Introduction
Oral squamous cell carcinoma (OSCC) represents 2–3%
of all human cancers and is the 6th most frequent type of cancer
worldwide (1,2). OSCC has a consistently poor prognosis
and remains a lethal disease in >50% of cases diagnosed annually
(3). The current management and
treatment for the majority of OSCC patients is surgery, and
postoperative concurrent chemoradiotherapy is a widely accepted
standard of care for high-risk OSCC (4). This treatment strategy has led to
significant improvements in locoregional control and disease-free
survival, but not in overall survival (OS) (5). The risk factors associated with lack of
improvement in OS remain unclear, but one risk factor has been
hypothesized to be distant metastasis (DM). In the head and neck
region, it was reported that the hypopharynx and supraglottis were
the sites with a higher risk of DM (9.4 and 8.9%, respectively),
whereas the oral cavity had a lower risk (3.2%) (6). It has been reported that DMs occur 1–76
months after radical surgery in ~10% of patients with OSCC
(7). As DMs reduce the patients'
quality of life and carry a poor prognosis, improved treatment of
DMs is an important consideration.
Cetuximab, an epidermal growth factor receptor
(EGFR) inhibitor, was approved for the treatment of locally
advanced (LA) and recurrent/metastatic (R/M) head and neck squamous
cell carcinoma (HNSCC) in December, 2012 in Japan. Phase III trials
demonstrated that cetuximab used in combination with radiotherapy
in LA HNSCC (8) and in combination
with platinum-based chemotherapy as first-line treatment for R/M
HNSCC (9) achieved a higher response
rate and a significant increase in OS. In addition, it has been
reported that cetuximab used in combination with paclitaxel is
effective when platinum-based chemotherapy fails in R/M HNSCC
(10,11). However, the efficacy of cetuximab for
DMs remains unclear.
The aim of this retrospective study was to assess
the efficacy and safety of cetuximab therapy in patients with LA
and R/M OSCC, with a specific focus on patients with DMs.
Patients and methods
Study design
We retrospectively reviewed records of patients with
confirmed unresectable LA and R/M OSCC who were treated with
cetuximab between December, 2012 and July, 2015 (cetuximab group).
This study was approved by the independent ethics committee of our
Nagasaki University Hospital. The endpoint of this trial was
defined as the time-to-disease progression, and we examined the
tumor response rate, progression-free survival (PFS), OS and
safety. Tumor response was assessed every 4–8 weeks with repeated
clinical and enhanced computed tomography (CT) assessments, until
progressive disease (PD) was observed; the best overall response
was evaluated according to the response evaluation criteria in
solid tumours for the duration of treatment (12). PFS was defined as the time from the
date of the first cetuximab administration to the date of PD or
relapse, whichever occurred first. OS was defined as the time from
the date of cetuximab administration to the date of death. Survival
distributions were calculated with the Kaplan-Meier method and
compared using the log-rank test. Two-sided confidence intervals
(CIs) were calculated according to Clopper and Pearson. Toxic
effects were evaluated according to the National Cancer
Institute-Common Terminology Criteria for Adverse Events, version
4.0 (13).
Treatment
The regimens used in our department were cetuximab
plus radiotherapy according to the Bonner trial (8), cetuximab plus cisplatin and
5-fluorouracil according to the EXTREME trial (9), and cetuximab plus paclitaxel (10,11). Prior
to drug administration, the serum levels of surfactant protein-A,
surfactant protein-D and Krebs von den Lungen-6 were assessed using
blood tests; plain radiography and CT of the chest were performed
to determine the presence of interstitial pneumonia. In addition,
the serum levels of tick, mammalian meat and flatfish antibodies
were assessed for allergy and specific IgG antibody titres.
Cetuximab was administered at a dose of 400 mg/m2 for the first
injection and 250 mg/m2 weekly thereafter. A total radiation dose
of 60–66 Gy was administered once daily in fractions of 1.8–2.0 Gy,
with five fractions administered per week. Cisplatin was
administered at a dose of 100 mg/m2 on day 1, and 5-fluorouracil
was administered at a dose of 1,000 mg/m2 for 4 days. Paclitaxel
was administered weekly at a dose of 60–80 mg/m2. Dosage was
decided according to the patient's general condition. Patients who
had at least stable disease (SD) received cetuximab therapy until
PD or unacceptable toxic effects occurred.
Results
Patient characteristics
The patient clinicopathological characteristics are
summarized in Table I. Over a period
of 2 years and 6 months, 21 patients were enrolled in the study;
47.6% of the patients were male and 52.4% were female, and 20
patients had a performance status of 0 or 1. The median age of the
patients was 73 years, (range, 51–88 years). The most common site
of the primary tumour was the mandibular gingiva (52.4%), followed
by the tongue (23.8%) and maxillary gingiva (9.52%). On
pathological examination, 16 of the 21 tumors were SCCs (76.2%) and
the remaining 5 were adenosquamous cell carcinoma (n=1, 4.76%),
ameloblastic carcinoma (n=1, 4.76%), myoepithelial carcinoma (n=1,
4.76%), undifferentiated carcinoma (4.76%) and unclear (n=1,
4.76%). The overexpression of EGFR was confirmed by immunostaining
in each of the 5 non-SCC cases prior to drug administration. There
were 3 patients with LA carcinoma (14.3%) and 18 with R/M carcinoma
(85.7%). A total of 18 patients had undergone previous therapy: 8
had undergone surgery alone (38.1%), 2 had undergone surgery and
adjuvant radiotherapy (9.5%), and 8 had undergone surgery and
adjuvant chemoradiotherapy (38.1%). With respect to initial
treatment regimens, of the 21 tumors, 9 had been treated with
cetuximab plus radiotherapy (42.8%), 9 with cetuximab plus
paclitaxel (42.8%), 2 with cetuximab plus cisplatin and
5-fluorouracil (9.5%) and 1 with cetuximab alone (4.8%). The median
number of treatment courses with cetuximab was 9 (range, 0–70).
 | Table I.Clinicopathological characteristics of
the 21 patients. |
Table I.
