Introduction
The prevalence of end-stage renal disease (ESRD) is
increasing, with the population of the affected individuals in the
USA almost doubling every 10 years (1). ESRD has now become a major health
problem worldwide. Dialysis is the most common treatment for ESRD,
while kidney transplantation is the most ideal treatment. However,
dialysis and transplantation have adverse effects, including
cardiovascular disease, infection and cancer (2,3). In
addition, cancer is increasingly recognized as a complication and a
major cause of mortality in patients with ESRD receiving renal
replacement therapy (RRT) (4). Since
the association between chronic uremia and malignant disease was
first reported in 1970 (5), it has
been supported by an increasing number of studies. In 1993, the
association between malignancy and dialysis was assessed by a
meta-analysis of 15 studies, whose results, however, were
contradictory (6). The pooled data
from 10 of the studies suggested an average relative risk of
malignancy of 7.6 for dialysis patients, while it was 0.98
according to the 5 remaining studies showing an unchanged risk
(6). The shortcomings of the above
mentioned meta-analysis were that the sample size of the majority
of the studies included was small and only a few types of cancer
were assessed, rendering the risk estimates obtained unreliable. In
addition, all the studies included were on Western populations,
while the analysis lacked information on non-Western patients. In
2007, a meta-analysis of 5 studies on the cancer risk in renal
transplant recipients (RTRs) showed that an extensive variety of
cancer types occurred with an increased incidence in RTRs (7). However, the study did not specifically
evaluate the risk of overall cancer or risk factors, including age,
gender, follow-up time or country. Moreover, the number of studies
included was small and all studies assessed were on Western
populations.
In the past few years, several studies using
registry data have provided convincing evidence for the increased
incidence of certain cancer types in patients receiving RRT
(4,8–16).
However, it remains to be determined whether meta-analysis of these
studies and others may provide results that are different from
those of previous meta-analyses. Although certain reviews have
reported on the association between RRT and the occurrence of
cancer (2,17,18),
meta-analysis of data from previous studies can increase
statistical power by pooling the results of individual studies.
Therefore, the present meta-analysis was performed to quantify the
cancer risk in patients receiving RRT, which may provide a
realistic perspective on the cancer risk associated with RRT in the
clinical setting.
Materials and methods
Conducting the study
The analysis and data presentation of the study were
performed according to the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses statement checklist (19).
Data sources and searches
Two of the investigators (W.S. and L.H.) searched
the PubMed and EMBASE databases for studies published before May
29, 2015 using search term combinations of ‘transplant OR
transplantation OR dialysis OR hemodialysis’, ‘neoplasia OR
neoplasm OR neoplasms OR carcinoma OR cancer OR cancers OR
malignancy OR malignancies OR tumor OR tumors’, ‘standardized
incidence rate (SIR) OR standardized incidence ratio’, and ‘RR OR
relative risk’. All eligible studies were retrieved and their
references were reviewed to identify additional relevant
studies.
Inclusion criteria
Studies were included in the present meta-analysis
when meeting the following criteria: i) Population-based cohort
studies on chronic dialysis patients or RTRs; ii) chronic dialysis
or renal transplantation were defined as exposure interests and
cancer as the outcome of interest; iii) SIR/standardized mortality
rate or relative risk with their 95% confidence intervals (CIs) of
overall cancer (or with data to calculate them) were provided; iv)
the patient cohort mainly comprised adults.
Exclusion criteria
The following types of study were excluded: Case
reports, reviews, conference reports, editorials, studies not
written in English, as well as studies on transplantation of organs
other than kidneys. Studies were excluded when the cancer diagnosis
had not been submitted to a cancer registry. If multiple studies on
the same trial were encountered, only the most recent study was
included in the present meta-analysis.
Data extraction and quality
evaluation
Two investigators (W.S. and L.H.) independently
extracted the following variables from the selected studies: Name
of first author, publication year, country, cohort entry criteria,
study period, sample size, mean age, percentage of males,
patient-years, mean follow-up time, number of cancers observed in
the cohort, as well as the SIRs and their 95% CIs of commonly known
cancer types and overall cancer. If the overall-cancer SIR estimate
including non-melanoma skin cancer (NMSC) as well as that excluding
NMSC were provided, both values were considered. The quality of the
cohort studies was assessed by each investigator independently
using the Newcastle-Ottawa quality assessment scale (NOS) (20). The NOS reflects the quality of
published non-randomized studies with regard to selection,
comparability and outcome. Studies meeting ≥5 NOS criteria were
considered to be of high quality. Discrepancies between the
findings of the two investigators were resolved by discussion.
Data synthesis and analysis
SIRs with 95% CIs for overall cancer were pooled
using a random-effects model for possible heterogeneity among
studies. Risks for specific cancer types were only combined in the
same method if data from ≥2 studies were available for a given type
of cancer. Heterogeneity was assessed by means of the χ2
test and quantified using I2 statistics.
I2-values of 25, 50 and 75% were considered to indicate
low, moderate and severe statistical heterogeneity, respectively.
To assess any potential confounding factors, including sample size,
gender, age, follow-up time and geographical region, subgroup
analyses were performed if ≥1 study took the above factors into
account; furthermore, combined cohort data were stratified into
those including or excluding NMSC. In addition, a sensitivity
analysis was performed to assess the influence of any individual
study on the overall estimate. Publication bias was evaluated using
Egger's test (21). All the analyses
were performed using Stata 10.0 software (StataCorp LP, College
Station, TX, USA) and all P-values were calculated as two-sided.
Unless otherwise specified, P<0.05 was considered to indicate a
statistically significant difference.
