Introduction
Progressive prostate cancer that has been treated
with androgen-deprivation therapy using an antiandrogen, such as
bicalutamide, occasionally causes antiandrogen withdrawal syndrome
(AWS), which results in a response involving a significant decline
in prostate-specific antigen (PSA) levels.
AWS is generally defined as subjective and/or
objective improvement following discontinuation of an antiandrogen
in patients with elevated PSA levels who are treated with combined
androgen blockade (CAB) using steroidal or non-steroidal
antiandrogens (1).
The duration of AWS varies considerably (1) and only a limited number of cases
exhibiting a complete response, in terms of PSA level decline, have
been reported.
We herein report the case of a patient with
metastatic prostate cancer who received CAB, with the longest
duration of a complete response reported to date.
Case report
A 72-year-old man visited the Cancer Institute
Hospital (Tokyo, Japan) on June 9, 2004, complaining of right hip
joint pain. Laboratory examination revealed a PSA level of 588.8
ng/ml and an elevated alkaline phosphatase level 539 IU/l (normal
range, 100–325 IU/l). Prostate biopsy revealed adenocarcinoma, with
a Gleason score of 4+3=7. Computed tomography (CT) and bone
scintigraphy scans revealed metastases to the right pelvis and
right femoral head (extent of disease I).
CAB consisting of a luteinizing hormone-releasing
hormone analog and bicalutamide 80 mg/day was immediately
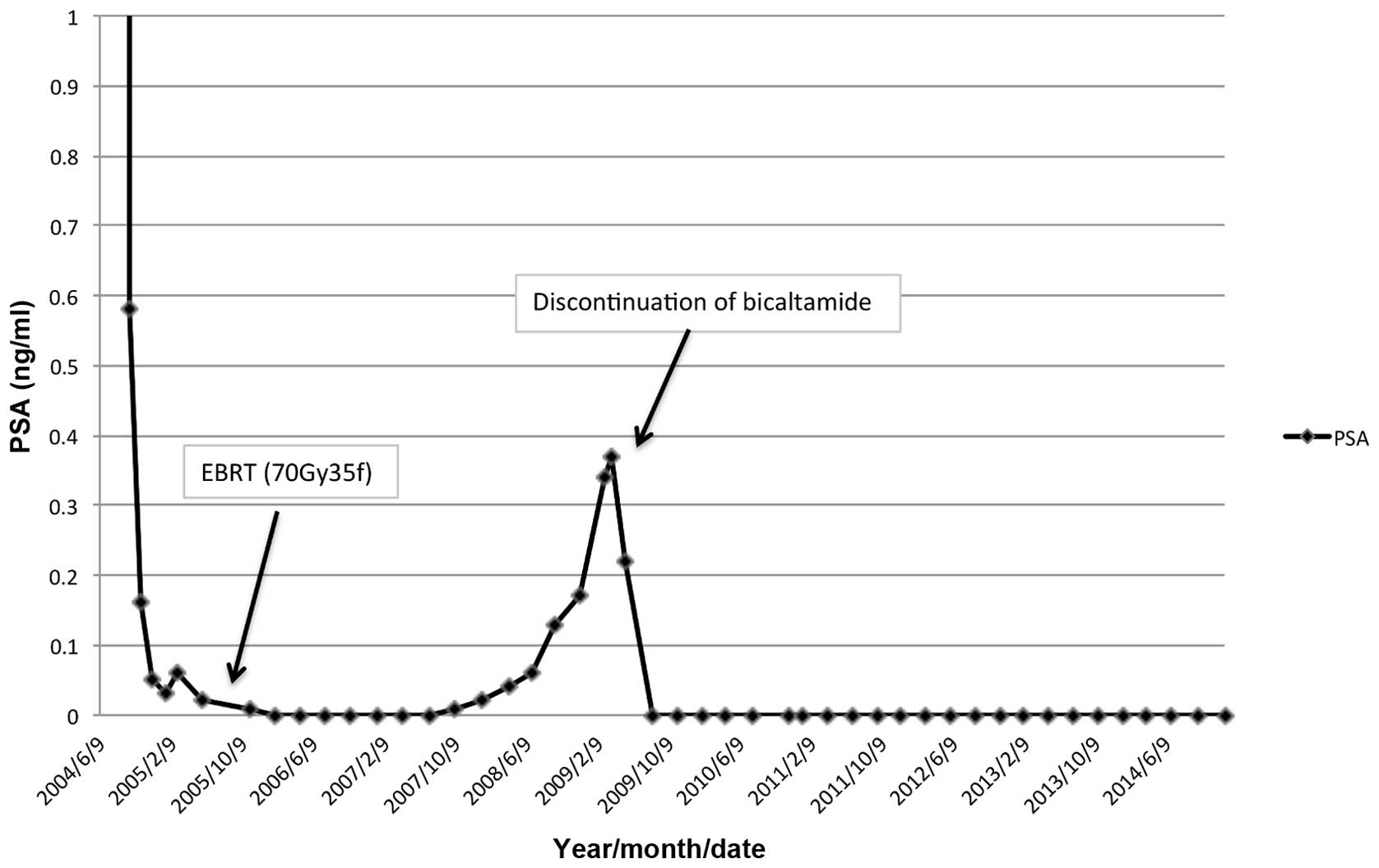
administered. The serum PSA level promptly decreased to 0.16 ng/m
in 3 months and had reached a nadir of 0.02 ng/m by May 26, 2005.
External beam radiation therapy (70 Gy) to the prostate was then
performed for local cancer control. The PSA level had decreased to
0.00 ng/ml by January 31, 2006. In addition, no bone metastases
were detected on bone scintigraphy.
The PSA level gradually increased to 0.34 ng/m by
March 3, 2009; however, no new lesions of distant metastasis were
detected by CT or bone scintigraphy. Bicalutamide was discontinued
to confirm AWS. The PSA level again decreased to 0.00 ng/ml by
August 18, 2009. To date, this undetectable PSA level has been
maintained, and no recurrence has been detected by bone
scintigraphy or CT for the past 6 years since the introduction of