Clinicopathological characteristics of
the 21 patients.
| Characteristics | No. of cases (%) |
|---|
| Gender |
|
| Male | 10 (47.6) |
|
Female | 11 (52.4) |
| Age, years |
|
|
Range | 51–88 |
|
Median | 73 |
| Primary site |
|
|
Tongue | 5 (23.8) |
|
Mandibular gingiva | 11 (52.4) |
|
Maxillary gingiva | 2 (9.52) |
| Oral
cavity floor | 1 (4.76) |
|
Intraosseous | 1 (4.76) |
|
Unknown | 1 (4.76) |
| Pathological
diagnosis |
|
|
SCC | 16 (76.2) |
|
Ameloblastic carcinoma | 1 (4.76) |
|
Myoepithelial carcinoma | 1 (4.76) |
| Adeno
SCC | 1 (4.76) |
|
Undifferentiated
carcinoma | 1 (4.76) |
|
Unclear | 1 (4.76) |
| Performance status
score |
|
| 0 | 11 (52.4) |
| 1 | 9 (42.9) |
| 2 | 1 (4.8) |
| Pattern of
disease |
|
| LA | 3 (14.3) |
|
R/M | 18 (85.7) |
| Previous
treatment |
|
| Surgery
alone | 8 (44.4) |
| Surgery
+ adjuvant RT | 2 (11.1) |
| Surgery
+ adjuvant CCRT | 8 (44.4) |
| Initial
treatment |
|
| Cet +
RT | 9 (42.8) |
| Cet +
TXL | 9 (42.8) |
| Cet +
FP | 2 (9.5) |
| Cet
alone | 1 (4.8) |
| Number of treatment
cycles |
|
|
Range | 0–70 |
|
Median | 9 |
Efficacy
The details of all cases and of DM cases are
presented in Table II. The overall
response rate was 57.1% (95% CI: 33.7–78.2%), with a complete
response (CR) rate of 33.3% (95% CI: 13.9–56.9%) and a partial
response (PR) rate of 23.8% (95% CI: 7.7–47.6%). The disease
control rate (PR plus SD) was 66.7% (95% CI: 42.7–85.4%).
Cetuximab-refractory tumors were observed in 2 patients. Although 5
patients initially achieved disease control, they developed PD.
Among these, 2 patients presented with brain metastasis during
cetuximab administration, although 1 achieved locoregional and lung
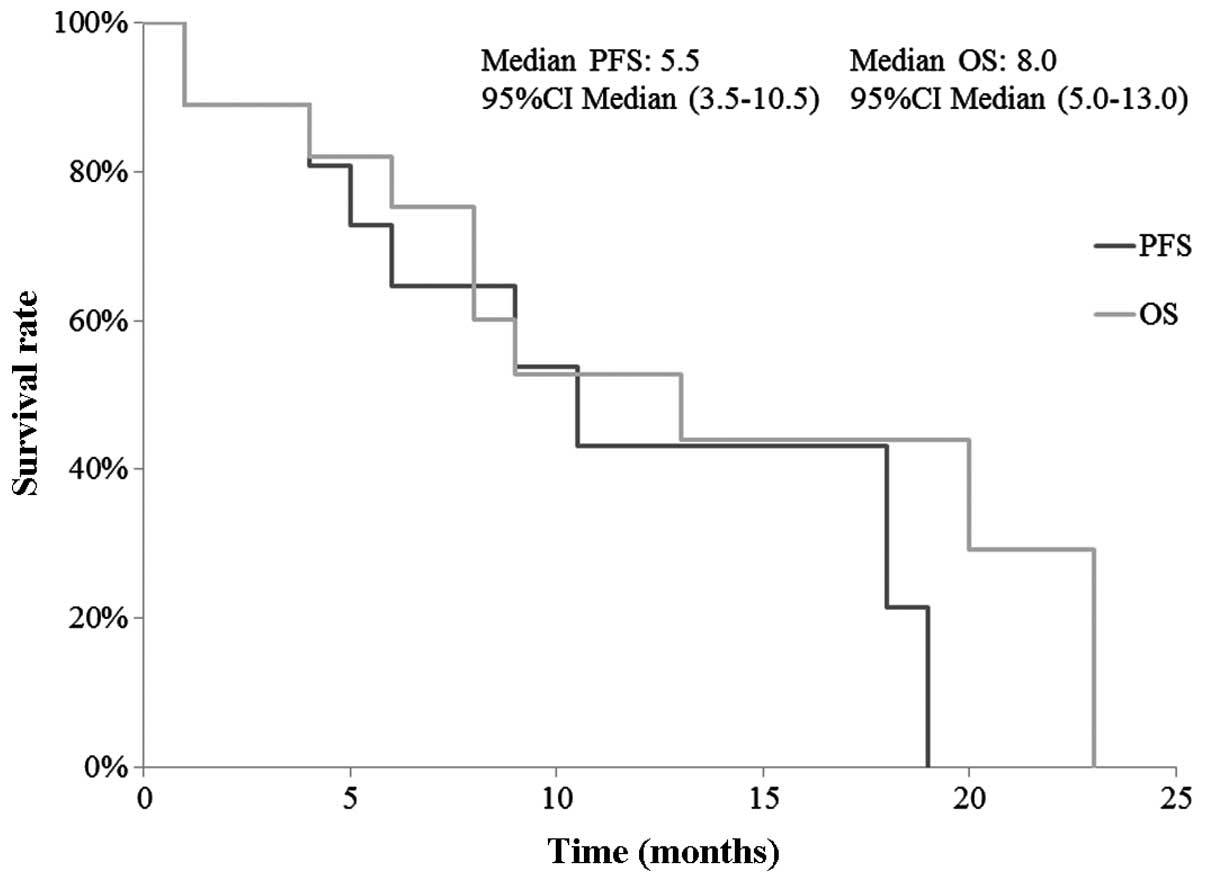
metastasis control. The 1-year PFS and OS were 43.1 and 52.7%,
respectively. The median PFS and OS were 5.5 months (95% CI:
3.5–10.5 months) and 8.0 months (95% CI: 5–13 months), respectively
(Fig. 1).
 | Table II.Tumor response. |
Table II.
