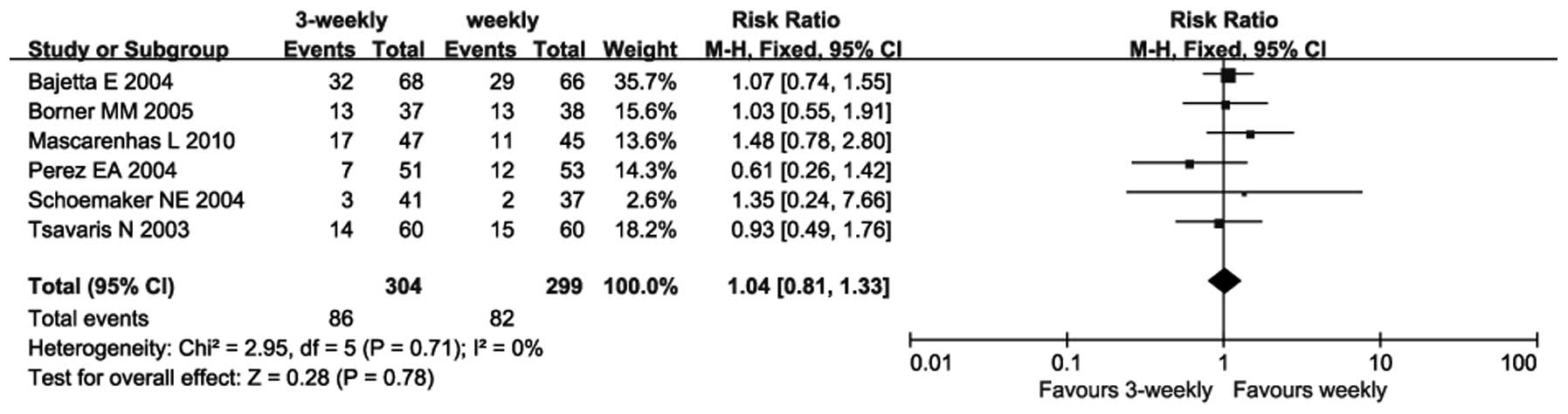
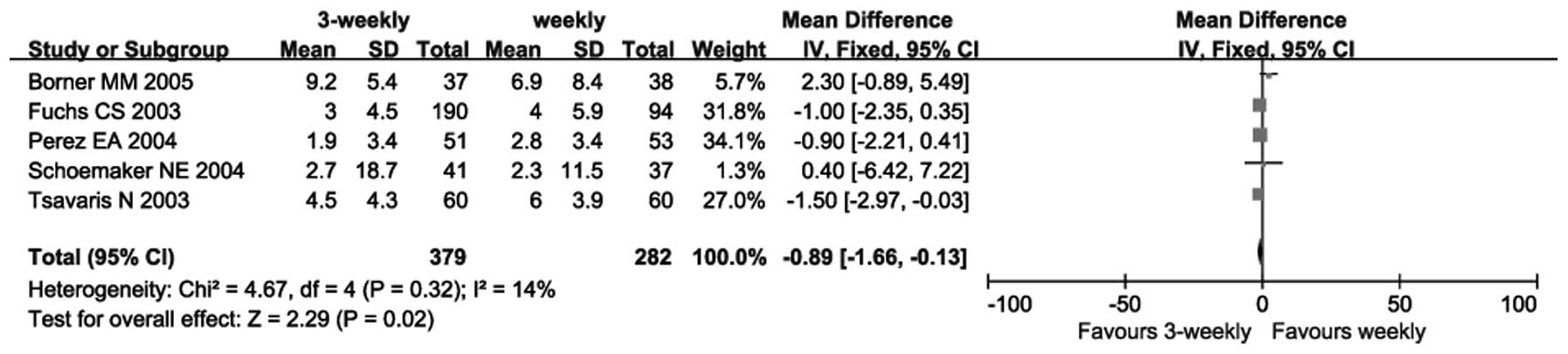
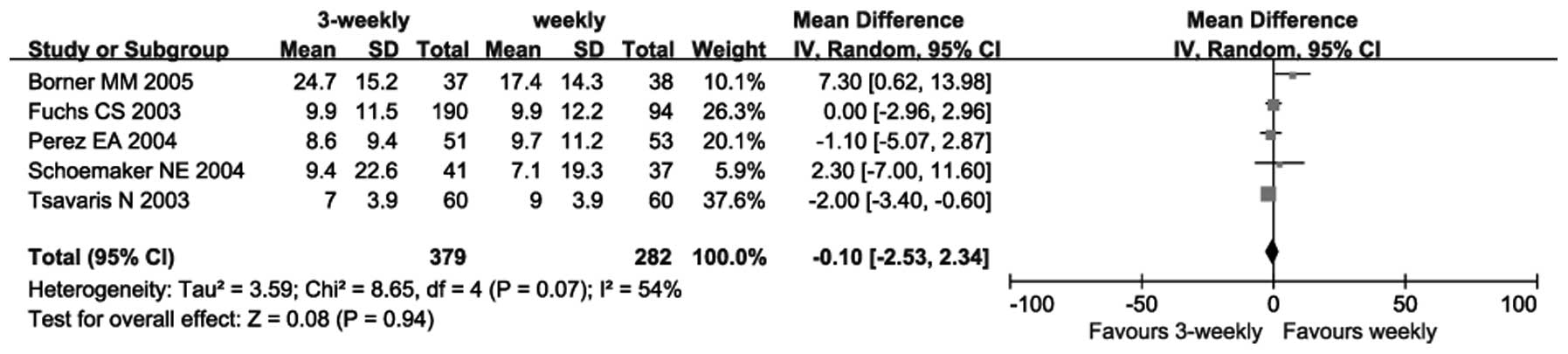
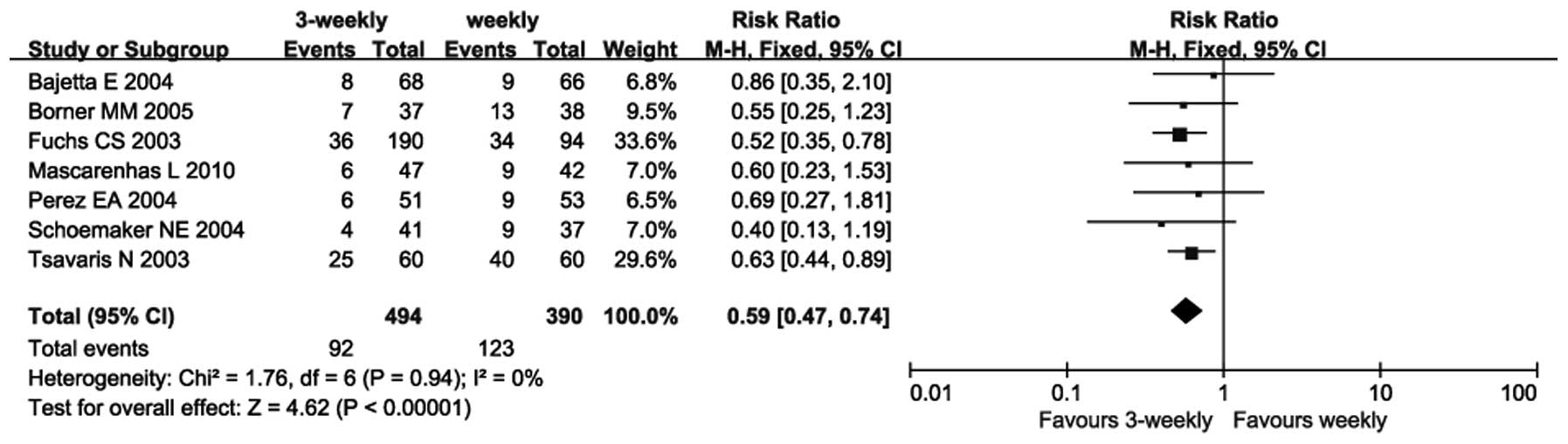
|
1
|
Pitot HC, Wender DB, O'Connell MJ,
Schroeder G, Goldberg RM, Rubin J, Mailliard JA, Knost JA, Ghosh C,
Kirschling RJ, et al: Phase II trial of irinotecan in patients with
metastatic colorectal carcinoma. J Clin Oncol. 15:2910–2919.
1997.PubMed/NCBI
|
|
2
|
Vanhoefer U, Harstrick A, Achterrath W,
Cao S, Seeber S and Rustum YM: Irinotecan in the treatment of
colorectal cancer: Clinical overview. J Clin Oncol. 19:1501–1518.
2001.PubMed/NCBI
|
|
3
|
Rivory LP, Bowles MR, Robert J and Pond
SM: Conversion of irinotecan (CPT-11) to its active metabolite,
7-ethyl-10-hydroxycamptothecin (SN-38), by human liver
carboxylesterase. Biochem Pharmacol. 52:1103–1111. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iyer L, King CD, Whitington PF, Green MD,
Roy SK, Tephly TR, Coffman BL and Ratain MJ: Genetic predisposition
to the metabolism of irinotecan (CPT-11). Role of uridine
diphosphate glucuronosyltransferase isoform 1A1 in the
glucuronidation of its active metabolite (SN-38) in human liver
microsomes. J Clin Invest. 101:847–854. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu ZY, Yu Q, Pei Q and Guo C:
Dose-dependent association between UGT1A1*28 genotype and
irinotecan-induced neutropenia: Low doses also increase risk. Clin
Cancer Res. 16:3832–3842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Innocenti F, Schilsky RL, Ramírez J,
Janisch L, Undevia S, House LK, Das S, Wu K, Turcich M, Marsh R, et
al: Dose-finding and pharmacokinetic study to optimize the dosing
of irinotecan according to the UGT1A1 genotype of patients with
cancer. J Clin Oncol. 32:2328–2334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KP, Kim HS, Sym SJ, et al: A UGT1A1*28
and *6 genotype-directed phase I dose-escalation trial of
irinotecan with fixed-dose capecitabine in Korean patients with
metastatic colorectal cancer. Cancer Chemother Pharmacol.
