Introduction
Breast cancer has a relatively favorable prognosis
compared with the prognosis of other cancers, such as lung, colon,
ovarian and pancreatic cancers. The survival of patients with
primary breast cancer has significantly improved due to recent
advances in therapeutic strategies. However, the treatment of
metastatic breast cancer (MBC) has not improved significantly and
the outcomes remain unsatisfactory. Although anthracyclines and
taxanes are the representative agents used as standard chemotherapy
in the adjuvant setting, these agents have also been used in
several cases of MBC (1–4) and selecting therapeutic regimens in this
setting may be difficult for physicians. In cases of
hormone-insensitive tumors, or cases of hormone-sensitive tumors
that exhibit resistance to endocrine therapy, cytotoxic agents that
have not been previously administered, or taxanes in combination
with molecular-targeted agents, are potential candidates (4–9). Recently,
taxanes in combination with bevacizumab, which targets the tumor
vasculature to reduce blood supply to the tumors (7–9), or
eribulin, which is an analogue of halichondrin B targeting tubulin
in tumor cells, have demonstrated favorable efficacy in improving
the prognosis of patients with MBC (10). While a number of these available
cytotoxic agents have been shown to exert a suppressive effect on
tumor growth, their effects are not maintained for a long period,
due to the acquisition of resistance to these agents by the tumor
cells, attenuated physical status of the patients or intolerable
treatment-related adverse effects. Thus, to prolong the survival of
the patients with MBC, alternative therapeutic strategies are
required to achieve a long-term response or enable long-term
administration of the regimens.
We previously reported that the administration of
one therapeutic regimen for ≥1 year was one of the most important
factors for long-term survival in the metastatic setting (11). We also demonstrated that more MBC
patients who survived for ≥60 months following MBC diagnosis had
received metronomic chemotherapy, compared with patients exhibiting
shorter survival (12). Metronomic
chemotherapy, which is defined as continuous or frequent treatment
with low doses of anticancer agents, is commonly used as palliative
care in patients who have been heavily pretreated with cytotoxic
drugs or who have poor performance status (13). Interestingly, metronomic chemotherapy
used for palliation has been reported to result in favorable tumor
responses and prolonged survival of the treated patients (14–17).
Metronomic chemotherapy may be continued over a long period of time
due to its minimal toxicity. However, this strategy has not been
investigated in detail in terms of its mechanism of action, outcome
and toxicity. The aim of this study, was to compare the
clinicopathological characteristics and clinical outcome between
patients with MBC who received metronomic chemotherapy and those
who received non-metronomic regimens.
Patients and methods
Patients
Data from 80 patients with advanced or recurrent
breast cancer, who were treated with chemotherapeutic regimens at
Kagawa University Hospital from February, 2005 to June, 2014, were
retrospectively analyzed. To assess the usefulness of metronomic
chemotherapy in the metastatic setting, the patients were divided
into two groups according to whether they had received metronomic
chemotherapy as treatment for MBC. The metronomic group included 52
patients who had received metronomic regimens at least once in the
metastatic setting, and the non-metronomic group, which included 28
patients who had not received a metronomic regimen but had received
other cytotoxic agents. The metronomic regimens included
capecitabine (1,800 mg/body/day) alone or in combination with
cyclophosphamide (100 mg/body/day) administered in cycles of 14
consecutive days repeated at 3-week intervals, or TS-1 (100
mg/body/day) alone or in combination with cyclophosphamide (100
mg/body/day) administered in cycles of 14 consecutive days repeated
at 3-week intervals. For some patients with hormone-sensitive
tumors, endocrine therapy consisting of either tamoxifen, letrozole
or exemestane, was added to the metronomic regimen at the
physician's discretion. For patients with human epidermal factor
receptor 2 (HER2)-overexpressing tumors, trastuzumab was added to
the metronomic regimen.
The present study was in compliance with the
guidelines of the Ethics Committee of Kagawa University Hospital
and conformed to the provisions of the Declaration of Helsinki in
1995. All the study patients provided written informed consent.
Evaluation of therapeutic
efficacy
Tumor response was assessed by physical examination
and computed tomography, magnetic resonance imaging or bone scan,
according to the Response Evaluation Criteria in Solid Tumors
(18), every 2–3 months during
treatment. Complete response (CR) was defined as the absence of
evidence of disease, partial response (PR) was defined as a
reduction in the product of the two largest perpendicular diameters
of the target lesions by ≥50%, and progressive disease (PD) was
defined as an increase in tumor size by ≥25%, or presence of a new
lesion. Clinical response that did not meet any of the
abovementioned definitions was classified as stable disease (SD).