Tumor response.
|
| All cases | DM cases |
|---|
|
|
|
|
|---|
| Tumor response | n=21 (%) | 95% CI | n=10 (%) | 95% CI |
|---|
| Best response |
|
|
|
|
| CR | 7
(33.3) | 13.9–56.9 | 4 (40.0) | 12.2–73.8 |
| PR | 5
(23.8) | 7.7–47.6 | 2 (20.0) | 2.5–55.6 |
| SD | 4
(19.0) | 4.9–42.5 | 2 (20.0) | 2.5–55.6 |
| PD | 2
(9.5) | 0.7–31.6 | 2 (20.0) | 2.5–55.6 |
| NE | 3
(14.3) | 3.0–36.5 | 4 (40.0) | 12.2–73.8 |
| Overall response
rate (CR+PR) | 12 (57.1) | 33.7–78.2 | 6 (60.0) | 26.7–87.8 |
| Disease control
rate (CR+PR+SD) | 16 (76.2) | 52.7–91.8 | 8 (80.0) | 43.2–98.5 |
A total of 10 patients treated with cetuximab
therapy had DMs. The most common distant site was the lung (70.0%),
followed by the upper mediastinal lymph nodes (10.0%),
parapharyngeal lymph nodes (10.0%), and multiple bone metastases on
the ribs, iliac bone and vertebrae (10.0%). The overall response
rate among patients with DMs was 60.0% (95% CI: 26.2–87.8%), with a
CR rate of 40.0% (95% CI: 12.2–73.8%) and a PR rate of 20.0% (95%
CI: 2.5–55.6%). The disease control rate was 70.0% (95% CI:
34.8–93.8%). No difference in the efficacy of cetuximab therapy was
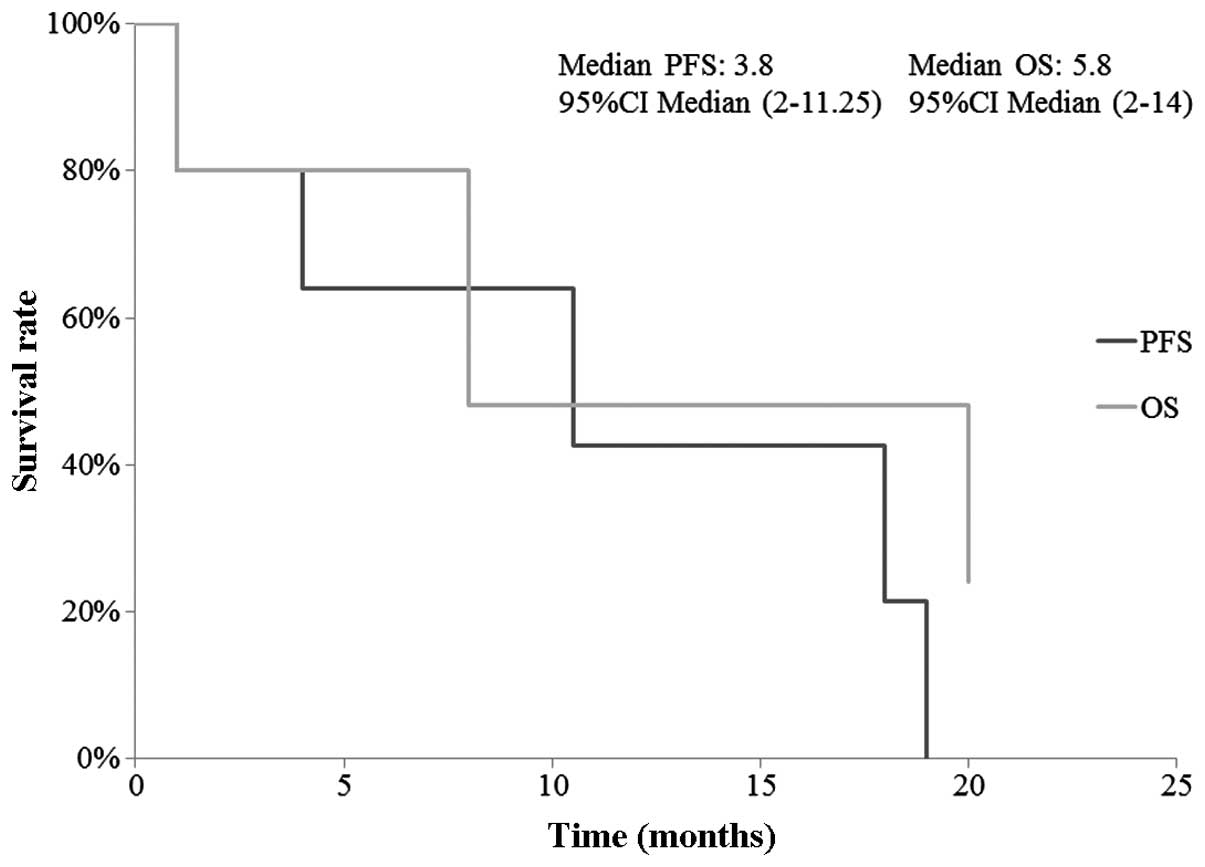
observed between different DM sites. The 1-year PFS and OS were
42.7 and 48.0%, respectively. The median PFS and OS were 3.8 months
(95% CI: 2–11.25 months) and 5.8 months (95% CI: 2–14 months),
respectively (Fig. 2). In addition,
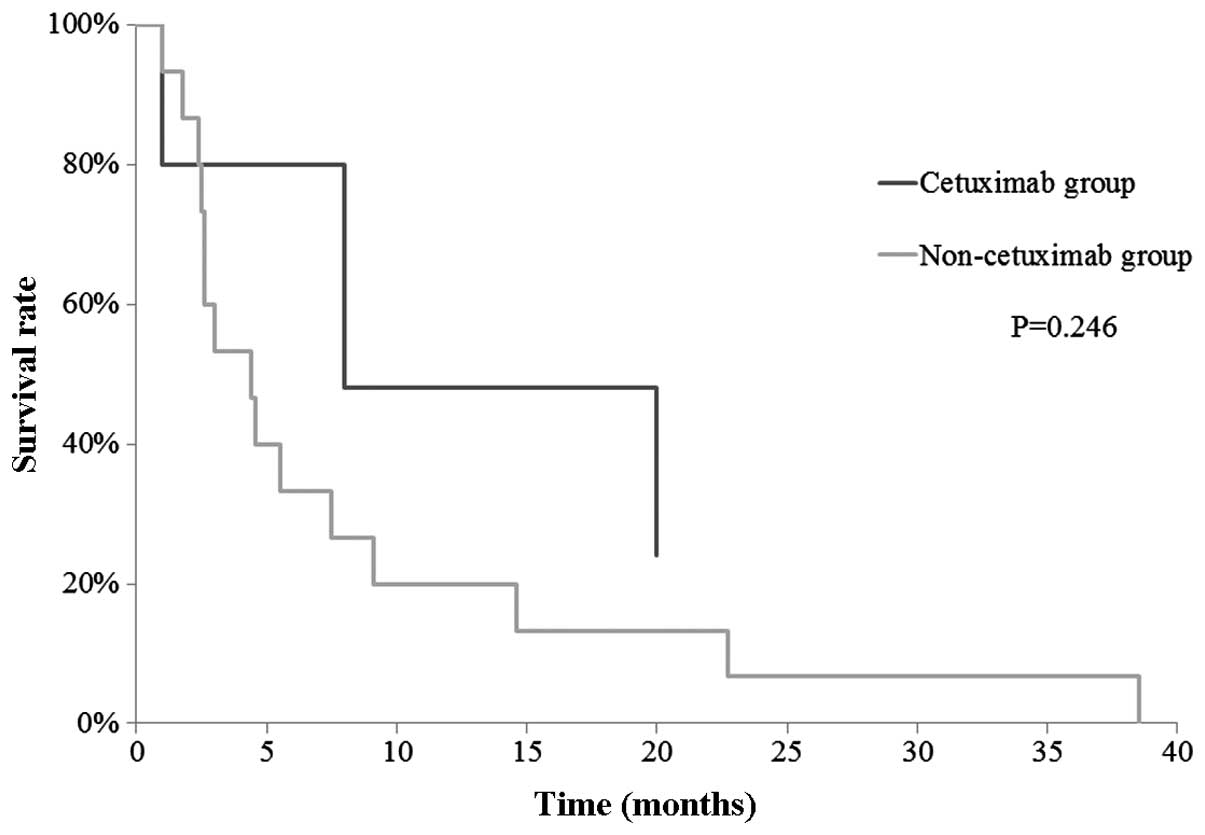
we retrospectively reviewed the records of patients with confirmed
DM between April, 2001 and November, 2012 before cetuximab was
