Introduction
Tumour angiogenesis is a process essential for
cancer cell proliferation, invasion and metastasis (1). The balance between pro- and
anti-angiogenic factors, including growth factors, cytokines and
chemokines, which is responsible for normal angiogenesis, is
disrupted during tumourigenesis (2,3).
Vascular endothelial growth factor (VEGF) is a
critical pro-angiogenic protein that drives tumour angiogenesis.
The biological functions of VEGF are mediated upon binding to type
III receptor tyrosine kinases, VEGF receptor (VEGFR)-1, VEGFR-2 and
VEGFR-3 (4–7).
VEGFR-1 plays an important role in tumour
progression and dissemination, and enhances tumour metastasis in
the lung via induction of matrix metalloproteinase 9 (8). The binding of VEGF to VEGFR-2 activates
multiple signalling pathways, resulting in upregulation of
endothelial cell proliferation, migration and survival and an
increase in vascular permeability. Expression of VEGFR-2 in
combination with VEGFR-3 is significantly upregulated in the tumour
vascular endothelium of the most common human solid tumours.
VEGFR-3 is largely confined to the lymphatic endothelium in adult
tissues, but its expression also plays a fundamental role in the
tumour microenvironment by promoting the sprouting of new lymphatic
vessels from pre-existing ones (7,9,10).
Microvessel density (MVD), as determined by the
expression of the endothelial antigens CD34 and CD105, is a direct
neoangiogenesis marker and an important prognostic indicator in
NSCLC. MVD has been shown to be correlated with the concentration
and expression of VEGF; it is also associated with enzymes involved
in the early stages of angiogenesis, tumour growth and occurrence
of distant metastasis (11–13).
There is plentiful literature regarding the
association of angiogenic factors with disease prognosis (4,14–20); however, only a few studies evaluate
the role of such factors in predicting response to chemotherapy
(21–25).
Platinum-based doublet chemotherapy is considered
the current standard of care for patients with stage III NSCLC;
however, a number of patients in our institution were historically
treated with the combination of paclitaxel-ifosfamide-cisplatin
(TIP), based on earlier study reports (26–28). In
the present study, the expression of VEGFR-1, VEGFR-2, VEGFR-3, and
the endothelial markers CD34 and CD105, was assessed in tumour
samples of patients with stage III NSCLC; the respective parameters
were further analyzed retrospectively in relation to response to
induction TIP chemotherapy.
Patients and methods
Study design
A total of 70 patients with stage IIIA NSCLC treated
with induction TIP chemotherapy at our institution between 1998 and
2008 were retrospectively analysed. The patients were staged
according to the American Joint Committee on Cancer/Union for
International Cancer Control tumour-node-metastasis staging system
(6th edition) (29) and classified
into two equal-sized groups (n=35) based on response to
chemotherapy (responders vs. non-responders). The responders and
non-responders were subsequently offered surgery and radiotherapy,
respectively. The groups were matched by pre-treatment patient and
tumour characteristics (Table I).
 | Table I.Patient characteristics and clinical
parameters. |
Table I.
Patient characteristics and clinical
parameters.
|
Characteristics | Group A
(responders), no. (%) | Group B
(non-responders), no. (%) |
|---|
| Total patients | 35 (100) | 35 (100) |
| Gender |
|
|
|
Male | 30 (87) | 26 (74) |
|
Female | 5
(13) | 9 (26) |
| Age (years) |
|
|
|
Median | 58 | 56 |
|
Range | 40–72 | 48–70 |
| Performance
status |
|
|
| 0 | 13 (37) | 11 (32) |
| 1 | 22 (63) | 24 (68) |
| Histology |
|
|
|
Adenocarcinoma | 20 (57) | 20 (57) |
|
Squamous cell Ca | 11 (32) | 13 (37) |
|
Large-cell Ca | 4
(11) | 2 (6) |
The study protocol was approved by the Ethics
Committee of Laiko General Hospital (Athens, Greece).
Chemotherapy regimen
TIP chemotherapy was administered according to the
following three-weekly schedule: Paclitaxel (Taxol®) was
administered at 135–215 mg m−2 over 1 h by intravenous
(i.v.) infusion on day 1, following premedication consisting of
dexamethasone 20 mg, dimethindene maleate (Fenistil®) 4
mg and ranitidine 50 mg; all were administered i.v. 1 h prior to
paclitaxel. Ifosfamide was administered at 4.5–6.0 g m−2
i.v. over 1 h divided between days 1 and 2 (2.25–3.0 g
m−2 per day) along with mesna uroprotection, 40% of the
ifosfamide dose, administered i.v. before and at 3 and 6 h after
ifosfamide. Cisplatin 80–100 mg m−2 was administered
i.v. over 30 min divided between days 1 and 2 (40–50 mg
m−2 per day), with adequate vigorous pre- and
post-hydration, furosemide and electrolyte replacement (20 mEq
potassium chloride and 8 mEq magnesium sulphate per litre of
post-hydration solution). For febrile neutropenia, primary
prophylaxis (filgrastim 5 mg/kg) was administered until recovery of
neutrophils. Dose modifications for all three chemotherapeutic
drugs were made in patients with chemotherapy-related toxicities.
All the toxicities were graded according to the Common Toxicity
Criteria for Adverse Events (30).
The patients received up to 4 cycles of chemotherapy and evaluation
of response was performed every 2 cycles by X-rays, computed
tomography (CT) scans and bone scans using the Response Evaluation
Criteria in Solid Tumours, version 1.0 (31). Patients showing complete or partial
response on induction chemotherapy were classified as responders
and were subsequently offered either lobectomy or pneumonectomy,
with resection of the involved lymph node stations. Non-responders
received ≤6 cycles of TIP and were offered radical
radiotherapy.