CR and PR were defined as objective response. CR, PR and SD were
defined as disease control rate (DCR), and CR, PR and SD observed
for ≥6 months were defined as clinical benefit rate (CBR). The
clinical outcomes examined in this study included time-to-treatment
failure (TTF), defined as the duration from initiation to
discontinuation of treatment, time-to-progression (TTP), defined as
the duration from initiation of treatment to disease progression or
death from any cause, and overall survival (OS), defined as the
duration from initiation of treatment to death from any cause.
Toxicity was assessed according to the National Cancer Institute
Common Toxicity Criteria, version 3.0 (19). For patients who had received ≥2
cytotoxic regimens for MBC, survival time was calculated from the
administration of the first metronomic regimen in the metronomic
group, or the most effective cytotoxic regimen in the
non-metronomic group.
Statistical analysis
The by Mann-Whitney U test or the standard
Chi-square test were used for comparisons between the two groups.
The effects of baseline characteristics, clinical response or
prognostic parameters on the risk of progression or death were
assessed using the Kaplan-Meier survival analysis and the log-rank
test of significance. A 95% confidence interval for the median of
each variable was computed using the method of Brookmeyer and
Crowley. P<0.05 was considered to indicate statistically
significant differences; all P-values were two-sided. The SPSS
statistical software system, version 23 (SPSS Inc., Tokyo, Japan)
was used for all calculations.
Results
Baseline characteristics of the
patients
The median age of the patients was 59 years (range,
32–82 years). Hormone-sensitive and HER2-overexpressing tumors
accounted for 59.0 and 21.9% of the cases, respectively (Table I). When the clinicopathological
characteristics were compared between patients who had received
metronomic regimens and those who had received a non-metronomic
regimen, the median age was significantly higher and the tumor
grade was significantly lower in the metronomic group compared with
those in the non-metronomic group (age, 60 vs. 52 years, P=0.0013;
and tumor grade, 2 vs. 3, P=0.025, respectively). The number of
chemotherapy regimens administered prior to the metronomic regimen,
or of other cytotoxic regimens that achieved the best response
among regimens that patients had received in the metastatic
setting, was higher in the metronomic compared with the
non-metronomic group (3 vs. 2, respectively; P=0.0018). Other
baseline characteristics, including disease-free interval (DFI),
number of disease sites, hormone receptor and HER2 status of the
tumor, prior anthracycline or taxane treatment and the proportion
of patients with visceral lesions, did not differ significantly
between the two groups.
 | Table I.Clinicopathological characteristics of
patients who received metronomic regimens and those who received
other regimens. |
Table I.
Clinicopathological characteristics of
patients who received metronomic regimens and those who received
other regimens.