AWS. The clinical course and change in serum PSA levels are shown
in Fig. 1.
Written informed consent was obtained from the
patient regarding the publication of the details of this case.
Discussion
AWS is a well-established phenomenon in prostate
cancer (1). Kelly and Sher first
reported AWS with the non-steroidal antiandrogen flutamide
(2); they reported a decrease of ≥50%
in the PSA level following discontinuation of flutamide in 10 of
their 35 patients (29%) (3).
Small et al first reported AWS with
bicalutamide in 1994 (4). The
association between antiandrogen withdrawal and a change in PSA
level is a general phenomenon observed for all antiandrogens. The
differences in the binding affinity of various antiandrogens are
unrelated. The response rate previously reported by Suzuki et
al was 15.5% for bicalutamide and 12.8% for flutamide, with no
significant difference between the two antiandrogens (5) and a reported mean response duration of
5.8±3.8 months. Noguchi et al reported the case of a patient
who exhibited a complete PSA decline for >2 years after the
discontinuation of a steroidal antiandrogen (6). Compared to these previously reported
studies, the response duration of the present case is the longest
reported to date.
The duration of antiandrogen use prior to withdrawal
was identified as an AWS predictor by Sator et al (7), who reported that only the duration of
prior antiandrogen exposure was a significant predictor of PSA
response after withdrawal. Additionally, a higher frequency of AWS
was observed in the group of patients who received antiandrogen for
a longer duration (>32 months prior to withdrawal). In the
present case, consistent with Sator's report, the patient received
bicalutamide for ~48 months prior to withdrawal.
The molecular mechanisms underlying AWS have not
been fully elucidated. Mutation of androgen receptors (ARs) is the
most supported hypothesis (8). In
this hypothesis, bicalutamide acts as an agonist for mutant ARs. In
the present case, a luteinizing hormone-releasing agonist was used
for >4 years, and external beam radiation (70 Gy/35 fx) was
performed prior to the discontinuation of bicalutamide. It is
suggested that, during this period, androgen-dependent and
hormone-sensitive tumor cells were eliminated, whereas a moderate
number of AR-mutated cells survived.