approved (non-cetuximab group), and a historical-control study was
performed to compare the OS between the cetuximab and non-cetuximab
groups in patients with DMs. The 1-year OS for each group was 48.0
and 20.0%, respectively. No significant difference was observed
(P=0.246), but the data indicated that cetuximab provided
additional benefits in patients with DMs (Fig. 3).
Safety
Treatment-related grade 3–5 adverse events were
reported in 9 patients (42.9%) (Table
III). There were 3 grade 3 infusion reactions (anaphylaxis and
dyspnea in all cases), 1 grade 4 reaction (anaphylaxis) and 1 grade
5 reaction (interstitial pneumonia) among patients receiving
cetuximab, who had to withdraw from therapy. Grade 3
hypophosphatemia, neutropenia, upper gastrointestinal hemorrhage
and liver toxicity were reported with the cetuximab plus paclitaxel
regimen; grade 3 diarrhea was reported with the cetuximab plus
cisplatin and 5-fluorouracil regimen; and grade 3 hypophosphatemia
was reported with the cetuximab plus radiotherapy regimen, which
led to the discontinuation of these drug combinations. Skin
reactions, including an acne-like rash and paronychia, were seen in
all patients; 11 patients had grade 1 (52.4%), 7 had grade 2
(33.3%), and 3 patients (14.3%) had non-evaluable infusion-related
reactions; no patient had an infusion-related reaction of grade
>3. Grade 1–2 treatment-related adverse events included
hypomagnesemia (47.6%), taste dysfunction (14.3%), alopecia (9.5%),
peripheral neuropathy (9.5%) and nausea (9.5%).
 | Table III.Adverse events. |
Table III.