| Characteristics | All patients | Patients receiving
METb | Patients not
receiving MET | P-value |
|---|
| Patient no. | 80 | 52 | 28 |
|
| Age, years | 59 (32–82) | 60 (40–81) | 51 (32–82) | 0.0013 |
| Tumor grade | 2 (1–3) | 2 (1–3) | 3 (1–3) | 0.025 |
| DFI, months | 37 (3–24) | 42 (10–241) | 37 (3–128) | 0.201 |
| Disease sites, n | 2 (1–6) | 2 (1–6) | 2 (1–6) | 0.869 |
| Visceral lesions,
% | 56.2 | 53.8 | 57.7 | 0.75 |
| AT pretreatment,
% | 40.7 | 39.2 | 42.3 | 0.872 |
| Hormone-sensitive
tumors, % | 59 | 63.5 | 50 | 0.258 |
| HER2-overexpressing
tumors, % | 21.9 | 23.4 | 19.3 | 0.743 |
| Triple-negative
tumors, % | 30.8 | 25 | 42.3 | 0.121 |
| Chemotherapy cycles
for BC, n | 4 (1–7) | 4 (1–7) | 3 (1–5) | 0.034 |
| Chemotherapy cycles
for MBC, n | 3 (1–7) | 3 (1–7) | 2 (1–4) | 0.0018 |
Efficacy of treatment and clinical
outcomes
When clinical outcomes were compared between
patients who had received metronomic regimens and those who had
received a non-metronomic regimen, overall response rate (ORR), CBR
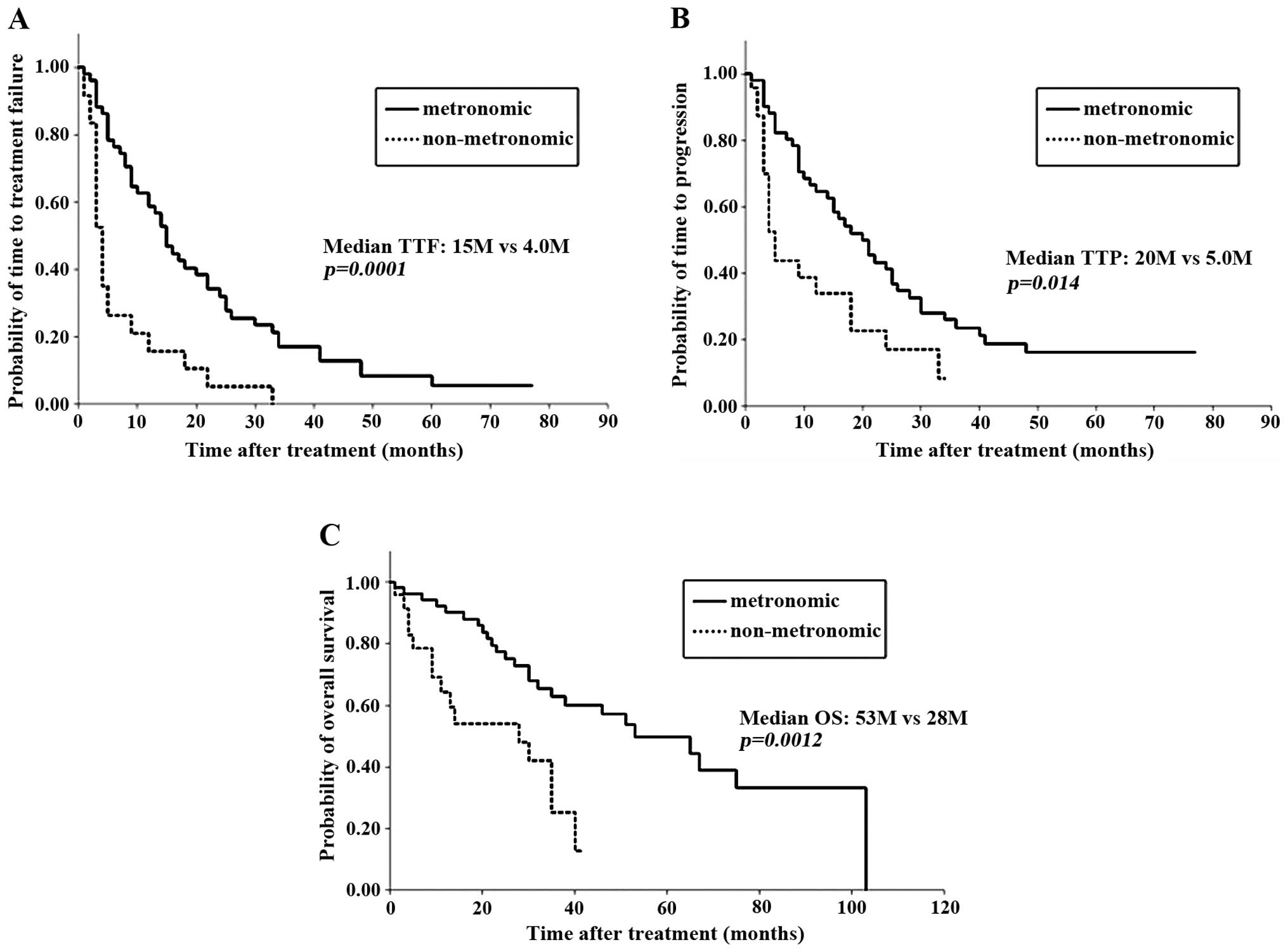
or DCR did not differ between the two groups (Table II). However, the median TTF, TTP and
OS were significantly longer in the metronomic group compared with
those in the non-metronomic group (TTF, 15 vs. 4 months, P=0.0001;
TTP, 20 vs. 5 months, P=0.014; and OS, 53 vs. 28 months, P=0.0012,
respectively; Table II and Fig. 1). The OS from the time of MBC
diagnosis also appeared to be significantly longer in the
metronomic group (P=0.0003). Therefore, metronomic regimens were
associated with a significantly more favorable prognosis in MBC,
regardless of the tumor response to treatment.
 | Table II.Clinical outcomes of patients who
received and those who did not receive metronomic regimen. |
Table II.