Takeshita et al reported a case in which AWS
and radiotherapy of the prostate contributed to the disappearance
of metastatic lymph nodes and a decline in PSA level to below the
detection limit for a long period (9). Culp et al recently reported that
local therapy for the prostate may confer a survival advantage to
selected patients with metastatic disease (10). Therefore, in the present case, AWS
combined with local treatment to the prostate (radiation therapy)
may achieve good cancer control.
The median overall survival following antiandrogen
withdrawal was 22 months (7). Small
et al reported that there was no significant difference
between the median survival times of AWS responders and
non-responders (11). However,
despite advanced disease, the patient in the present case has
survived for >10 years since the diagnosis. It is thus
considered that AWS has contributed to the prolongation of the
patient's life expectancy and has improved his quality of life.
In conclusion, although the long response duration
seen in the present case is rare, the AWS phenomenon should be
considered, even in patients with advanced disease.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Glossary
Abbreviations
Abbreviations:
|
AWS
|
antiandrogen withdrawal syndrome
|
|
PSA
|
prostate-specific antigen
|
|
CAB
|
combined androgen blockade
|
|
CT
|
computed tomography
|
|
AR
|
androgen receptor
|
References
|
1
|
Paul R and Breul J: Antiandrogen
withdrawal syndrome associated with prostate cancer therapies:
Incidence and clinical significance. Drug Saf. 23:381–390. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kelly WK and Scher HI: Prostate-specific
antigen decline after antiandrogen withdrawal: The flutamide
withdrawal syndrome. J Urol. 149:607–609. 1993.PubMed/NCBI
|
|
3
|
Scher HI and Kelly WK: Flutamide
withdrawal syndrome: Its impact on clinical trials in
hormone-refractory prostate cancer. J Clin Oncol. 11:1566–1572.
1993.PubMed/NCBI
|
|
4
|
Small EJ and Carroll PR: Prostate-specific
antigen decline after casodex withdrawal: Evidence for an
antiandrogen withdrawal syndrome. Urology. 43:408–410. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki H, Okihara K, Miyake H, Fujisawa M,
Miyoshi S, Matsumoto T, Fujii M, Takihana Y, Usui T, Matsuda T, et
al: Alternative nonsteroidal antiandrogen therapy for advanced
prostate cancer that relapsed after initial maximum androgen
blockade. J Urol. 180:921–927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noguchi K, Teranishi J, Uemura H, Fujikawa
N, Saito K and Murai T: Complete response, as determined by
prostate-specific antigen level, to chlormadinone acetate
withdrawal persisting longer than 2 years in patients with advanced
prostate cancer: Two case reports. Int J Urol. 13:1259–1261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sartor AO, Tangen CM, Hussain MH,
Eisenberger MA, Parab M, Fontana JA, Chapman RA, Mills GM, Raghavan
D and Crawford ED: Southwest Oncology Group: Antiandrogen
withdrawal in castrate-refractory prostate cancer: A Southwest
oncology group trial (SWOG 9426). Cancer. 112:2393–2400. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hara T, Miyazaki J, Araki H, Yamaoka M,
Kanzaki N, Kusaka M and Miyamoto M: Novel mutations of androgen
receptor: A possible mechanism of bicalutamide withdrawal syndrome.
Cancer Res. 63:149–153. 2003.PubMed/NCBI
|
|
9
|
Takeshita H, Kawakami S and Fukui I:
Profound bicalutamide withdrawal syndrome in a hormone-refractory
T4N1 prostate cancer permitting both salvage radiotherapy and
cessation of hormonal therapy. Int J Urol. 16:337–338. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Culp SH, Schelhammer PF and Williams MB:
Might men diagnosed with metastatic prostate cancer benefit from
definitive treatment of the primary tumor? A SEED-based study. Eur
Urol. 65:1058–1066. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Small EJ and Srinivas S: The antiandrogen
withdrawal syndrome. Experience in a large cohort of unselected
patience with advanced prostate cancer. Cancer. 76:1428–1434. 1995.
View Article : Google Scholar : PubMed/NCBI
|