Results
Description of included studies
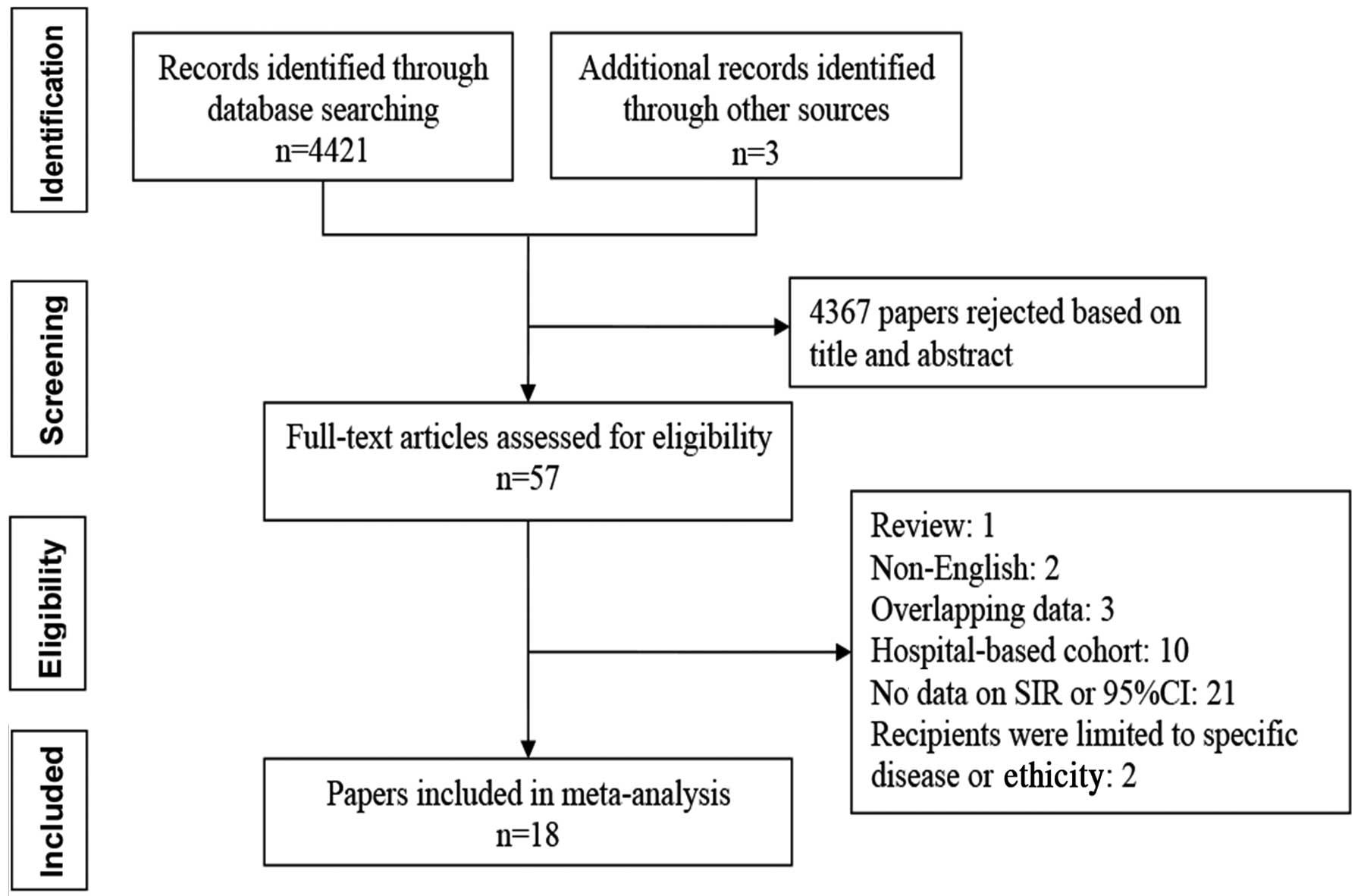
A total of 4,424 studies were identified via the
search strategy applied, which is outlined in Fig. 1, and the full-text version of 57
studies was retrieved. Of these, 39 were excluded, as they
comprised 3 duplicate studies, 2 studies not written in English, 1
review, 21 from which the SIR and 95% CI could not be calculated,
10 studies on hospital-based cohorts and 2 on irrelevant topics.
Finally, 22 cohort studies contained in 18 studies were included in
the present meta-analysis (8–16,22–30). The
main characteristics of the studies included are presented in
Table I. The earliest study began in
1989 (22) and the latest ended in
2015 (11). All the studies were
population-based. Of the patients included, 1,443,684 received
dialysis and 85,035 were RTRs. On average, dialysis patients were
15.8 years older than RTRs. The average duration of follow-up was
more than twice as long after transplantation (7.17 years) compared
to patients on dialysis (2.60 years from first dialysis). The
present meta-analysis included 75,336 cases of cancer identified in
a total of 1,528,719 individuals. According to the NOS, all cohort
studies were of high quality (data not shown).
 | Table I.Characteristics of studies included
in the present meta-analysis. |
Table I.
Characteristics of studies included
in the present meta-analysis.