Clinical outcomes of patients who
received and those who did not receive metronomic regimen.
| Outcomes | Patients receiving
MET | Patients not
receiving MET | P-value |
|---|
| Response rate, % | 36 | 52 | 0.167 |
| Clinical benefit
rate, % | 68 | 53.8 | 0.368 |
| Disease control rate,
% | 74 | 61.5 | 0.346 |
| Median TTF,
months | 15 |
4 | 0.0001 |
| Median TTP,
months | 20 |
5 | 0.014 |
| Median OS,
months | 53 | 28 | 0.0012 |
| Median OS from MBC,
months | 103 | 30 | 0.0003 |
To identify the factors responsible for the
favorable clinical outcome with metronomic regimens,
clinicopathological factors and clinical outcomes were compared
between patients who responded to metronomic regimens and
non-responders. All but one of the patients who responded to
metronomic regimens had hormone-sensitive tumors, whereas patients
with hormone-sensitive tumors accounted for <50% of the patients
who failed to respond to this regimen (94.4 vs. 47.1%,
respectively, P=0.0008; Table III).
One patient without luminal-type tumor who responded to the
metronomic regimen had an HER2-overexpressing tumor. Of note, none
of the patients with TN tumors responded to metronomic regimens.
Other factors, such as age, tumor grade, DFI, HER2 status of the
tumor and prior treatment, did not differ significantly between the
two groups. When comparing clinical outcomes between patients who
responded and those who did not respond to a metronomic regimen,
all prognostic factors, i.e., TTF, TTP and OS, were significantly
longer in responders compared with non-responders (TTF, 33 vs. 9
months, P=0.0002; TTP, 41 vs. 11 months, P=0.0023; and OS, not
reached vs. 30 months, P=0.00002, respectively; Table IV). These data suggested that a
favorable response to a metronomic regimen is an important
prognostic factor and that patients with TN cancer are unlikely to
benefit from metronomic regimens. To confirm that patients with TN
tumors did not benefit from metronomic regimens, baseline
characteristics and clinical outcomes were compared between
patients with TN tumors and those with non-TN tumors in the
metronomic group. The median age was significantly higher and DFI
was shorter in patients with TN tumors compared with non-TN tumors
(age, 70 vs. 60 years, P=0.015; and DFI, 63 vs. 23 months,
P=0.0002, respectively; data not shown). Other baseline
characteristics did not differ between patients with TN and those
with non-TN tumors. Patients with non-TN tumors (luminal-type
tumors accounting for 94% of the non-TN tumors) were able to
benefit significantly from metronomic regimens: ORR, 47.4%; TTP, 24
months; and OS >5 years (Table V).
By contrast, patients with TN tumors are unlikely to benefit from
such regimens, as they are not expected to survive for >2
years.
 | Table III.Comparison of clinical outcomes of
patients achieving CR and those not achieving CR. |
Table III.
Comparison of clinical outcomes of
patients achieving CR and those not achieving CR.
| Outcomes | Responders | Non-responders | P-value |
|---|
| Patient no. | 18 | 34 |
|
| Age, years | 59.5 (36–82) | 60.5 (43–81) | 0.35 |
| Tumor grade | 1 (1–3) | 2 (1–3) | 0.36 |
| DFI, months | 60 (24–172) | 32 (10–241) | 0.61 |
| Disease sites,
n | 2 (1–5) | 2 (1–6) | 0.328 |
| Visceral lesions,
% | 55.5 | 52.9 | 0.859 |
| AT pretreatment,
% | 44.4 | 38.2 | 0.667 |
| Hormone-sensitive
tumors, % | 94.4 | 47.1 | 0.0008 |
| HER2-overexpressing
tumors, % | 33.3 | 18.8 | 0.276 |
| Triple-negative
tumors, % | 0 | 38.2 | 0.0027 |
| Chemotherapy cycles
for BC, n | 3.82±1.92 | 4.44±1.97 | 0.25 |
| Chemotherapy cycles
for MBC, n | 3.12±1.64 | 3.97±2.01 | 0.1 |
 | Table IV.Clinical outcomes of patients who
responded and those who did not respond to metronomic
chemotherapy. |
Table IV.
