wild-type. However, given the small sample size of BRAF mutation
carriers, this result may not be entirely reliable. Taken together,
BRAF mutations do not appear to be a predictive biomarker in
current meta-analysis, but they do have predictive value for poor
prognosis.
The meta-analysis performed in the present study had
certain limitations. First, the trial results included in this
meta-analysis were extracted from published data, rather than being
based on data of the individual patients. Secondly, only studies
with full published text were included in this analysis: All
presentations at conferences were excluded. It is possible that the
results of the full publication may differ from conference
presentations due to updating of the data. Finally, inevitable
variations existed among the studies, including the study design,
basic therapies, follow-up intervals and type of therapy. All these
factors could potentially affect the results of the
meta-analysis.
In conclusion, the meta-analysis performed in the
present study indicated that adding cetuximab to chemotherapy
significantly improves OS in patients with mCRC who lack any RAS
mutations (i.e., either in the KRAS exon 2 or any other RAS
mutation). Individuals who carry the KRAS exon 2 wild-type, but who
also have any novel type of RAS mutation, receive distinctly less
benefit from cetuximab-based treatment compared with those without
any RAS mutations. This meta-analysis also reveals that the BRAF
V600E mutation may be associated with a poorer response and worse
survival rates in wild-type KRAS mCRC patients treated with
cetuximab. Given the limited number of studies included in the
present meta-analysis, our findings need to be further explored and
verified in larger randomized studies. Furthermore, all-RAS
mutation and BRAF testing should be undertaken prior to the
administration of cetuximab in order to obtain valuable prognostic
and predictive information that may drive treatment decisions.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Köhne CH, Láng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejpar S, et al: Cetuximab plus irinotecan, fluorouracil and
leucovorin as first-line treatment for metastatic colorectal
cancer: Updated analysis of overall survival according to tumor
KRAS and BRAF mutation status. J Clin Oncol. 29:2011–2019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bokemeyer C, Bondarenko I, Hartmann JT, de
Braud F, Schuch G, Zubel A, Celik I, Schlichting M and Koralewski
P: Efficacy according to biomarker status of cetuximab plus
FOLFOX-4 as first-line treatment for metastatic colorectal cancer:
The OPUS study. Ann Oncol. 22:1535–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maughan TS, Adams RA, Smith CG, Meade AM,
Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL,
et al: Addition of cetuximab to oxaliplatin-based first-line
combination chemotherapy for treatment of advanced colorectal
cancer: Results of the randomised phase 3 MRC COIN trial. Lancet.
377:2103–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tveit KM, Guren T, Glimelius B, Pfeiffer
P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund
E, et al: Phase III trial of cetuximab with continuous or
intermittent fluorouracil, leucovorin and oxaliplatin (Nordic FLOX)
versus FLOX alone in first-line treatment of metastatic colorectal
cancer: The NORDIC-VII study. J Clin Oncol. 30:1755–1762. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Final results from PRIME: Randomized phase III
study of panitumumab with FOLFOX4 for first-line treatment of
metastatic colorectal cancer. Ann Oncol. 25:1346–1355. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Price TJ, Peeters M, Kim TW, Li J, Cascinu
S, Ruff P, Suresh AS, Thomas A, Tjulandin S, Zhang K, et al:
Panitumumab versus cetuximab in patients with
chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal
cancer (ASPECCT): A randomised, multicentre, open-label,
non-inferiority phase 3 study. Lancet Oncol. 15:569–579. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bokemeyer C, Van Cutsem E, Rougier P,
Ciardiello F, Heeger S, Schlichting M, Celik I and Köhne CH:
Addition of cetuximab to chemotherapy as first-line treatment for
KRAS wild-type metastatic colorectal cancer: Pooled analysis of the
CRYSTAL and OPUS randomised clinical trials. Eur J Cancer.
48:1466–1475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Cutsem E, Lenz HJ, Köhne CH, Heinemann
V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken
JH and Ciardiello F: Fluorouracil, leucovorin and irinotecan plus
cetuximab treatment and RAS mutations in colorectal cancer. J Clin
Oncol. 33:692–700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stintzing S, von Fischer Weikersthal L,
Decker T, Vehling-Kaiser U, Jäger E, Heintges T, Stoll C, Giessen
C, Modest DP, Neumann J, et al: FOLFIRI plus cetuximab versus
FOLFIRI plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer-subgroup analysis of patients with
KRAS: Mutated tumours in the randomised German AIO study KRK-0306.
Ann Oncol. 23:1693–1699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai
SY, Ye QH, Yu Y, Xu B, Qin XY and Xu J: Randomized controlled trial
of cetuximab plus chemotherapy for patients with KRAS wild-type
unresectable colorectal liver-limited metastases. J Clin Oncol.
31:1931–1938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bokemeyer C, Köhne CH, Ciardiello F, Lenz
HJ, Heinemann V, Klinkhardt U, Beier F, Duecker K, van Krieken JH
and Tejpar S: FOLFOX4 plus cetuximab treatment and RAS mutations in
colorectal cancer. Eur J Cancer. 51:1243–1252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Mahoney MR, O'Neil BH, Shaw JE, Polite BN, Hochster
HS, Atkins AN, et al: CALGB/SWOG 80405: Phase III trial of
irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin
(mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients
(pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma
of the colon or rectum (MCRC). ASCO Meeting Abstracts.
32:LBA32014.
|
|
22
|
Tveit K, Guren T, Glimelius B, Pfeiffer P,
Sorbye H, Pyrhonen S, Kure E, Ikdahl T, Skovlund T and
Christoffersen T: Randomized phase III study of
5-fluorouracil/folinate/oxaliplatin given continuously or
intermittently with or without cetuximab, as first-line treatment
of metastatic colorectal cancer: The NORDIC VII study
(NCT00145314), by the Nordic colorectal cancer biomodulation group.
ASCO Meeting Abstracts. 29:3652011.
|
|
23
|
Pietrantonio F, Petrelli F, Coinu A, Di
Bartolomeo M, Borgonovo K, Maggi C, Cabiddu M, Iacovelli R, Bossi
I, Lonati V, et al: Predictive role of BRAF mutations in patients
with advanced colorectal cancer receiving cetuximab and
panitumumab: A meta-analysis. Eur J Cancer. 51:587–594. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui D, Cao D, Yang Y, Qiu M, Huang Y and
Yi C: Effect of BRAF V600E mutation on tumor response of anti-EGFR
monoclonal antibodies for first-line metastatic colorectal cancer
treatment: A meta-analysis of randomized studies. Mol Biol Rep.
41:1291–1298. 2014. View Article : Google Scholar : PubMed/NCBI
|