Introduction
Colorectal cancer (CRC) is one of the most commonly
occurring cancers worldwide (1). CRC
represents the third largest of the leading causes of
cancer-associated mortality in Western countries (2). In recent times there has been a marked
increase in the incidence of mortality from CRC in Japan (3,4). At
present, cytotoxic agents, including irinotecan, oxaliplatin and
fluoropyrimidines, have extended the median survival times (MSTs)
of patients with advanced CRC (5–12). Despite
these advances, advanced CRC remains incurable in the majority of
patients.
Cetuximab is an antibody that specifically inhibits
epithelial growth factor receptors. It was initially approved for
patients with metastatic CRC lesions who expressed epidermal growth
factor receptors, who had failed to respond to irinotecan
(topoisomerase inhibitor) therapy (13). The randomized phase III CRYSTAL
(cetuximab combined with irinotecan in first-line therapy for
metastatic CRC) trial demonstrated the efficacy of first-line
treatment with cetuximab plus infusional fluorouracil, leucovorin
and irinotecan in patients with metastatic CRC tumors lacking
mutations in the Kirsten rat sarcoma viral oncogene homolog
(KRAS) gene at codons 12 and 13 (hereafter, referred to as
KRAS wild-type) (14). In
addition, the OPUS (oxaliplatin and cetuximab in first-line
treatment of metastatic CRC) study demonstrated that the addition
of cetuximab to another standard first-line regimen, fluorouracil,
leucovorin and oxaliplatin, markedly improved the response rate and
progression-free survival (PFS) time in patients with metastatic
KRAS wild-type CRC (15).
Currently, the efficacy and safety of first-line
cetuximab-based combination chemotherapies has not been well
described in Japan (16). The aim of
the present study was to evaluate the efficacy, the safety and the
rate of conversion into resectable status of a first-line
chemotherapy that included cetuximab in patients with advanced or
metastatic KRAS wild-type CRC in Japan.
Materials and methods
Patient eligibility
This prospective multicenter observational study was
conducted at 13 affiliated medical institutions. Institutional
review boards at each study site approved the study protocol. A
total of 64 patients were enrolled in the study between May 2010
and October 2013. The patients met the following eligibility
criteria: i) histologically confirmed advanced or metastatic
KRAS wild-type CRC; and ii) cetuximab-based chemotherapies
were administered as a first-line treatment.
Study design and assessment
In first-line treatments that included cetuximab,
essentially, an initial dose of 400 mg/m2 cetuximab was
infused over a 2 h period, and subsequently, 250 mg/m2
cetuximab was infused over a 1 h period weekly. Adverse events were
graded according to the National Cancer Institute Common
Terminology Criteria for Adverse Events, version 4.0. The Response
Evaluation Criteria in Solid Tumors (RECIST), version 1.1, was used
to evaluate the chemotherapeutic response (17). Responses were defined as either a
complete response (CR) or a partial response (PR). The Kaplan-Meier
method was used to estimate the PFS and the overall survival (OS)
rates, and the log-rank test was used to examine statistical
significance. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed with JMP
12 software (SAS Institute, Inc., Cary, NC, USA).