Clinical outcomes of patients who
responded and those who did not respond to metronomic
chemotherapy.
| Outcomes | Responders | Non-responders | P-value |
|---|
| Response rate,
% | 100 |
0 |
|
| Clinical benefit
rate, % | 100 | 47.1 | 0.0002 |
| Disease control
rate, % | 100 | 58.8 | 0.0023 |
| Median TTF,
months | 33 |
9 | 0.00002 |
| Median TTP,
months | 41 | 11 |
0.000008 |
| Median OS,
months | Not reached | 30 | 0.00002 |
| Median OS from MBC,
months | Not reached | 40 | 0.0033 |
 | Table V.Clinical outcomes of patients with TN
cancer and those with non-TN cancer following metronomic
chemotherapy. |
Table V.
Clinical outcomes of patients with TN
cancer and those with non-TN cancer following metronomic
chemotherapy.
| Outcomes | Non-TN | TN | P-value |
|---|
| Response rate,
% | 47.4 | 0 | 0.0026 |
| Clinical benefit
rate, % | 76.3 | 38.5 | 0.0042 |
| Disease control
rate, % | 81.6 | 53.8 | 0.036 |
| Median TTF,
months | 16 | 7 | 0.0014 |
| Median TTP,
months | 24 | 8 | 0.0002 |
| Median OS,
months | 67 | 16 |
0.0000007 |
| Median OS from MBC,
months | 108 | 20 |
0.00000002 |
Adverse events
To assess the toxicity of the treatment,
treatment-related adverse events were compared between patients who
received metronomic regimens and those who did not receive this
type of regimen. The proportion of patients with severe adverse
events (grades 3 and 4) was significantly lower in the metronomic
group compared with that in the non-metronomic group (19.2 vs.
42.2%, respectively, P=0.0314; Table
VI). In the metronomic group, 10 cases with severe adverse
events included 7 cases of leukopenia, 1 case of febrile
neutropenia, 1 case of hemorrhagic cystitis and 1 case of
thrombocytopenia. In the non-metronomic group, 11 cases of severe
adverse events included 5 cases of leukopenia, 1 case of hand-foot
syndrome, 1 case of congestive heart failure, 1 case of neuropathy,
1 case of pneumonia, 1 case of proteinuria and 1 case of anorexia.
Of the severe adverse events, 2 in the metronomic and 4 in the
non-metronomic group led to discontinuation of treatment (Table VI). The proportion of patients with
any grade of treatment-related adverse events was significantly
lower in the metronomic group compared with that in the
non-metronomic group (36.5 vs. 61.6%, respectively; P=0.0376).
 | Table VI.Adverse events in patients who
received metronomic regimens and in those who did not receive this
type of regimen. |
Table VI.
Adverse events in patients who
received metronomic regimens and in those who did not receive this
type of regimen.
| Adverse events | Patients receiving
MET (n=52) | Patients not
receiving MET (n=26) | P-value |
|---|
| Any grade, n
(%) | 19 (36.5) | 16 (61.5) | 0.0376 |
| Grade 3/4, n
(%) | 10 (19.2) | 11 (42.4) | 0.0314 |
|
Leukopenia | 7 | 5 |
|
|
Cystitis | 1a | – |
|
| Febrile
neutropenia | 1 | – |
|
|
Thrombocytopenia | 1a | – |
|
|
Hand-foot syndrome | – | 1 |
|
| Heart
failure | – | 1a |
|
|
Neuropathy | – | 1a |
|
|
Pneumonia | – | 1a |
|
|
Proteinuria | – | 1 |
|
|
Anorexia | – | 1a |
|
Discussion
As chemotherapy agents used in the adjuvant setting,
anthracyclines and taxanes are included in standard regimens, based
on abundant evidence from a number of clinical trials over the past
several decades, showing significant reduction in the risk of
relapse or death from breast cancer (1–3). By
contrast, there is no standard regimen recommended in the
metastatic setting. In cases with life-threatening metastatic
lesions or rapidly growing tumors, regimens that are expected to
control lesions quickly, such as simultaneous administration of
taxanes and anthracyclines, or taxanes in combination with either
gemcitabine, capecitabine or bevacizumab, should be applied
(4–9,20–23). However, a proportion of the patients
exhibit disease progression during or after receiving these
cytotoxic regimens, even if favorable combinations are selected. We
previously demonstrated that long-term administration of one
regimen was crucial for long-term survival of patients with MBC
(11). To prolong the duration of
treatment or TTF, chemotherapeutic regimens that are effective but
less toxic are required.