71:1609–1617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goetz MP, McKean HA, Reid JM, et al:
UGT1A1 genotype-guided phase I study of irinotecan, oxaliplatin,
and capecitabine. Invest New Drugs. 31:1559–1567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phelps MA and Sparreboom A: Irinotecan
pharmacogenetics: A finished puzzle? J Clin Oncol. 32:2287–2289.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rothenberg ML, Eckardt JR, Kuhn JG, Burris
HA III, Nelson J, Hilsenbeck SG, Rodriguez GI, Thurman AM, Smith
LS, Eckhardt SG, et al: Phase II trial of irinotecan in patients
with progressive or rapidly recurrent colorectal cancer. J Clin
Oncol. 14:1128–1135. 1996.PubMed/NCBI
|
|
11
|
Armand JP, Extra YM, Catimel G, Abigerges
D, Marty M and Clavel M: Rationale for the dosage and schedule of
CPT-11 (irinotecan) selected for phase II studies, as determined by
European phase I studies. Ann Oncol. 7:837–842. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bajetta E, Di Bartolomeo M, Mariani L,
Cassata A, Artale S, Frustaci S, Pinotti G, Bonetti A, Carreca I,
Biasco G, et al: Randomized multicenter phase II trial of two
different schedules of irinotecan combined with capecitabine as
first-line treatment in metastatic colorectal carcinoma. Cancer.
100:279–287. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borner MM, Bernhard J, Dietrich D, et al:
A randomized phase II trial of capecitabine and two different
schedules of irinotecan in first-line treatment of metastatic
colorectal cancer: Efficacy, quality-of-life and toxicity. Ann
Oncol. 16:282–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fuchs CS, Moore MR, Harker G, Villa L,
Rinaldi D and Hecht JR: Phase III comparison of two irinotecan
dosing regimens in second-line therapy of metastatic colorectal
cancer. J Clin Oncol. 21:807–814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mascarenhas L, Lyden ER, Breitfeld PP, et
al: Randomized phase II window trial of two schedules of irinotecan
with vincristine in patients with first relapse or progression of
rhabdomyosarcoma: A report from the Children's Oncology Group. J
Clin Oncol. 28:4658–4663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perez EA, Hillman DW, Mailliard JA, Ingle
JN, Ryan JM, Fitch TR, Rowland KM, Kardinal CG, Krook JE, Kugler JW
and Dakhil SR: Randomized phase II study of two irinotecan
schedules for patients with metastatic breast cancer refractory to
an anthracycline, a taxane, or both. J Clin Oncol. 22:2849–2855.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schoemaker NE, Kuppens IE, Moiseyenko V,
Glimelius B, Kjaer M, Starkhammer H, Richel DJ, Smaaland R,
Bertelsen K, Poulsen JP, et al: A randomised phase II multicentre
trial of irinotecan (CPT-11) using four different schedules in
patients with metastatic colorectal cancer. Br J Cancer.
91:1434–1441. 2004.PubMed/NCBI
|
|
18
|
Tsavaris N, Ziras N, Kosmas C, Giannakakis
T, Gouveris P, Vadiaka M, Dimitrakopoulos A, Karadima D, Rokana S,
Papalambros E, et al: Two different schedules of irinotecan
(CPT-11) in patients with advanced colorectal carcinoma relapsing
after a 5-fluorouracil and leucovorin combination. A randomized
study. Cancer Chemother Pharmacol. 52:514–519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
andomized trials. BMJ. 18:d59282011. View Article : Google Scholar
|
|
20
|
Rothenberg ML, Cox JV, DeVore RF,
Hainsworth JD, Pazdur R, Rivkin SE, Macdonald JS, Geyer CE Jr,
Sandbach J, Wolf DL, et al: A multicenter, phase II trial of weekly
irinotecan (CPT-11) in patients with previously treated colorectal
carcinoma. Cancer. 85:786–795. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rougier P, Van Cutsem E, Bajetta E,
Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg
H, Wils J, et al: Randomised trial of irinotecan versus
fluorouracil by continuous infusion after fluorouracil failure in
patients with metastatic colorectal cancer. Lancet. 352:1407–1412.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoskins JM, Goldberg RM, Qu P, Ibrahim JG
and McLeod HL: UGT1A1*28 genotype and irinotecan-induced
neutropenia: Dose matters. J Natl Cancer Inst. 99:1290–1295. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masuda N, Kudoh S and Fukuoka M:
Irinotecan (CPT-11): Pharmacology and clinical applications. Crit
Rev Oncol Hematol. 24:3–26. 1996. View Article : Google Scholar : PubMed/NCBI
|