Results
Patients and treatment
Baseline demographic characteristics are shown in
Table I. The median age was 66 years
(range: 33–85); 42 patients (65.6%) were men; and the Eastern
Cooperative Oncology Group-Performance Status Scale was 0 in 40
patients (62.5%) and 1 in 18 patients (28.1%). Overall, 43 patients
had metastatic liver lesions; 21 patients had postoperative lesion
recurrence; and 43 patients had advanced primary CRC. Among the 21
patients with postoperative recurrences, 15 (71.4%) were diagnosed
with an unresectable lesion. Among the 43 patients with advanced
primary CRC, initially, 33 patients (76.7%) were diagnosed as
unresectable.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | n=64 (cases) |
|---|
| Age (years) | 66 (33–85) |
| Gender
(male/female) | 42/22 |
| PS
(0/1/2/3/unknown) | 40/18/3/1 |
| Disease
statusa |
|
| Postoperative
recurrence | 21 |
|
Resectable/unresectable | 6/15 |
| Advanced primary
cases | 43 |
|
Resectable/unresectable | 10/33 |
| Combined
regimens |
|
|
FOLFOX | 29 |
|
FOLFIRI | 11 |
|
XELOX | 14 |
|
Others | 10 |
| Resection following
chemotherapy |
|
| Yes | 29 |
| No | 35 |
Cetuximab was added to first-line treatments,
administered as follows: 29 patients (45.3%) received a combination
of infusional fluorouracil, leucovorin and oxaliplatin; 14 patients
(21.9%) received a combination of capecitabine and oxaliplatin; and
10 patients (15.6%) received a combination of infusional
fluorouracil, leucovorin and irinotecan. The median follow-up
period following the beginning of treatment was 1,093 days (range:
385–1,592). The median duration of the first-line cetuximab-based
treatment was 14 weeks (range: 2–87).
Efficacy
The efficacy of the cetuximab-based treatment is
summarized in Table II. The overall
response rate (CR plus PR) was 50% (32/64 patients). Confirmed CRs
or PRs occurred in 29 patients (63.1%) who received
oxaliplatin-based treatment, and in three patients (25.0%) who
received irinotecan-based treatment. Initially, 48 patients were
diagnosed with unresectable lesions. Among these, 13 lesions