Metronomic chemotherapy, which is defined as
continuous or frequent treatment with low doses of anticancer
agents, has been reported to result in favorable tumor responses
and prolonged survival (13). We
previously reported that the proportion of patients who received a
metronomic regimen as the most effective regimen was two-thirds of
the long-term survivors (65.3%), who survived for ≥50 months after
diagnosis with MBC, which was double that of the non-long-term
survivors, who succumbed to the disease within 50 months (12). The mechanisms of inhibition of tumor
growth by metronomic chemotherapy remain to be determined.
Administration of low doses of cytotoxic agents is not expected to
cause potent cytotoxic activity against tumor cells. However,
metronomic chemotherapy has been shown in a preclinical study to
elicit anti-angiogenic effects, abrogating tumor growth (14). Therefore, this treatment may continue
to suppress tumor growth over a long period. In the present study,
we aimed to identify a population of patients who may benefit from
metronomic chemotherapy by retrospectively comparing baseline
characteristics and clinical outcomes of patients with MBC who had
been treated with metronomic regimens and those of patients who had
been treated with non-metronomic regimens.
Among the baseline characteristics assessed in this
study, median age was significantly higher, tumor grade was lower
and the number of prior cytotoxic regimens was higher in the
metronomic group compared with those in the non-metronomic group
(Table I). The data suggest that
metronomic regimens tended to be administered to patients who were
elderly or had attenuated physical status, or to patients who had
been heavily pretreated. As regards clinical outcome, all
prognostic parameters (TTF, TTP and OS) after metronomic regimens
or after the most effective of the cytotoxic regimens that patients
had received, were significantly prolonged in the metronomic group
compared with those in the non-metronomic group (Table II). By contrast, ORR did not differ
significantly between the two groups. These data suggest that
long-term treatment by one regimen is an important factor
responsible for favorable prognosis, rather than temporary tumor
response to different agents. In addition, the data suggest that
good tumor responses to potent cytotoxic agents that have toxic
side effects may not result in favorable outcomes in patients with
MBC. Furthermore, we compared the clinical outcomes of patients who
responded to metronomic regimens and those of patients who did not
respond, in order to determine whether tumor response to metronomic
regimens is required for favorable prognosis. All the prognostic
factors assessed were found to be significantly better in
responders compared with non-responders (Table IV). Moreover, survival time from MBC
diagnosis was significantly prolonged in responders compared with
that in non-responders, regardless of treatment line of the regimen
for MBC. It was noted that almost all patients (94.4%) who
responded to metronomic regimens had hormone-sensitive luminal-type
tumors (Table III). By contrast,
none of patients with TN tumors responded to this type of regimen.
A comparison of clinical outcomes between patients with non-TN
tumors and patients with TN tumors in the metronomic group revealed
that all prognostic factors, including TTF, TTP and OS, were
significantly prolonged in patients with non-TN tumors compared
with those in patients with TN tumors (Table V). These data suggest that metronomic
regimens may be indicated for luminal-type breast cancer, but not
for TN breast cancer.
As described above, not only long-term treatment
with one regimen, but also a less toxic regimen, is crucial for
favorable prognosis in MBC. The proportion of patients who
experienced treatment-related adverse events was significantly
lower in the metronomic group compared with that in the
non-metronomic group (36.5 vs. 61.5%, respectively; Table VI). Furthermore, serious grade 3/4
adverse events were less frequently observed in the metronomic
compared with the non-metronomic group (19.2 vs. 42.4%,
respectively). The results suggested that metronomic chemotherapy
is less toxic and more tolerable in heavily pretreated patients
with MBC.