| First author,
year | Country | Cohort entry
criterion | Study period | Sample size, n | Mean age,
years | Men,% | Patient-years,
n | Mean follow-up
time, years | Cancers, n | (Ref.) |
|---|
| Port, 1989 | United States | Dialysis | 1973–1984 | 4,161 | 52 | 56.8 | NA | NA | 63 | (22) |
| Maisonneuve,
1999 | Australia and | Dialysis | 1980–1994 | 13,497 | 49 | 55.7 |
34,456 | 2.6 |
500 | (30) |
|
| New Zealand |
|
|
| Europe | Dialysis | 1980–1994 | 296,903 | 52 | 58.4 |
858,532 | 2.9 |
6,849 |
|
|
| United States | Dialysis | 1980–1994 | 521,404 | 58 | 53.4 | 1,152,047 | 2.2 | 17,695 |
|
| Birkeland,
2000 | Denmark | Dialysis | NA-1995 | 3,592 | 50.2 | 60 |
8,043 |
2.26 |
110 | (23) |
| Stewart, 2009 | Australia | Dialysis | 1982–2003 | 23,764 | 54.5 | 54 |
63,431 | 2.7 |
1,018 |
(8) |
| Lin, 2012 | China | Dialysis | 1997–2008 | 92,348 | 60.4 | 48.5 |
409,909 | 4.4 |
4,328 |
(9) |
| Loy, 2013 | Singapore | Dialysis | 1998–2007 | 5,505 | 58.1 | 52.23 | NA | 3.9 |
267 | (10) |
| Butler, 2015 | United States | Dialysis | 1996–2009 | 482,510 | 67 | 51.6 |
988,395 | 2.5 | 35,767 | (11) |
| Hoshida, 1997 | Japan |
Transplantation | 1970–1995 | 1,744 | 36a | 66.2 |
12,982 | 7.4 | 46 | (24) |
| Birkeland,
2000 | Denmark |
Transplantation | NA-1995 | 1,821 | 39 | 60.6 |
13,734 | 7.5 |
209 | (23) |
| Kyllönen, 2000 | Finland |
Transplantation | 1964–1997 | 2,890 | 41.5 | 59.5 |
20,817 | 7.2 |
230 | (25) |
| Adami, 2003 | Sweden |
Transplantation | 1970–1997 | 5,004 | 46 | 60 |
36,963 | 6.8 |
639 | (26) |
| Végso, 2007 | Hungary |
Transplantation | 1973–2007 | 2,535 | 53.1 | NA | NA | 9.8 |
193 | (27) |
| Villeneuve,
2007 | Canada |
Transplantation | 1981–1998 | 11,155 | NA | 63.2 |
81,237 | 7.3 |
778 | (28) |
| Serraino, 2007 | Italy |
Transplantation | 1988–2004 | 1,829 | NA | 65.3 |
16,196 | 7.3a |
104 | (29) |
| Stewart, 2009 | Australia |
Transplantation | 1982–2003 | 8,173 | 41.9 | 59 |
49,357 | 6 |
770 |
(8) |
| Collett, 2010 | United Kingdom |
Transplantation | 1980–2007 | 25,104 | NA | NA | NA | NA |
1,982 | (12) |
| Li, 2012 | China |
Transplantation | 1997–2008 | 4,716 | 44.1 | 52.5 |
22,556 | 4.8 |
320 | (13) |
| Cheung, 2012 | China |
Transplantation | 1972–2011 | 4,895 | 43.7 | 58.6 |
40,246 | 8.2 |
299 | (14) |
| Piselli, 2013 | Italy |
Transplantation | 1997–2009 | 7,217 | NA | 64.2 | NA | 5.5 |
395 | (15) |
| Krynitz, 2013 | Sweden |
Transplantation | 1970–2008 | 7,952 | 47a | 62 | NA | 9.7 |
2,774 | (16) |
Overall cancer risk in RRT
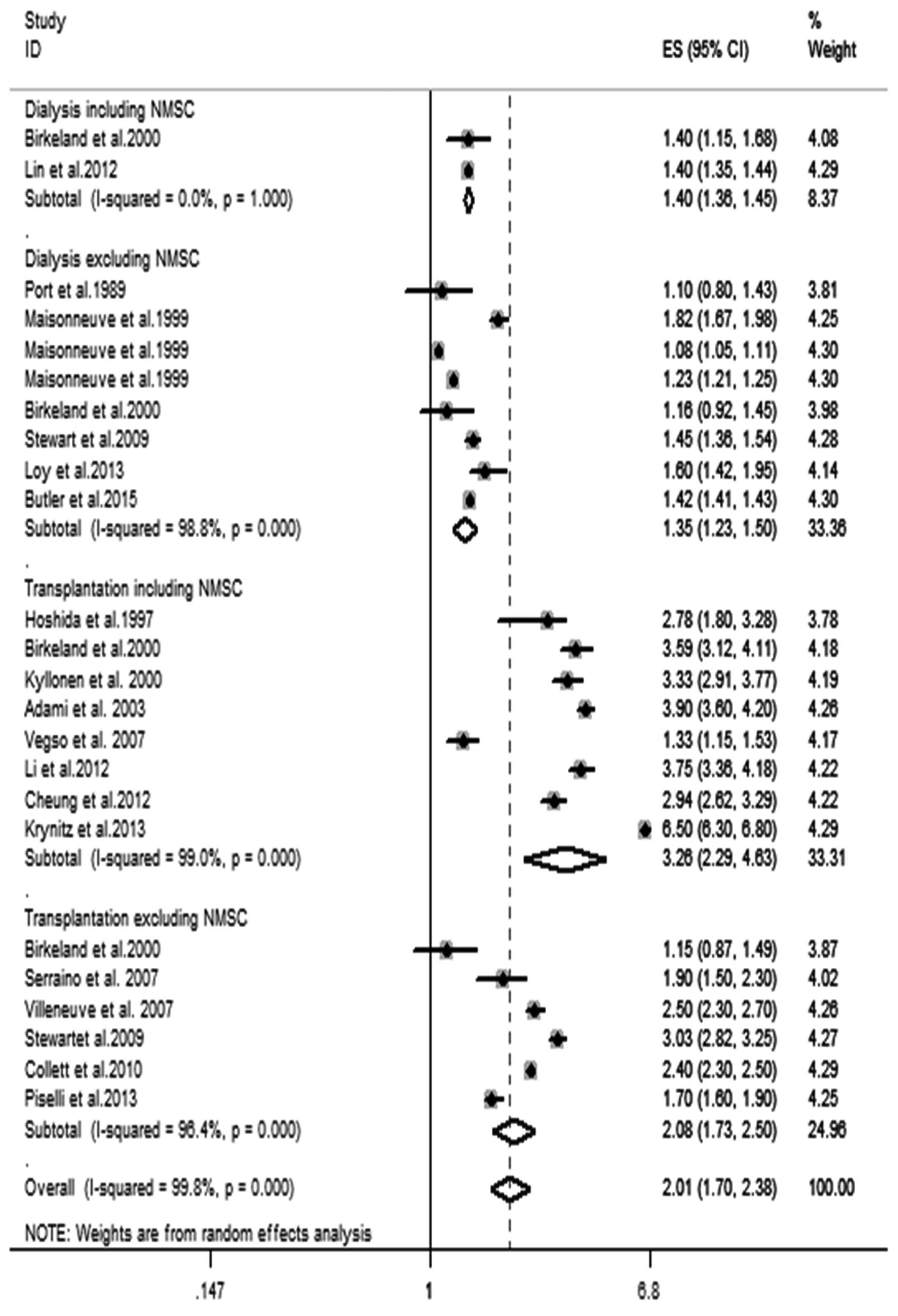
As shown in Fig. 2,
RRT was significantly associated with an increased risk for overall
cancer. The pooled SIR of overall cancer for patients receiving
dialysis including NMSC, dialysis excluding NMSC, transplantation
including NMSC and transplantation excluding NMSC and RRT were 1.40
(95% CI, 1.36–1.45), 1.35 (95% CI, 1.23–1.50), 3.26 (95% CI,
2.29–4.63), 2.08 (95% CI, 1.73–2.50), 2.01 (95% CI, 1.70–2.38),
respectively. Significant heterogeneity was observed in the pooled
analysis (I2=99.8%; P<0.001). To explore possible
sources of the heterogeneity, subgroup analyses were performed with
regard to sample size, gender, age, follow-up time and geographical
region. Subgroup analysis with regard to sample size showed that
for dialysis, the pooled SIR was increased in studies with a sample
size of ≥10,000 patients (excluding NMSC), and that for
transplantation, the pooled SIR was increased in studies with a
sample size of ≥5,000 patients. Subgroup analysis with regard to
gender showed that female patients with RRT had a significantly
higher risk for overall cancer compared with that of male patients.