Adverse events.
| Adverse events | Cet+RT (n=9) | Cet+TXL (n=9) | Cet+FP (n=2) | Cet (n=1) |
|---|
| Grade ≥3 |
|
|
|
|
|
Infusion reaction | 1 | 3 |
|
|
|
Neutropenia |
| 1 |
|
|
|
Hypophosphatemia | 1 |
|
|
|
| Upper
gastrointestinal hemorrhage |
| 1 |
|
|
|
Interstitial pneumonia | 1 |
|
|
|
| Liver
toxicity |
| 1 |
|
|
|
Mucositis | 1 |
|
|
|
|
Diarrhea |
|
| 1 |
|
| Grade 1–2 |
|
|
|
|
|
Acne-like rash | 7 | 8 | 2 | 1 |
|
Nausea |
|
| 2 |
|
|
Paronychia |
| 2 |
|
|
|
Peripheral neuropathy |
| 2 |
|
|
|
Hypomagnesemia | 4 | 5 | 1 |
|
|
Alopecia |
| 1 |
|
|
| Taste
dysfunction | 3 |
|
|
|
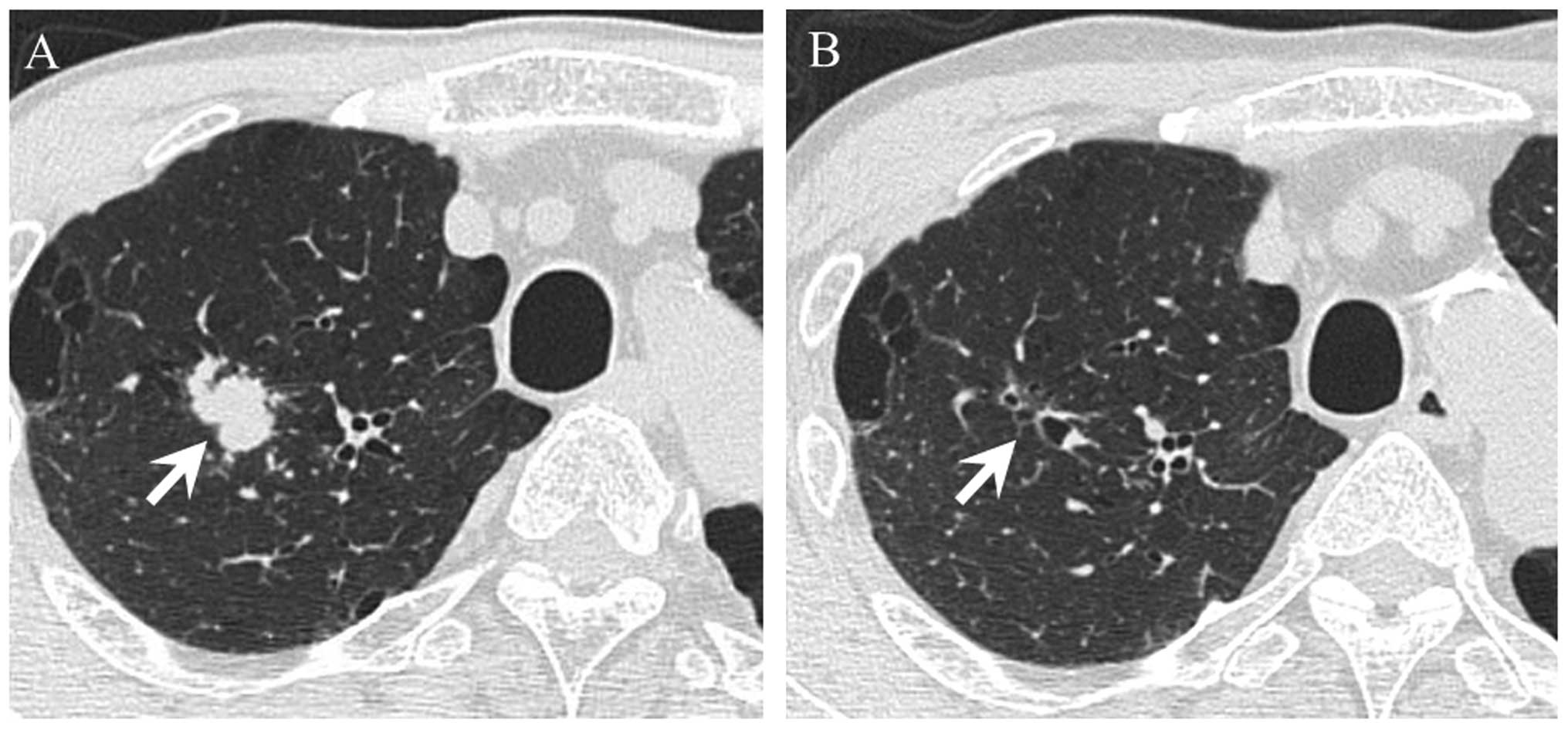
Case report
A representative case report in which CR was
achieved with cetuximab therapy for a DM is shown. A 60-year old
man was diagnosed with SCC of the mandible (T4N0M0, stage IVA) and
underwent segmental mandibulotomy, modified radical neck dissection
and reconstruction with fibular osteocutaneous free flaps. However,
a DM occurred in the lung fields 10 months after surgery. As the
patient experienced failure of a platinum-based chemoradiotherapy,
cetuximab plus paclitaxel was administered (Fig. 4A and B). Following administration of
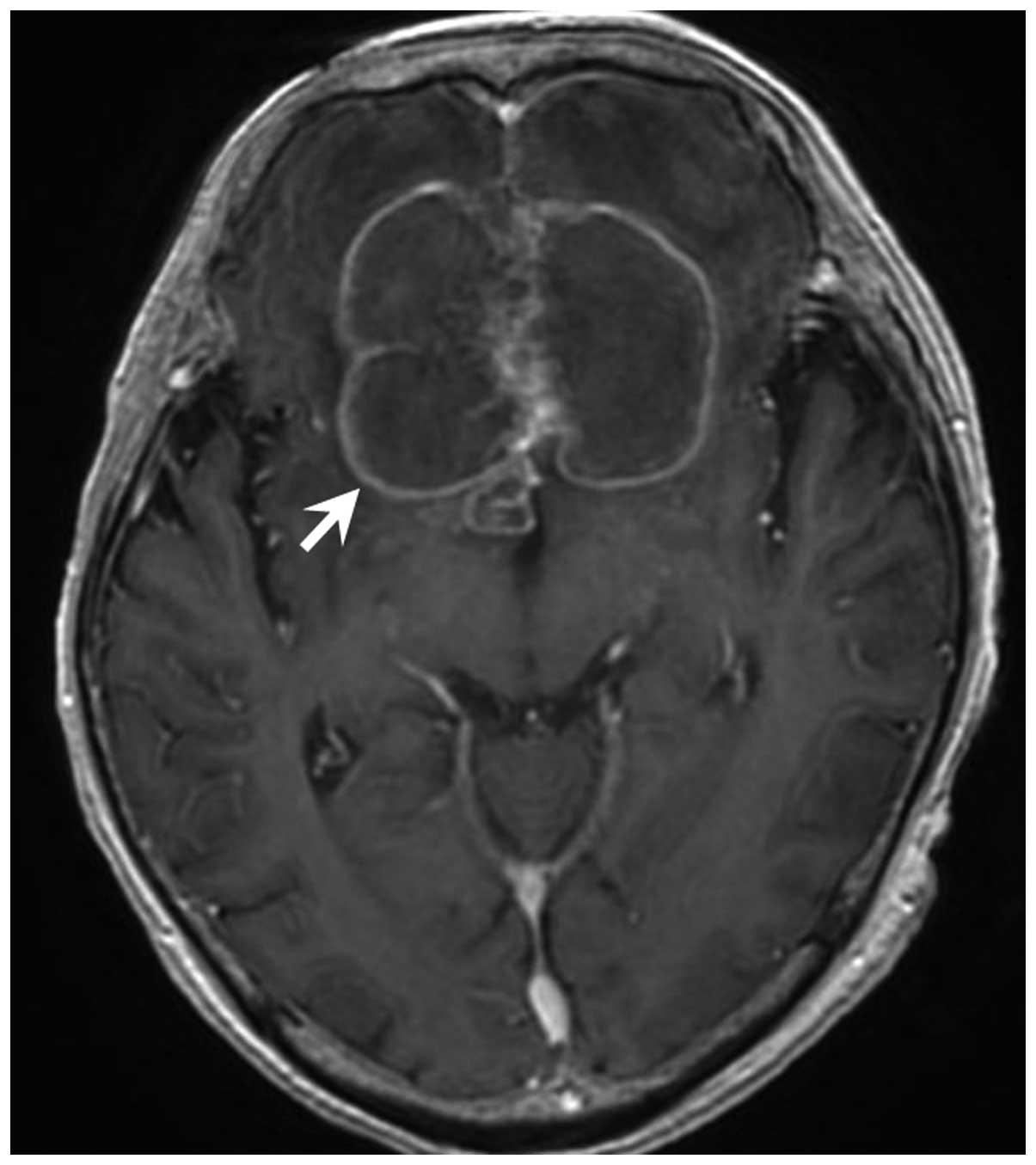
57 courses, brain metastases were detected by enhanced magnetic
resonance imaging, despite better locoregional and lung DM control
(Fig. 5). Palliative surgery for the
brain metastases was performed at the department of neurosurgery,
and metastasis of oral SCC to the brain was pathologically
diagnosed. Following surgery, best supportive care was provided at
another hospital.
Discussion
Further investigation of the effects of cetuximab
therapy on OSCC may allow identification of new treatment
possibilities, particularly with respect to DM. Therefore, the aim