In conclusion, metronomic regimens demonstrated
favorable activity and minimal toxicity in patients with MBC,
regardless of prior treatment, treatment line and disease sites. In
particular, this type of regimen is indicated for patients with
luminal-type breast cancer and results in long-term survival,
particularly in responders. However, since a metronomic regimen is
unlikely to be effective in patients with TN tumors, alternative
therapeutics are required for this subpopulation.
Acknowledgements
The present study was supported in part by a
Grant-in-Aid for Scientific Research from the Ministry of
Education, Science, Sports and Culture, Japan (grant no.
15591340).
Glossary
Abbreviations
Abbreviations:
|
CBR
|
clinical benefit rate
|
|
CR
|
complete response
|
|
DCR
|
disease control rate
|
|
DFI
|
disease-free interval
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
MBC
|
metastatic breast cancer
|
|
ORR
|
overall response rate
|
|
OS
|
overall survival
|
|
PD
|
progressive disease
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
TTF
|
time-to-treatment failure
|
|
TN
|
triple-negative
|
|
TTP
|
time-to-progression
|
References
|
1
|
Smith I and Chua S: Medical treatment of
early breast cancer. IV: Neoadjuvant treatment. BMJ. 332:223–224.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bria E, Nistico C, Cuppone F, Carlini P,
Ciccarese M, Milella M, Natoli G, Terzoli E, Cognetti F and
Giannarelli D: Benefit of taxanes as adjuvant chemotherapy for
early breast cancer: Pooled analysis of 15,500 patients. Cancer.
106:2337–2344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buzdar AU, Singletary SE, Valero V, Booser
DJ, Ibrahim NK, Rahman Z, Theriault RL, Walters R, Rivera E, Smith
TL, et al: Evaluation of paclitaxel in adjuvant chemotherapy for
patients with operable breast cancer: Preliminary data of a
prospective randomized trial. Clin Cancer Res. 8:1073–1079.
2002.PubMed/NCBI
|
|
4
|
O'Shaughnessy J, Miles D, Vukelja S,
Moiseyenko V, Ayoub JP, Cervantes G, Fumoleau P, Jones S, Lui WY,
Mauriac L, et al: Superior survival with capecitabine plus
docetaxel combination therapy in anthracycline-pretreated patients
with advanced breast cancer: Phase III trial results. J Clin Oncol.
20:2812–2823. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Albain KS, Nag SM, Calderillo-Ruiz G,
Jordaan JP, Llombart AC, Pluzanska A, Rolski J, Melemed AS,
Reyes-Vidal JM, Sekhon JS, et al: Gemcitabine plus paclitaxel
versus paclitaxel monotherapy in patients with metastatic breast
cancer and prior anthracycline treatment. J Clin Oncol.
26:3950–3957. 2009. View Article : Google Scholar
|
|
6
|
Nielsen DL, Bjerre KC, Jakobsen EH, Cold
S, Stenbygaard L, Sørensen PG, Kamby C, Møller S, Jørgensen CLT and
Andersson M: Gemcitabine plus docetaxel versus docetaxel in
patients with predominantly human epidermal growth factor receptor
2-negative locally advanced or metastatic breast cancer: A
randomized, phase III study by the Danish Breast Cancer Cooperative
Group. J Clin Oncol. 29:4748–4754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brufsky AM, Hurvitz S, Perez E, Swamy R,
Valero V, O'Neill V and Rugo HS: RIBBON-2: A randomized,
double-blind, placebo-controlled, phase III trial evaluating the
efficacy and safety of bevacizumab in combination with chemotherapy
for second-line treatment of human epidermal growth factor receptor
2-negative metastatic breast cancer. J Clin Oncol. 29:4286–4293.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robert NJ, Diéras V, Glaspy J, Brufsky AM,
Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et
al: RIBBON-1: Randomized, double-blind, placebo-controlled, phase
III trial of chemotherapy with or without bevacizumab for
first-line treatment of human epidermal growth factor receptor
2-negative, locally recurrent or metastatic breast cancer. J Clin
Oncol. 29:1252–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cortes J, O'Shaughnessy J, Loesch D, Blum
JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V,
Delozier T, et al: Eribulin monotherapy versus treatment of
physician's choice in patients with metastatic breast cancer
(EMBRACE): A phase 3 open-label randomised study. Lancet.