Furthermore, subgroup analysis regarding patient age indicated that
the risk of cancer was particularly high in the lowest age group
and progressively decreased with age. Among RTRs, the risk for
cancer was highest in the first year after transplantation with
inclusion of NMSC (SIR=24.75; 95% CI, 7.63–80.21) and subsequently
decreased in the following years. Furthermore, the risk for cancer
excluding NMSC was highest in the first year after dialysis
(SIR=2.16; 95% CI, 1.53–3.04) and progressively decreased with
follow-up duration compared with the general population.
Stratification based on geographical region indicated that the
pooled SIR in non-Asian populations of RTRs was higher than that in
Asian RTRs. No significant change in the majority of subgroup
analyses for heterogeneity was observed (Table II).
 | Table II.Subgroup analyses of overall cancer
risk in patients who received renal replacement therapy. |
Table II.
Subgroup analyses of overall cancer
risk in patients who received renal replacement therapy.
|
| Including NMSC | Excluding NMSC |
|---|
|
|
|
|
|---|
|
|
|
|
| Heterogeneity |
|
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Characteristic | No. studies | (Refs.) | Pooled SIR
(95%CI) | I2
(%) |
P-valuea | No. studies | (Refs.) | Pooled SIR
(95%CI) | I2
(%) |
P-valuea |
|---|
| Sample size, n |
|
|
Dialysis |
|
|
<10,000 | 1 | (23) | 1.40
(1.15–1.68) | NA | NA | 3 | (10,22,23) | 1.29
(1.00–1.67) | 74.8 |
0.019 |
|
≥10,000 | 1 | (9) | 1.40
(1.35–1.44) | NA | NA | 5 | (8,11,30) | 1.37
(1.22–1.54) | 99.3 | <0.001 |
|
Transp |
|
|
<5,000 | 6 | (13,14,23–25,27) | 2.81
(2.07–3.82) | 96.7 | <0.001 | 2 | (23,29) | 1.49
(0.91–2.43) | 87.8 |
0.004 |
|
≥5,000 | 2 | (16,26) | 5.04
(3.06–8.32) | 99.3 | <0.001 | 4 | (8,12,15,28) | 2.36
(1.94–2.87) | 97.1 | <0.001 |
| Gender |
|
|
Dialysis |
|
|
Men | 2 | (9,23) | 1.45
(1.23–1.71) |
0.0 |
0.464 | 5 | (10,23,30) | 1.36
(1.13–1.65) | 86.3 | <0.001 |
|
Women | 2 | (9,23) | 1.59
(1.50–1.69) |
0.0 |
0.585 | 5 | (10,23,30) | 1.50
(1.28–1.76) | 95.0 | <0.001 |
|
Transp |
|
|
Men | 4 | (13,14,23,24) | 2.95
(2.54–3.42) | 56.1 |
0.077 | 2 | (23,28) | 2.10
(1.32–3.35) | 87.9 |
0.004 |
|
Women | 4 | (13,14,23,24) | 3.84
(3.03–4.86) | 78.9 |
0.003 | 2 | (23,28) | 2.28
(2.03–2.57) |
0.0 |
0.777 |
| Age at first
dialysis, years |
|
|
0–34 | 1 | (9) | 9.20
(7.68–10.93) | NA | NA | 4 | (10,30) | 4.09
(2.59–6.47) | 93.0 | <0.001 |
|
35–64 | 0 | NA | NA | NA | NA | 4 | (10,30) | 1.88
(1.41–2.50) | 96.9 | <0.001 |
|
≥65 | 1 | (9) | 0.80
(0.76–0.84) | NA | NA | 4 | (10,30) | 1.21
(1.11–1.32) | 78.2 |
0.003 |
| Age at transp,
years |
|
|
0–20 | 2 | (13,14) | 21.97
(5.72–84.4) | 61.5 |
0.107 | 0 | NA | NA | NA | NA |
|
30–45 | 1 | (24) | 4.78
(2.73–8.38) | NA | NA | 1 | (28) | 3.33
(2.87–3.83) | NA | NA |
|
>60 | 1 | (13) | 2.31
(1.74–3.07) | NA | NA | 1 | (28) | 1.69
(1.44–1.96) | NA | NA |
| Follow-up time |
|
| Within
1 year of first dialysis | 1 | (9) | 8.30
(7.70–8.92) | NA | NA | 4 | (10,30) | 2.16
(1.53–3.04) | 94.0 | <0.001 |
| Year 2
after first dialysis | 1 | (9) | 3.90
(3.62–4.19) | NA | NA | 4 | (10,30) | 1.47
(1.22–1.77) | 95.4 | <0.001 |
| Years
3–5 after first dialysis | 0 | NA | NA | NA | NA | 4 | (10,30) | 1.32
(1.07–1.63) | 96.8 | <0.001 |
| Within
1 year of transp | 3 | (13,14,24) | 24.75
(7.63–80.21) | 95.6 | <0.001 | 1 | (28) | 2.97
(1.42–1.95) | NA | NA |
| Years
1–5 after transp | 3 | (13,14,24) | 6.45
(4.16–9.99) | 90.3 | <0.001 | 1 | (28) | 2.39
(2.13–2.67) | NA | NA |
| Years
5–10 after transp | 3 | (13,14,24) | 2.40
(1.28–4.53) | 93.9 | <0.001 | 1 | (28) | 2.61
(2.30–2.94) | NA | NA |
| Region |
|
|
Dialysis |
|
|
Asian | 1 | (9) | 1.40
(1.35–1.44) | NA | NA | 1 | (10) | 1.60
(1.42–1.95) | NA | NA |
|
Non-Asian | 1 | (23) | 1.40
(1.15–1.68) | NA | NA | 7 | (8,11,22,23,30) | 1.33
(1.19–1.47) | 99.0 | <0.001 |
|
Transp |
|
|
Asian | 3 | (13,14,24) | 3.19
(2.62–3.89) | 80.9 |
0.005 | 0 | NA | NA | NA | NA |
|
Non-Asian | 5 | (16,23,25–27) | 3.33
(2.05–5.41) | 99.3 | <0.001 | 6 | (8,12,15,23,28,29) | 2.08
(1.73–2.50) | 96.4 | <0.001 |
Risk of specific cancer types in
patients with RRT
Subgroup analyses were performed for specific cancer
types reported in ≥1 study (Table
III). The meta-analyses showed that myeloma and melanoma as
well as cancer of the thyroid gland, kidney, thyroid and other
endocrine glands, tongue, bladder, cervix of the uterus, penis
scrotum and liver were more frequently observed in dialysis
patients compared to the entire population. However, no increase in
the risk of leukemia, Hodgkin's lymphoma (HL) and non-Hodgkin's
lymphoma (NHL), as well as breast, colorectal, intestinal, stomach,
lung, body of uterus or prostate cancer was observed in dialysis
patients. Furthermore, the meta-analyses demonstrated that
transplantation was associated with an increased risk of KS, NMSC,
melanoma, leukemia, malignant lymphoma, myeloma, NHL and HL, as
well as cancer of the lip, skin, kidney, anus, thyroid, bladder,
liver, cervix, stomach, esophagus, pancreas and lung, whereas no
increased risk of cancer of the larynx, ovary, uterus, prostate,
breast, body of uterus, colon/rectum and brain was observed. As
only 1 study reported on specific cancer types, formal
meta-analyses were not performed.