of the present study was to assess the efficacy and safety of
cetuximab therapy in patients with LA and R/M OSCC in our
department, with a specific focus on patients with DMs.
EGFR is overexpressed in 80–90% of HNSCC cells, and
its activation acts as a prognostic indicator, predicting poor
survival and/or more advanced disease stage, promotion of tumor
motility and invasiveness (14). Agra
et al reported that patients whose tumours did not express
EGFR had better treatment results following salvage surgery, with a
disease-specific survival (DSS) rate of 64.3% at 3 years, while
those whose tumours expressed EGFR had a DSS rate of 27.2% at 3
years (15). A phase III trial of the
Radiation Therapy Oncology Group indicated that EGFR expression,
which varied considerably among HNSCCs, was a strong independent
prognostic indicator for OS and disease-free survival and a robust
predictor of locoregional relapse, and that blockade of EGFR
signaling sensitizes cells to the effects of radiation (16). Moreover, cetuximab represents a
promising growth-inhibitory agent that may affect cellular
proliferation, apoptosis and chemoradiosensitivity in SCC cell
lines of the head and neck in vitro (17,18). From
these reports, inhibition of EGFR has shown strong clinical
evidence of improving clinical outcome. The results of our study
confirm its therapeutic efficiency in OSCC patients, with an
overall response rate of 52.4%, a CR rate of 28.6% and a median OS
of 7.0 months. In cases with DMs, cetuximab therapy achieved an
overall response rate of 50.0%, a CR rate of 40.0% and a median OS
of 8.0 months. Specifically, cetuximab therapy was shown to result
in improved 1-year OS compared to the 1-year OS of the
non-cetuximab therapy group using a historical-control study.