377:914–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kontani K, Hashimoto SI, Murazawa C,
Norimura S, Tanaka H, Ohtani M, Fujiwara-Honjo N, Date M, Houchi H
and Yokomise H: Metronomic chemotherapy for metastatic breast
cancer to prolong time to treatment failure to 12 months or more.
Mol Clin Oncol. 1:225–230. 2013.PubMed/NCBI
|
|
12
|
Kontani K, Hashimoto S, Murazawa C,
Norimura S, Tanaka H, Ohtani M, Fujiwara-Honjo N, Date M, Teramoto
K, Houchi H and Yokomise H: Factors responsible for long-term
survival in metastatic breast cancer. World J Surg Oncol.
12:3442014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oostendorp LJ, Stalmeier PF, Donders AR,
van der Graaf WT and Ottevanger PB: Efficacy and safety of
palliative chemotherapy for patients with advanced breast cancer
pretreated with anthracyclines and taxanes: A systematic review.
Lancet Oncol. 12:1053–1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kerbel RS and Kamen BA: The
anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer.
4:423–436. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colleoni M, Rocca A, Sandri MT, Zorzino L,
Masci G, Nolè F, Peruzzotti G, Robertson C, Orlando L, Cinieri S,
et al: Low-dose oral methotrexate and cyclophosphamide in
metastatic breast cancer: Antitumor activity and correlation with
vascular endothelial growth factor levels. Ann Oncol. 13:73–80.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Colleoni M, Orlando L, Sanna G, Rocca A,
Maisonneuve P, Peruzzotti G, Ghisini R, Sandri MT, Zorzino L, Nolè
F, et al: Metronomic low-dose oral cyclophosphamide and
methotrexate plus or minus thalidomide in metastatic breast cancer:
Antitumor activity and biological effects. Ann Oncol. 17:232–238.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong NS, Buckman RA, Clemons M, Verma S,
Dent S, Trudeau ME, Roche K, Ebos J, Kerbel R, Deboer GE, et al:
Phase I/II trial of metronomic chemotherapy with daily dalteparin
and cyclophosphamide, twice-weekly methotrexate, and daily
prednisone as therapy for metastatic breast cancer using vascular
endothelial growth factor and soluble vascular endothelial growth
factor receptor levels as markers of response. J Clin Oncol.
28:723–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumors:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
US Department of Health and Human
Services. Common Terminology Criteria for Adverse Events (CTCAE).
version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdfAccessed.
January 14–2014
|
|
20
|
Jassem J, Pieńkowski T, Płuzańska A, Jelic
S, Gorbunova V, Mrsic-Krmpotic Z, Berzins J, Nagykalnai T, Wigler
N, Renard J, et al: Doxorubicin and paclitaxel versus fluorouracil,
doxorubicin, and cyclophosphamide as first-line therapy for women
with metastatic breast cancer: Final results of a randomized phase
III multicenter trial. J Clin Oncol. 19:1707–1715. 2001.PubMed/NCBI
|
|
21
|
Bonneterre J, Dieras V, Tubiana-Hulin M,
Bougnoux P, Bonneterre ME, Delozier T, Mayer F, Culine S, Dohoulou
N and Bendahmane B: Phase II multicentre randomised study of
docetaxel plus epirubicin vs 5-fluorouracil plus epirubicin and
cyclophosphamide in metastatic breast cancer. Br J Cancer.
91:1466–1471. 2004.PubMed/NCBI
|
|
22
|
Sledge GW, Neuberg D, Bernardo P, Ingle
JN, Martino S, Rowinsky EK and Wood WC: Phase III trial of
doxorubicin, paclitaxel and the combination of doxorubicin and
paclitaxel as front-line chemotherapy for metastatic breast cancer:
An intergroup trial (E1193). J Clin Oncol. 21:588–592. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paridaens R, Biganzoli L, Bruning P, Klijn
JG, Gamucci T, Houston S, Coleman R, Schachter J, Van Vreckem A,
Sylvester R, et al: Paclitaxel versus doxorubicin as first-line
single-agent chemotherapy for metastatic breast cancer: A European
Organization for Research and Treatment of Cancer Randomized Study
with cross-over. J Clin Oncol. 18:724–733. 2000.PubMed/NCBI
|