 | Table III.Pooled risks of specific cancer types
in patients with renal replacement therapy. |
Table III.
Pooled risks of specific cancer types
in patients with renal replacement therapy.
|
|
|
|
| Heterogeneity |
|---|
|
|
|
|
|
|
|---|
| Type/site of
malignancy | No. studies | (Refs.) | Pooled SIR
(95%CI) | I2
(%) |
P-valuea |
|---|
| Dialysis |
|
| Thyroid
gland | 3 | (8,9,23) | 4.92
(1.43–16.93) | 93.3 | <0.001 |
|
Kidney | 9 | (8–11,22,23,30) | 4.87
(4.14–5.72) | 93.6 | <0.001 |
|
Myeloma | 4 | (10,30) | 4.15
(3.1–5.56) | 90.5 | <0.001 |
|
Melanoma | 2 | (22,23) | 2.83
(1.28–6.23) |
0.0 |
0.652 |
|
Thyroid/other endocrine
glands | 4 | (10,30) | 2.57
(1.82–3.63) | 77.5 |
0.004 |
|
Bladder | 9 | (8–11,22,23,30) | 2.51
(1.85–3.41) | 97.8 | <0.001 |
|
Tongue | 5 | (9,10,30) | 1.8
(1.43–2.26) | 49.0 |
0.097 |
| Cervix
of uterus | 5 | (9,10,30) | 1.76
(1.09–2.86) | 94.8 | <0.001 |
| Penis
scrotum | 4 | (9,10,30) | 1.75
(1.36–2.27) |
0.0 |
0.828 |
|
Liver | 5 | (9,10,30) | 1.39
(1.28–1.51) |
7.4 |
0.364 |
| HL | 4 | (9,30) | 1.58
(0.94–2.66) | 70.4 |
0.017 |
|
NHL | 7 | (10,11,22,23,30) | 1.16
(0.86–1.55) | 91.5 | <0.001 |
|
Breast | 8 | (9–11,22,23,30) | 1.15
(0.9–1.46) | 98.0 | <0.001 |
|
Colorectal | 2 | (9,11) | 1.13
(0.90–1.43) | 95.1 | <0.001 |
|
Intestinal | 4 | (10,30) | 1.12
(0.91–1.39) | 95.8 | <0.001 |
|
Stomach | 6 | (9,10,22,30) | 1.03
(0.71–1.50) | 96.5 | <0.001 |
|
Leukemia | 5 | (9,10,30) | 1.02
(0.55–1.9) | 95.9 | <0.001 |
|
Lung | 7 | (9–11,22,30) | 0.98
(0.77–1.24) | 98.7 | <0.001 |
| Body of
uterus | 6 | (9,10,22,30) | 0.96
(0.78–1.19) | 65.5 |
0.013 |
|
Prostate | 7 | (9–11,22,30) | 0.87
(0.69–1.09) | 95.5 | <0.001 |
|
Transplantation |
|
| Kaposi
sarcoma | 4 | (12,15,16,29) | 59.48
(24.43–144.86) | 93.1 | <0.001 |
|
Lip | 7 | (12,15,16,23,25,26,28) | 29.74
(16.96–52.17) | 95.7 | <0.001 |
|
NMSC | 6 | (12–14,23,25,26) | 15.18
(8.08–28.52) | 98.9 | <0.001 |
|
Skin | 2 | (16,27) | 11.84
(0.6–233.25) | 99.8 | <0.001 |
|
Kidney | 11 | (8,12–16,23–26,28) | 9.7
(5.69–16.53) | 97.1 | <0.001 |
|
Anus | 2 | (12,16) | 9.4 (6.5–13.6) |
0.0 |
0.403 |
|
Malignant lymphoma | 2 | (13,24) | 6.65
(2.97–14.89) | 50.2 |
0.156 |
|
NHL | 9 | (12–16,
23,26–29) | 6.05
(4.11–8.9) | 94.8 | <0.001 |
| HL | 5 | (12,15,16,23,28) | 4.85
(2.97–7.9) | 45.8 |
0.117 |
| Thyroid
gland | 11 | (8,12–16,23–28) | 3.75
(2.5–5.62) | 74.8 | <0.001 |
|
Bladder | 11 | (8,12–16, 23–28) | 3.15
(1.27–7.8) | 98.0 | <0.001 |
|
Myeloma | 3 | (12,16,28) | 2.96
(1.94–4.52) | 47.1 |
0.151 |
|
Liver | 9 | (12–16,24,27–29) | 2.52
(1.71–3.73) | 73.7 | <0.001 |
| Oral
cavity | 5 | (12–16) | 2.38
(1.22–4.64) | 86.1 | <0.001 |
| Cervix
of uterus | 3 | (12,16,28) | 2.20
(1.56–3.10) |
0.0 |
0.732 |
|
Melanoma | 9 | (8,12–16,23,27,28) | 2.05
(1.52–2.78) | 52.7 |
0.031 |
|
Stomach | 8 | (12–16,24,26,28) | 1.92
(1.6–2.31) |
0.0 |
0.779 |
|
Leukemia | 7 | (12–16,23,28) | 1.62
(1.23–2.14) |
5.4 |
0.386 |
|
Esophagus | 6 | (12–16,28) | 1.61
(1.22–2.13) |
0.0 |
0.680 |
|
Pancreas | 6 | (12–16,28) | 1.55
(1.19–2.0) |
0.0 |
0.511 |
|
Lung | 8 | (12–16,27–29) | 1.52
(1.15–1.99) | 84.2 | <0.001 |
|
Larynx | 4 | (13,15,16,28) | 1.53
(0.84–2.79) | 18.8 |
0.297 |
|
Ovary | 2 | (15,28) | 1.39
(0.69–2.77) |
0.0 |
0.702 |
|
Uterus | 4 | (12,14,24,28) | 1.37
(0.75–2.51) | 46.4 |
0.133 |
|
Prostate | 6 | (12,14–16,27,28) | 1.14
(0.94–1.37) | 41.5 |
0.129 |
|
Breast | 9 | (12,14–16,23–25,27,28) | 1.13
(0.99–1.29) |
9.1 |
0.360 |
| Body of
uterus | 2 | (15,16) | 1.09
(0.66–1.8) |
0.0 |
0.631 |
|
Colon/rectum | 4 | (12,15,27,28) | 1.06
(0.66–1.72) | 87.7 | <0.001 |
|
Brain | 3 | (16,23,28) | 1.00
(0.64–1.57) |
0.0 |
0.593 |
Sensitivity analysis
Sensitivity analyses were performed by excluding 1
study at a time. The SIRs were similar without significant
fluctuation, ranging from 1.99 (95% CI, 1.78–2.24) to 2.17 (95% CI,
1.81–2.59) (data not shown).
Reporting bias
Egger's test indicated the presence of a publication
bias regarding the primary outcome (P=0.05).
Discussion
The present meta-analysis demonstrated that dialysis
and transplantation were associated with an increased risk of
overall cancer and the majority of specific cancer types. Compared
with the general population, the risk of overall cancer including
NMSC was 1.4-fold increased and that excluding NMSC was 1.35-fold
increased for patients receiving dialysis, while the risk of
overall cancer including NMSC was 3.26-fold increased and that