(27.1%) were converted to a resectable status following treatment
with a cetuximab-based combination chemotherapy. Among these 13
patients with converted lesions, 10 had been initially diagnosed
with non-resectable liver metastases. The conversion rate was
higher in the oxaliplatin-based group (12/30; 40.0%) compared with
the irinotecan-based group (1/12; 8.3%).
 | Table II.Efficacy of cetuximab added to
different combination treatments. |
Table II.
Efficacy of cetuximab added to
different combination treatments.
|
| Patients |
|
|---|
|
|
|
|
|---|
| Response rate | No. | Percentage of total
(%) | CR + PR (%) |
|---|
| Total cases
(n=64) |
|
|
|
| CR | 1 | 1.6 | 50.0 |
| PR | 31 | 48.4 |
|
| SD | 20 | 31.2 |
|
| PD | 8 | 12.5 |
|
| NE | 4 | 6.2 |
|
| Oxaliplatin-based
(n=46) |
|
|
|
| CR | 1 | 2.2 | 63.1 |
| PR | 28 | 60.9 |
|
| SD | 14 | 30.4 |
|
| PD | 1 | 2.2 |
|
| NE | 2 | 4.3 |
|
| Irinotecan-based
(n=12) |
|
|
|
| CR | 0 | 0.0 | 25.0 |
| PR | 3 | 25.0 |
|
| SD | 3 | 25.0 |
|
| PD | 6 | 50.0 |
|
| NE | 0 | 0.0 |
|
| Others (n=6) |
|
|
|
| CR | 0 |
0.0 |
0.0 |
| PR | 0 |
0.0 |
|
| SD | 3 | 50.0 |
|
| PD | 1 | 16.7 |
|
| NE | 2 | 33.3 |
|
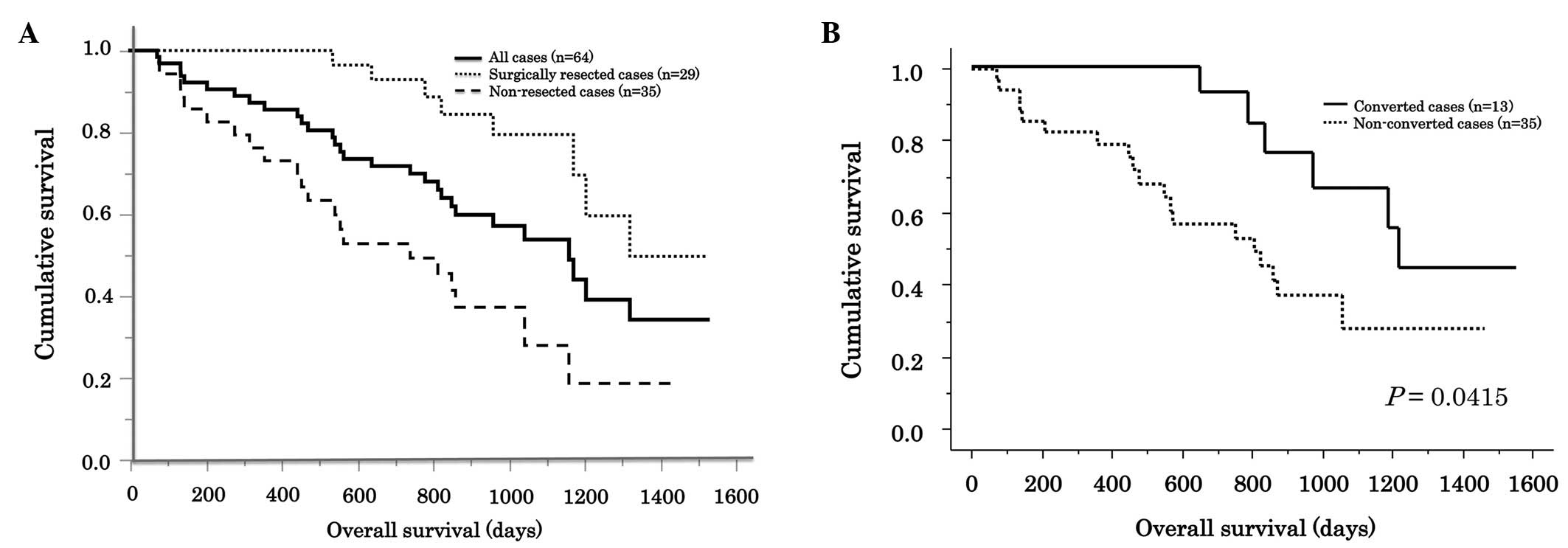
The MST was 1,189 days. Patients who had received
surgical resections following treatment (n=29) had a significantly
longer MST compared with patients with surgically unresectable
lesions (MST: 1,339 and 806 days, respectively; P=0.0006; Fig 1A). In patients with initially
unresectable lesions (n=48), the MST was 872 days. In this
subgroup, patients with tumors that converted to a surgical
resectable status had a significantly longer MST compared with
patients with unresectable lesions (MST: 1,551 and 806 days,
respectively; P=0.0415; Fig 1B). The
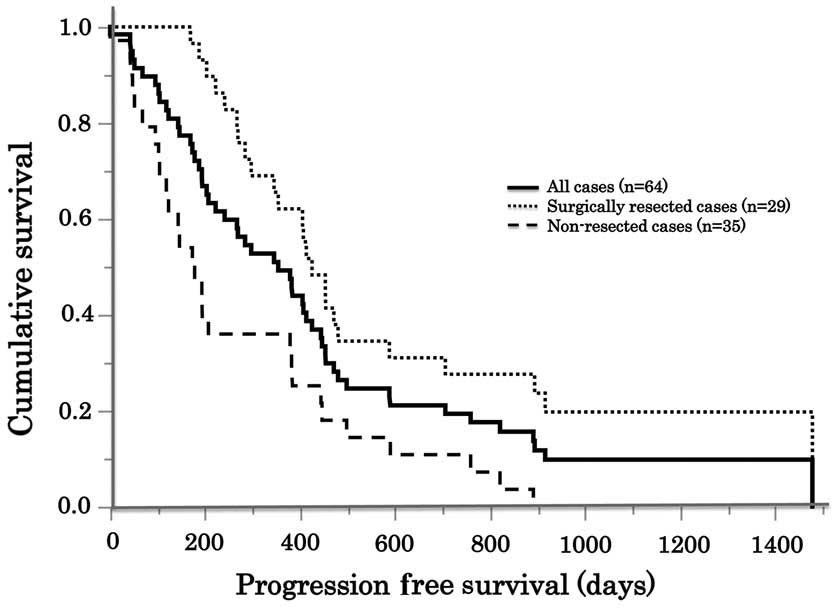
overall median PFS time was 359 days. Patients who received
surgical resections following treatment (n=29) had a significantly
longer median PFS time (432 days) compared with patients with
surgically unresectable lesions (n=35; median PFS time, 196 days;
P=0.0056; Fig 2).