Although the evidence was weak due to the non-uniform regimens in
our study, the observed clinical outcomes were comparable to those
reported by previous studies (9–11,19). However, 5 patients experienced PD, and
2 patients presented with brain metastasis during cetuximab
administration. Distant brain metastases from oral SCC are
extremely rare, and it has been reported that brain metastases
comprise 0.4–5.5% of all DMs (7,20–22). In brain metastases from HNSCC, it has
been reported that human papillomavirus-positive HNSCCs are more
prone to metastasize to distant and unusual sites (22,23).
However, it has been reported that adding cetuximab to radiotherapy
plus cisplatin significantly prolonged the PFS and OS in human
papillomavirus-positive HNSCC (24).
Although it currently remains unclear, the association between
cetuximab and brain metastasis may be associated with the
re-overexpression of the human papillomavirus-related gene, which
was once decreased by cetuximab.
Although cetuximab is expected to provide
significant therapeutic benefits, infusion reactions have been
reported during the administration or post-administration of
monoclonal antibodies. The incidence of all-grade and grade 3–4
infusion reactions has been reported to be 13.5 and 2.9%,
respectively, in the Bonner et al trial (8), and 10 and 2.3%, respectively, in the
EXTREME trial (9), whereas Touma
et al reported rates of 19.3 and 6.6%, respectively
(25). Due to these reasons, it has
been reported the IgE antibodies were shown to be specific for the
oligosaccharide galactose-α-1,3-galactose (α-gal), which is present
on the Fab portion of the cetuximab heavy chain (26), and IgE antibody formation against
α-gal is associated with tick bites and ingestion of mammalian meat
(27,28). In our study, the incidence of grade
3–4 infusion reactions was highly comparable to that reported by
previous studies, but the association with allergy to α-gal was
negative. interstitial pneumonia has been reported to be a rare
adverse event of cetuximab (29). The
incidence of all-grade and grade 3–4 infusion reactions has been
reported to be 1.2 and 0.7%, respectively, and 10 patients
succumbed to this condition in a Japanese post-marketing
surveillance of cetuximab in patients with metastatic colorectal
cancer (30). In our study, one
patient succumbed to acute respiratory distress syndrome, which
occurred due to an interstitial pneumonia flare-up.
Cetuximab-induced lung disease was diagnosed based on the clinical
course and findings. Regarding the representative adverse events
observed in our study, a grade 3–4 acne-like rash was not observed,
but grade 3 hypophosphatemia, neutropenia, upper gastrointestinal
hemorrhage and liver toxicity were observed. Although neutropenia
is a known adverse event of cetuximab, hypophosphatemia, upper
gastrointestinal hemorrhage and liver toxicity have not been
reported in other HNSCC studies with cetuximab (8–11,31). Most of these adverse events, including
neutropenia, were observed with the cetuximab plus paclitaxel
regimen. Hitt et al reported that grade 3–4 adverse events
were observed in 30 of 46 patients (65%), including an acne-like
rash (24%), neutropenia (13%), and neuropathy/paresthesia (11%)
(10); and Péron et al
reported that 20 of 42 patients (48%) received a dose reduction or
cessation of treatment after dose reduction (11). In our study, 6 of 9 patients (66.6%)
received dose reduction or discontinued treatment due to severe
adverse events, possibly attributed to an interaction of paclitaxel
and cetuximab.
To date, there are few treatment options for
patients with DM following failure of platinum-containing
chemotherapy. Our study suggests that cetuximab is expected to have
significant therapeutic efficiency in patients with unresectable LA
and R/M OSCC, including in those with DMs, although unacceptable or
severe adverse events may often occur.
Glossary
Abbreviations
Abbreviations:
|
CI
|
confidence interval
|
|
CR
|
complete response
|
|
CT
|
computed tomography
|
|
DM
|
distant metastasis
|
|
DSS
|
disease-specific survival
|
|
EGFR
|
epidermal growth factor receptor
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
LA
|
locally advanced
|
|
R/M
|
recurrent/metastatic
|
|
OS
|
overall survival
|
|
OSCC
|
oral squamous cell carcinoma
|
|
PD
|
progressive disease
|
|
PFS
|
progression-free survival
|
|
PR
|
partial response
|
|
SCC
|
squamous cell carcinoma
|
|
SD
|
stable disease
|
|
PS
|
performance status
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rogers SN, Brown JS, Woolgar JA, Lowe D,
Magennis P, Shaw RJ, Sutton D, Errington D and Vaughan D: Survival
following primary surgery for oral cancer. Oral Oncol. 45:201–211.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernier J, Cooper JS, Pajak TF, van
Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem
J, Ang KK and Lefèbvre JL: Defining risk levels in locally advanced
head and neck cancers: A comparative analysis of concurrent
postoperative radiation plus chemotherapy trials of the EORTC
(#22931) and RTOG (#9501). Head Neck. 27:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper JS, Zhang Q, Pajak TF, Forastiere
AA, Jacobs J, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al: Long-term follow-up of the RTOG 9501/intergroup phase III
trial: Postoperative concurrent radiation therapy and chemotherapy
in high-risk squamous cell carcinoma of the head and neck. Int J
Radiat Oncol Biol Phys. 84:1198–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim JY, Lim YC, Kim SH, Kim JW, Jeong HM
and Choi EC: Predictive factors of isolated distant metastasis
after primary definitive surgery without systemic treatment for
head and neck squamous cell carcinoma. Oral Oncol. 46:504–508.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi M, Aoki T, Nakamura N, Carreras
J, Kajiwara H, Kumaki N, Inomoto C, Ogura G, Kikuchi T, Kikuti YY,
et al: Clinicopathological analysis of 502 patients with oral
squamous cell carcinoma with special interest to distant
metastasis. Tokai J Exp Clin Med. 39:178–185. 2014.PubMed/NCBI
|
|
8
|
Bonner JA, Harari PM, Giralt J, Azamia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vermonken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hitt R, Irigoyen A, Cortes-Funes H, Grau
JJ, García-Sáenz JA and Cruz-hernandez JJ: Spanish Head and Neck
Cancer Cooperative Group (TTCC): Phase II study of the combination
of cetuximab and weekly paclitaxel in the first-line treatment of
patients with recurrent and/or metastatic squamous cell carcinoma
of head and neck. Ann Oncol. 23:1016–1022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Péron J, Ceruse P, Lavergne E, Buiret G,
Pham BN, Chabaud S, Favier B, Girodet D, Zrounba P, Ramade A and
Fayette J: Paclitaxel and cetuximab combination efficiency after
the failure of a platinum-based chemotherapy in
recurrent/metastatic head and neck squamous cell carcinoma.