excluding NMSC was 2.08-fold increased for patients with
transplantation. Therefore, the risk of cancer for patients
receiving transplantation was higher than that for patients
receiving dialysis.
Similar to other published meta-analyses of this
type (31,32), the present study had a high level of
heterogeneity. Subgroup analyses were performed to explore the
sources of this heterogeneity. Stratification of subjects by
gender, in agreement with previous studies (9,10,13,14,23,30),
showed that the SIR of all cancers in female patients with RRT was
higher than that in male patients, suggesting that female RRT
patients require a higher level of cancer surveillance.
Stratification of subjects by age showed that the risk for all
cancers was higher in younger patients and decreased with age in
patients with RRT, whether those receiving dialysis or
transplantation, similar to the findings of several large studies
(9,10,13,14,24,28,30).
These age-associated observations may have resulted from the
following major aspects: First, the higher cancer risk in younger
patients receiving RRT compared with that in older patients was
likely to be due to the low incidence rate of cancer among young
individuals in the general population. Second, the age phenomenon
may be attributed to the fact that younger patients may have been
affected by considerably more serious viral-associated cancer,
against which they tend to lack immunity compared to older
patients. Therefore, the discrepancy in cancer risk may disappear
with advancing age (33). Finally,
the risk of cancer stands in competition with the risk of other
chronic illnesses, such as cardiovascular diseases, in the older
population. Thus, younger patients receiving RRT require more
intensive cancer surveillance than older patients.
Grouping of studies by follow-up time showed that
the cancer risk was highest in the first year of receiving dialysis
or after renal transplantation and decreased over subsequent years.
This result was compatible with the findings of previous studies
(9,10,13,14,24,28,30).
Only 1 study reported that the SIR was highest at 10 years
post-transplantation (4). The reason
for this may be that ESRD is an important risk factor for cancer
(34). A further explanation may be
the increased amount of medical surveillance. In addition, the
increased rate of cancer diagnosis may have been due to undetected
cancers being already present prior to RRT. Furthermore, recent
increases in the risk of cancer in RTRs may be associated with the
effects of more potent immunosuppressive treatments, which are
increasingly used for prevention and treatment of acute rejection.
The mean duration from initiation of dialysis to the detection of
cancer was 2.8 years according to the study by Vajdic et al
(4) and 3.6 years in the study by Loy
et al (10). The mean time
interval between renal transplantation and tumor development ranged
from 4.9 to 9.4 years (4,14,25,27),
suggesting that RTRs have a risk of cancer occurring after a number
of years. Therefore, regular follow-up is warranted if cancer is
not found during early screening.
Grouping of studies by region showed that the risk
for overall cancer remained significantly elevated in the Asian and
non-Asian populations receiving RRT, although no significant
difference between them was observed. However, the distribution of
cancer types in Asian RTRs differed from that in non-Asian RTRs.
Numerous studies have shown that in Western countries, RTRs are at
greater risk of developing NMSC (16,25,26). By
contrast, NHL as well as renal and bladder cancer had the highest
SIRs in Hong Kong (14), and kidney
cancer was the most common cancer type in Taiwan (13) and Japan (24). These results emphasize the requirement
for vigilant cancer surveillance following transplantation.
The present study also observed a strongly increased
risk of site-specific cancer in RRT patients. For dialysis
patients, the risk was highest for thyroid gland and kidney cancer
as well as myeloma (ESRD-associated cancer types) (8). For kidney transplantation, the most
common cancer types were KS, lip cancer and NMSC (immune
deficiency-associated cancer types) (8); this finding was in agreement with the
study by Engels et al (35),
who reported that the cancer risk was most pronounced for KS, lip
cancer and NMSC among solid organ transplant recipients in the USA.