Adverse events
Adverse events were graded for all treatment groups
(Table III). No
treatment-associated mortalities were identified during the
therapy. The most frequent grade 3/4 adverse event was neutropenia,
which occurred in 20.3% of the patients. The incidence of grade 3/4
skin toxicity was 17.2% (11/64 patients). Among skin toxicities,
acneiform eruption was the most common (7/64 patients; 10.9%).
Grade 4 skin toxicities were not observed in the present study.
 | Table III.Adverse events. |
Table III.
Adverse events.
|
| Grade 1–2 | Grade 3–4 | Total |
|---|
|
|
|
|
|
|---|
| Adverse event | n | % | n | % | n | % |
|---|
| Skin toxicity |
|
|
|
|
|
|
| All
(maximum toxicity) | 28 | (43.8) | 11 | (17.2) | 39 | (60.9) |
|
Acneiform eruption | 28 | (43.8) | 7 | (10.9) | 35 | (54.7) |
|
Rhagades | 18 | (28.1) | 4 | (6.2) | 22 | (34.3) |
|
Peronychia | 23 | (35.9) | 2 | (3.1) | 25 | (39.0) |
| Hematological
toxicity |
|
|
|
|
|
|
|
Neutropenia | 12 | (18.9) | 13 | (20.3) | 25 | (39.0) |
|
Leukopenia | 12 | (18.9) | 12 | (18.9) | 24 | (37.8) |
|
Thrombocytopenia | 13 | (20.3) | 2 | (3.1) | 15 | (23.4) |
|
Anemia | 6 | (9.4) | 0 | (0.0) | 6 | (9.4) |
| Others |
|
|
|
|
|
|
|
Peripheral neuropathy | 24 | (37.5) | 3 | (4.7) | 27 | (42.2) |
|
Fatigue | 16 | (25.0) | 2 | (3.1) | 18 | (28.1) |
|
Anorexia | 11 | (17.2) | 1 | (1.6) | 12 | (18.9) |
|
Stomatitis | 8 | (12.5) | 2 |
(3.1) | 10 | (15.6) |
| Nausea,
vomiting | 5 | (7.8) | 1 | (1.6) | 6 | (9.4) |
Discussion
In the present study, it was identified that, among
patients with advanced or metastatic KRAS wild-type CRC,
one-half (50%) responded well to cetuximab-based therapies, whereas
approximately one-third (27%) of unresectable lesions were
converted to resectable status. These findings were consistent with
results obtained from the CRYSTAL trial, which revealed that the
addition of cetuximab to a first-line regimen improved the clinical
outcome in patients with advanced KRAS wild-type CRC
(14). In another study, the vascular
endothelial growth factor-A antibody, bevacizumab, combined with a
first-line regimen was demonstrated to be effective for patients
with advanced CRC in a phase III trial (18). However, results obtained from the
FIRE-3 trial suggested that cetuximab may be preferable to
bevacizumab in combination therapies as a first-line regimen for
patients with KRAS wild-type tumors (19).