Anticancer Drug. 23:996–1001. 2012.
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, et al: New response evaluation
criteria in solid tumours: Revised RECIST guideline (version 1.1).
Eur J Cancer. 45:228–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laskin JJ and Sandler AB: Epidermal growth
factor receptor: A promising target in solid tumours. Cancer Treat
Rev. 30:1–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Agra IM, Carvalho AL, Pinto CA, Martins
EP, Filho JG, Soares FA and Kowalski LP: Biological markers and
prognosis in recurrent oral cancer after salvage surgery. Arch
Otolaryngol Head Neck Surg. 134:743–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R,
Hammond EH, Fu KK and Milas L: Impact of epidermal growth factor
receptor expression on survival and pattern of relapse in patients
with advanced head and neck carcinoma. Cancer Res. 62:7350–7356.
2002.PubMed/NCBI
|
|
17
|
Huang SM, Bock JM and Harari PM: Epidermal
growth factor receptor blockade with C225 modulates proliferation,
apoptosis and radiosensitivity in squamous cell carcinomas of the
head and neck. Cancer Res. 59:1935–1940. 1999.PubMed/NCBI
|
|
18
|
Zhang N, Erjala K, Kulmala J, Qiu X,
Sundvall M, Elenius K and Grénman R: Concurrent cetuximab,
cisplatin and radiation for squamous cell carcinoma of the head and
neck in vitro. Radiother Oncol. 92:388–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vermorken JB, Herbst RS, Leon X, Amellal N
and Baselga J: Overview of the efficacy of cetuximab in recurrent
and/or metastatic squamous cell carcinoma of the head and neck in
patients who previously failed platinum-based therapies. Cancer.
112:2710–2719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hasegawa T, Tanakura M, Takeda D,
Sakakibara A, Akashi M, Minamikawa T and Komori T: Risk factors
associated with distant metastasis in patients with oral squamous
cell carcinoma. Otolaryngol Head Neck Surg. 152:1053–1060. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sumioka S, Sawai NY, Kishino M, Ishihama
K, Minami M and Okura M: Risk factors for distant metastasis in
squamous cell carcinoma of the oral cavity. J Oral Maxillofac Surg.
71:1291–1297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bulut OC, Lindel K, Hauswald H, Brandt R,
Klauschen F, Wolf J, Wolf T, Plinkert PK, Simon C, Weichert W and
Stenzinger A: Clinical and molecular characteristics of HNSCC
patients with brain metastases: A retrospective study. Eur Arch
Otorhinolaryngol. 271:1715–1722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruzevick J, Olivi A and Westra WH:
Metastatic squamous cell carcinoma to the brain: An unrecognized
pattern of distant spread in patients with HPV-related head and
neck cancer. J Neurooncol. 112:449–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan
PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar
AK, et al: Randomized phase III trial of concurrent accelerated
radiation plus cisplatin with or without cetuximab for stage III to
IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 32:2940–2950.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Touma W, Koro SS, Ley J, Wildes TM, Michel
L, Tao Y and Adkins D: Risk factors for and pre-medications to
prevent cetuximab-induced infusion reactions in patients with
squamous cell carcinoma of the head and neck. Oral Oncol.
50:895–900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung CH, Mirakhur B, Chan E, Le QT,
Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, et
al: Cetuximab-induced anaphylaxis and IgE specific for
galactose-alpha-1,3-galactose. N Engl J Med. 358:1109–1117. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Commins SP, James HR, Kelly LA, Pochan SL,
Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark
E, et al: The relevance of tick bites to the production of IgE
antibodies to the mammalian oligosaccharide
galactose-α-1,3-galactose. J Allergy Clin Immunol.
127:1286–1293.e6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Commins SP, Satinover SM, Hosen J, Mozena
J, Borish L, Lewis BD, Woodfolk JA and Platts-Mills TA: Delayed
anaphylaxis, angioedema, or urticaria after consumption of red meat
in patients with IgE antibodies specific for
galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 123:426–433.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
De Vos FY, Driessen CM, Jaspers HC, van
Herpen CM and Simons B: Cetuximab-induced pneumonitis in head and
neck cancer patient. Oral Oncol. 48:e17–e18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishiguro M, Watanabe T, Yamaguchi K, Satoh
T, Ito H, Seriu T, Sakata Y and Sugihara K: A Japanese
post-marketing surveillance of cetuximab (Erbituxw®) in patients
with metastatic colorectal cancer. Jpn J Clin Oncol. 42:287–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baselga J, Trigo JM, Bourhis J, Tortochaux
J, Cortés-Funes H, Hitt R, Gascón P, Amellal N, Harstrick A and
Eckardt A: Phase II multicenter study of the antiepidermal growth
factor receptor monoclonal antibody cetuximab in combination with
platinum-based chemotherapy in patients with platinum-refractory
metastatic and/or recurrent squamous cell carcinoma of the head and
neck. J Clin Oncol. 23:5568–5577. 2005. View Article : Google Scholar : PubMed/NCBI
|