Following kidney transplantation, there was an obvious increase in
the incidence of a wide variety of cancer types, several of which
were also increased in dialysis patients. The magnitude and breadth
of the increased cancer risk following transplantation suggested
that immune deficiency is the underlying cause. The results of the
pooled analysis of the present study are similar to those of the
previous meta-analysis by Grulich et al (7) for transplantation, with the exception of
laryngeal and colorectal cancer. Moreover, the present study
further evaluated risk factors, including age, gender and follow-up
time, for the development of cancer in RRT recipients. However, the
results of the present meta-analysis showed no statistically
significant association between RRT and breast, body of uterus,
colorectal and prostate cancers. In addition, the present study
reported an increased risk of HL, NHL, leukemia and lung cancer for
transplantation but not for dialysis. Of note, there was
significant heterogeneity among the majority of the studies on
various organ-specific cancers.
RRT is linked with cancer via the following
potential mechanisms: i) Underlying renal disease is a possible
explanation for the increased cancer risk, for example, acquired
cystic kidney disease is associated with an increased occurrence of
renal cell carcinoma (36). ii)
Carcinogenesis is linked to medications administered for treatment
of renal disease, such as azathioprine for skin cancers (37) and lymphomas (38). iii) It was reported that long-term
hemodialysis may suppress the DNA repair system of lymphocytes
(39), and plasma glutathione
peroxidase deficiency caused by renal dysfunction may lead to DNA
damage (40), which impairs the
defense of the organism against oncogenic viral infections and a
variety of nonviral tumor antigens. iv) Bioincompatibility of the
dialysis membrane may lead to the release of cytokines,
predisposing to tumor formation (41). v) Carcinogenesis is also associated
with lifestyle and other cancer risk factors, including age, gender
and smoking. vi) The correlation of cancer with dialysis may be
attributable to a coincidental association due to detection bias.
vii) As for RTRs, in addition to all the aforementioned risk
factors associated with dialysis, anti-rejection drugs profoundly
suppress immunity and may themselves be carcinogenic (42). Other than cancers occurring de
novo following transplantation, recurrence of preexisting
cancers and cancers from donor organs should also be taken into
account.
Several limitations of the present meta-analysis
should be acknowledged. First, certain studies, which failed to
provide data to calculate the SIR were not included in the
meta-analysis, which may have reduced the power of the analysis.
Furthermore, significant heterogeneity was observed among the
studies. Thus, subgroup analysis was performed to determine the
sources of heterogeneity. However, the variables examined did not
fully constitute the source of heterogeneity, suggesting that other
unknown confounding variables may be the source of heterogeneity.
However, sensitivity analyses demonstrated that the results were
robust. In addition, the observational nature of the studies
included in the present meta-analysis was likely to have caused
bias. Specifically, risk factors of cancers, including lifestyle,
smoking, alcohol use, immunosuppressive agents and ultraviolet
exposure were not taken into account by most studies. As a result,
relevant confounding factors could not be considered. Therefore,
well-designed studies considering more covariates are required to
investigate the association between RRT and the risk of cancer.
Furthermore, as certain studies included NMSC, the cancer incidence
may have been overestimated. Finally, the present meta-analysis had
the limitation of publication bias, as negative trials are less
likely to be reported.
In conclusion, the present meta-analysis
demonstrated that patients with RRT (particularly transplantation)
are at an increased risk of overall cancer as well as a wide range
of cancer types, particularly of the thyroid gland, kidney and
myeloma for dialysis and KS, lip and NMSC for transplantation.
Screening for cancer should be individualized and based on a
reasonable life expectancy. However, these conclusions should be
drawn cautiously due to high heterogeneity and publication bias as
well as the limited amount of data on certain types of cancer. To
further assess the link between RRT and cancer, additional large
and well-designed prospective studies are required.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81200531 to S.G. and
grant nos. 81470948 and 81270770 to G.X.).
References
|
1
|
Collins AJ, Foley RN, Gilbertson DT and
Chen SC: The state of chronic kidney disease, ESRD, and morbidity
and mortality in the first year of dialysis. Clin J Am Soc Nephrol.
4(Suppl 1): S5–S11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mandayam S and Shahinian VB: Are chronic
dialysis patients at increased risk for cancer? J Nephrol.
21:166–174. 2008.PubMed/NCBI
|
|
3
|
Briggs JD: Causes of death after renal
transplantation. Nephrol Dial Transplant. 16:1545–1549. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vajdic CM, McDonald SP, McCredie MR, van
Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM
and Grulich AE: Cancer incidence before and after kidney
transplantation. JAMA. 296:2823–2831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Penn I and Starzl TE: Malignant lymphomas
in transplantation patients: A review of the world experience. Int
J Clin Pharmacol Ther Toxicol. 3:49–54. 1970.
|
|
6
|
Marple JT and MacDougall M: Development of
malignancy in the end-stage renal disease patient. Semin Nephrol.
13:306–314. 1993.PubMed/NCBI
|
|
7
|
Grulich AE, van Leeuwen MT, Falster MO and
Vajdic CM: Incidence of cancers in people with HIV/AIDS compared
with immunosuppressed transplant recipients: A meta-analysis.
Lancet. 370:59–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stewart JH, Vajdic CM, van Leeuwen MT,
Amin J, Webster AC, Chapman JR, McDonald SP, Grulich AE and
McCredie MR: The pattern of excess cancer in dialysis and
transplantation. Nephrol Dial Transplant. 24:3225–3231. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ
and Fang TC: Increased risk of cancer in chronic dialysis patients:
A population-based cohort study in Taiwan. Nephrol Dial Transplant.