In the CRYSTAL trial, the median PFS time was 9.9
months, and the median OS time was 24.9 months for patients with
KRAS wild-type CRC who received a cetuximab-based treatment
(14). Similarly, in the OPUS trial,
the median PFS time was 8.3 months, and the median OS time was 22.8
months for patients with KRAS wild-type CRC who received a
cetuximab-based treatment (15). In
the current study, the median PFS time was 11.0 months, and the
median OS time was 39.6 months. Additionally, patients who received
oxaliplatin-based treatments had a median PFS time of 14.4 months
and a median OS time of 44.6 months. However, due to the relatively
small number of patients who received oxaliplatin-based treatment,
these outcomes may have been overestimated. Nevertheless, our
results have revealed treatment efficacy comparable with previous
reports.
In the present study, 29 patients received surgical
resections following first-line cetuximab-based combination
chemotherapies. The rate of true conversion from non-resectable to
resectable status was identified to be 27.1% (13/48 patients). This
finding was consistent with results from a previous phase II trial,
which assessed cetuximab in a neoadjuvant treatment for
non-resectable colorectal liver metastases (the CELIM trial)
(20). In that trial, the true
conversion rate from non-resectable to resectable lesions was 28.0%
(19/68 patients). In the present study, 31 patients initially had
non-resectable liver metastases. Among these, 10 patients became
resectable following first-line cetuximab combined therapies.
Therefore, in a setting similar to that of the CELIM trial, the
conversion rate in the present study was 32.2% (10/31 patients).
These changes in resectability also provided satisfactory treatment
efficacy, comparable with those observed previously (20).
Adverse events, particularly skin reactions, are
important factors that influence treatment compliance for
cetuximab-based treatments. The incidence of grade 3/4 skin
reactions was reported to be 18–40% in previous studies (13–15,19,20).
In the present study, the incidence of grade 3/4 skin toxicity was
17.2%. Notably, no grade 4 skin toxicity was observed. These
results suggested that adverse events associated with a first-line
cetuximab-based treatment were limited, and comparable with those
described in previous reports.
In conclusion, the present study has demonstrated
that cetuximab-based therapies represent a promising first-line
regimen for advanced or metastatic KRAS wild-type CRC in
Japan. Additionally, a low incidence of serious toxicities was
observed.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colorectal Cancer Estimated Incidence
Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
|
|
5
|
Cassidy J, Twelves C, Van Cutsem E, Hoff
P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, et
al: First-line oral capecitabine therapy in metastatic colorectal
cancer: A favorable safety profile compared with intravenous
5-fluorouracil/leucovorin. Ann Oncol. 13:566–575. 2002. View Article : Google Scholar
|
|
6
|
Cunningham D, Pyrhönen S, James RD, Punt
CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham
CA, Awad L, et al: Randomised trial of irinotecan plus supportive
care versus supportive care alone after fluorouracil failure for
patients with metastatic colorectal cancer. Lancet. 352:1413–1418.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rougier P, Van Cutsem E, Bajetta E,
Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg
H, Wils J, et al: Randomised trial of irinotecan versus
fluorouracil by continuous infusion after fluorouracil failure in
patients with metastatic colorectal cancer. Lancet. 352:1407–1412.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saltz LB, Cox JV, Blanke C, Rosen LS,
Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta
N, et al: Irinotecan plus fluorouracil and leucovorin for
metastatic colorectal cancer. Irinotecan study group. N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan and oxaliplatin combinations in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 22:23–30.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uehara K and Nagino M: Neoadjuvant
treatment for locally advanced rectal cancer: A systematic review.
Surg Today. 2015.(Epub ahead of print).
|
|
13
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar
|
|
14
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saeki H, Emi Y, Kumashiro R, Otsu H,
Kawano H, Ando K, Ida S, Kimura Y, Tokunaga E, Oki E, et al: Impact
of second-line and later cetuximab-containing therapy and KRAS
genotypes in patients with metastatic colorectal cancer: A
multicenter study in Japan. Surg Today. 44:1457–1464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumour response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol.
11:38–47. 2010. View Article : Google Scholar : PubMed/NCBI
|