27:1585–1590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loy EY, Choong HL and Chow KY: Cancer
among end-stage renal disease patients on dialysis. Ann Acad Med
Singapore. 42:640–645. 2013.PubMed/NCBI
|
|
11
|
Butler AM, Olshan AF, Kshirsagar AV,
Edwards JK, Nielsen ME, Wheeler SB and Brookhart MA: Cancer
incidence among US Medicare ESRD patients receiving hemodialysis,
1996–2009. Am J Kidney Dis. 65:763–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collett D, Mumford L, Banner NR, Neuberger
J and Watson C: Comparison of the incidence of malignancy in
recipients of different types of organ: A UK registry audit. Am J
Transplant. 10:1889–1896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li WH, Chen YJ, Tseng WC, Lin MW, Chen TJ,
Chu SY, Hwang CY, Chen CC, Lee DD, Chang YT, et al: Malignancies
after renal transplantation in Taiwan: A nationwide
population-based study. Nephrol Dial Transplant. 27:833–839. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheung CY, Lam MF, Chu KH, Chow KM, Tsang
KY, Yuen SK, Wong PN, Chan SK, Leung KT, Chan CK, et al:
Malignancies after kidney transplantation: Hong Kong renal
registry. Am J Transplant. 12:3039–3046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piselli P, Serraino D, Segoloni GP, et al:
Risk of de novo cancers after transplantation: Results from
a cohort of 7217 kidney transplant recipients, Italy 1997–2009. Eur
J Cancer. 49:336–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krynitz B, Edgren G, Lindelöf B, Baecklund
E, Brattström C, Wilczek H and Smedby KE: Risk of skin cancer and
other malignancies in kidney, liver, heart and lung transplant
recipients 1970 to 2008 - a Swedish population-based study. Int J
Cancer. 132:1429–1438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stengel B: Chronic kidney disease and
cancer: A troubling connection. J Nephrol. 23:253–262.
2010.PubMed/NCBI
|
|
18
|
Izzedine H and Perazella MA:
Onco-nephrology: An appraisal of the cancer and chronic kidney
disease links. Nephrol Dial Transplant. 30:1979–1988. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Egger M, Smith Davey G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Port FK, Ragheb NE, Schwartz AG and
Hawthorne VM: Neoplasms in dialysis patients: A population-based
study. Am J Kidney Dis. 14:119–123. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Birkeland SA, Løkkegaard H and Storm HH:
Cancer risk in patients on dialysis and after renal
transplantation. Lancet. 355:1886–1887. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoshida Y, Tsukuma H, Yasunaga Y, Xu N,
Fujita MQ, Satoh T, Ichikawa Y, Kurihara K, Imanishi M, Matsuno T
and Aozasa K: Cancer risk after renal transplantation in Japan. Int
J Cancer. 71:517–520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kyllönen L, Salmela K and Pukkala E:
Cancer incidence in a kidney-transplanted population. Transpl Int.
13(Suppl 1): S394–S398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adami J, Gäbel H, Lindelöf B, Ekström K,
Rydh B, Glimelius B, Ekbom A, Adami HO and Granath F: Cancer risk
following organ transplantation: A nationwide cohort study in
Sweden. Br J Cancer. 89:1221–1227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Végso G, Tóth M, Hídvégi M, Toronyi E,
Langer RM, Dinya E, Tóth A, Perner F and Járay J: Malignancies
after renal transplantation during 33 years at a single center.
Pathol Oncol Res. 13:63–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Villeneuve PJ, Schaubel DE, Fenton SS,
Shepherd FA, Jiang Y and Mao Y: Cancer incidence among Canadian
kidney transplant recipients. Am J Transplant. 7:941–948. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serraino D, Piselli P, Busnach G, Burra P,
Citterio F, Arbustini E, Baccarani U, De Juli E, Pozzetto U,
Bellelli S, et al: Risk of cancer following immunosuppression in
organ transplant recipients and in HIV-positive individuals in
southern Europe. Eur J Cancer. 43:2117–2123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maisonneuve P, Agodoa L, Gellert R,
Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney
AP, Briggs D, et al: Cancer in patients on dialysis for end-stage
renal disease: An international collaborative study. Lancet.
354:93–99. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan L, Chen P, Chen EZ, Gu A and Jiang ZY:
Risk of bladder cancer in renal transplant recipients: A
meta-analysis. Br J Cancer. 110:1871–1877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Z, Lin F, Qin B, Liang Y and Zhong R:
Polymyositis/dermatomyositis and malignancy risk: A metaanalysis
study. J Rheumatol. 42:282–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heidland A, Bahner U and Vamvakas S:
Incidence and spectrum of dialysis-associated cancer in three
continents. Am J Kidney Dis. 35:347–351; discussion 352–353. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vamvakas S, Bahner U and Heidland A:
Cancer in end-stage renal disease: Potential factors
involved-editorial. Am J Nephrol. 18:89–95. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Engels EA, Pfeiffer RM, Fraumeni JF Jr,
Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly
AR, Clarke CA, et al: Spectrum of cancer risk among US solid organ
transplant recipients. JAMA. 306:1891–1901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marple JT, MacDougall M and Chonko AM:
Renal cancer complicating acquired cystic kidney disease. J Am Soc
Nephrol. 4:1951–1956. 1994.PubMed/NCBI
|
|
37
|
Taylor AE and Shuster S: Skin cancer after
renal transplantation: The causal role of azathioprine. Acta Derm
Venereol. 72:115–119. 1992.PubMed/NCBI
|
|
38
|
Silman AJ, Petrie J, Hazleman B and Evans
SJ: Lymphoproliferative cancer and other malignancy in patients
with rheumatoid arthritis treated with azathioprine: A 20 year
follow up study. Ann Rheum Dis. 47:988–992. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vamvakas S, Bahner U, Becker P, Steinle A,
Götz R and Heidland A: Impairment of DNA repair in the course of
long-term hemodialysis and under cyclosporine immunosuppression
after renal transplantation. Transplant Proc. 28:3468–3473.
1996.PubMed/NCBI
|
|
40
|
Yoshimura S, Suemizu H, Nomoto Y, Sakai H,
Katsuoka Y, Kawamura N and Moriuchi T: Plasma glutathione
peroxidase deficiency caused by renal dysfunction. Nephron.
73:207–211. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akizawa T, Kinugasa E and Koshikawa S:
Increased risk of malignancy and blood-membrane interactions in
uraemic patients. Nephrol Dial Transplant. 9(Suppl 2): S162–S164.
1994.
|
|
42
|
Gutierrez-Dalmau A and Campistol JM:
Immunosuppressive therapy and malignancy in organ transplant
recipients: A systematic review. Drugs. 67:1167–1198. 2007.
View Article : Google Scholar : PubMed/NCBI
|