Immunocytochemistry
Tumour samples were obtained at the time of
diagnosis via bronchoscopy. The tumour specimens were initially
fixed in 10% neutral buffered formaldehyde and then embedded in
paraffin wax. Sections (4 µm) were cut consecutively.
Immunohistochemistry was performed on the most representative areas
of viable tumour cells, avoiding areas of extensive necrosis or
haemorrhage at the Pathology Department of the National and
Kapodistrian University of Athens.
The antibodies used were Monoclonal rabbit
VEGFR-1/Flt1 (dilution 1:50-1:100; cat. no. RP 077), policlonal
rabbit VEGFR-2/Flk1 (dilution 1:50-1:100; cat. no. RP 07),
policlonal rabbit VEGFR-3/Flt4 (dilution 1:50-1:10; cat. no. RP135)
(all from Diagnostic BioSystems, CA, USA), rabbit recombinant
monoclonal CD-105 (dilution 1:5-1:10; cat. no. M3527) and
monoclonal mouse CD-34 (Class II Clone QBEnd-10; cat. no. GA632)
(both from Dako, Glostrup, Denmark).
The results were recorded by two experienced
pathologists by independently counting the percentage of positive
cells and the intensity of staining in each section (1+, mild; 2+,
moderate; and 3+, intense). The immunostaining scores were
calculated by multiplying the percentage of labeled cells by the
intensity of staining. MVD was evaluated on immunostained sections
with CD34 and CD105 and it was determined in the three areas of
maximal vascularization by using the criteria of Weidner et
al (32).
The specificity of the immunohistochemical
procedures was verified by using negative and positive control
sections. The negative controls for each tissue were prepared by
omitting the primary antibody. Sections from human placenta and
tonsils were used as positive controls.
Statistical analysis
Descriptive statistics were used to present the main
statistical measures of the parameters under investigation. The
statistical measures used were frequencies and percentages for the
discrete variables, and descriptive statistics [mean, median,
standard error of the mean (SEM) and range] for the continuous
parameters. In cases where the normal distribution assumption was
rejected via the Kolmogorov-Smirnov test, the Mann-Whitney test was
implemented to compare the marker distribution between the two
patient groups. The Chi-square test was also used to test the
association between two discrete variables. Survival rates were
estimated with the Kaplan-Meier product limit method and compared
with the log-rank test. All analyses were implemented at a
significance level of α=5% with the use of the SPSS v16.0 software
(SPSS Inc., Chicago, IL, USA).
Results
Analysis of immunostaining scores of
angiogenic factors in NSCLC
The tumour samples from 70 NSCLC patients exhibited
an overall mean immunostaining score of 7.83 (SEM=0.87) for the
angiogenic factor VEGFR-1, 5.56 (SEM=0.79) for VEGFR-2 and 15.86
(SEM=1.49) for VEGFR-3. The overall mean value of the endothelial
antigen CD34 was 16.29 (SEM=1.29). Table
II describes the overall mean, median and SEM values of the
immunostaining scores of the lung tissue expression of VEGFR-1,
VEGFR-2, VEGFR-3 and the endothelial marker CD34.
 | Table II.Descriptive table depicting the
results of immunostaining scores of each examined parameter in 70
patients with non-small-cell lung cancer. |
Table II.
Descriptive table depicting the
results of immunostaining scores of each examined parameter in 70
patients with non-small-cell lung cancer.
| Parameters | Mean | Median | SEM |
|---|
| VEGFR-1 | 7.83 | 5.0 | 0.87 |
| VEGFR-2 | 5.56 | 5.0 | 0.79 |
| VEGFR-3 | 15.86 | 15.0 | 1.49 |
| CD34 | 16.29 | 15.0 | 1.29 |
The expression of the angiogenic marker CD105
exhibited a multivariate distribution. In total, 81.4% of the
patients did not express the CD105 antigen on the endothelial cells
of tumour tissue, whereas 18.6% exhibited immunostaining for the
same endothelial marker (Table
III).
 | Table III.Descriptive table depicting the
results of immunostaining scores of the endothelial antigen CD105
in 70 patients with non-small-cell lung cancer. |
Table III.
Descriptive table depicting the
results of immunostaining scores of the endothelial antigen CD105
in 70 patients with non-small-cell lung cancer.
|
| Response |
|
|---|
|
|
|
|
|---|
| Antigen | NR | R | Total |
|---|
| CD105 |
|
|
|
| Score
0 |
|
|
|
| Patient
no. (%) | 32 (91.4) | 25 (71.4) | 57 (81.4) |
| Score
10 |
|
|
|
| Patient
no. (%) | 3 (8.6) | 10 (28.6) | 13 (18.6) |
| Total |
|
|
|
| Patient
no. (%) | 35 (100.0) | 35 (100.0) | 70 (100.0) |
Variability in expression patterns of
angiogenic factors between responders and non-responders
Table IV describes
the mean value, standard deviation and SEM value of the
immunostaining scores of VEGFR-1, VEGFR-2, VEGFR-3 and CD34 between
responders and non-responders to chemotherapy.
 | Table IV.Descriptive table depicting the
results of immunostaining scores of each examined parameter in the
responder and non-responder groups of non-small-cell lung cancer
patients. |
Table IV.
Descriptive table depicting the
results of immunostaining scores of each examined parameter in the
responder and non-responder groups of non-small-cell lung cancer
patients.
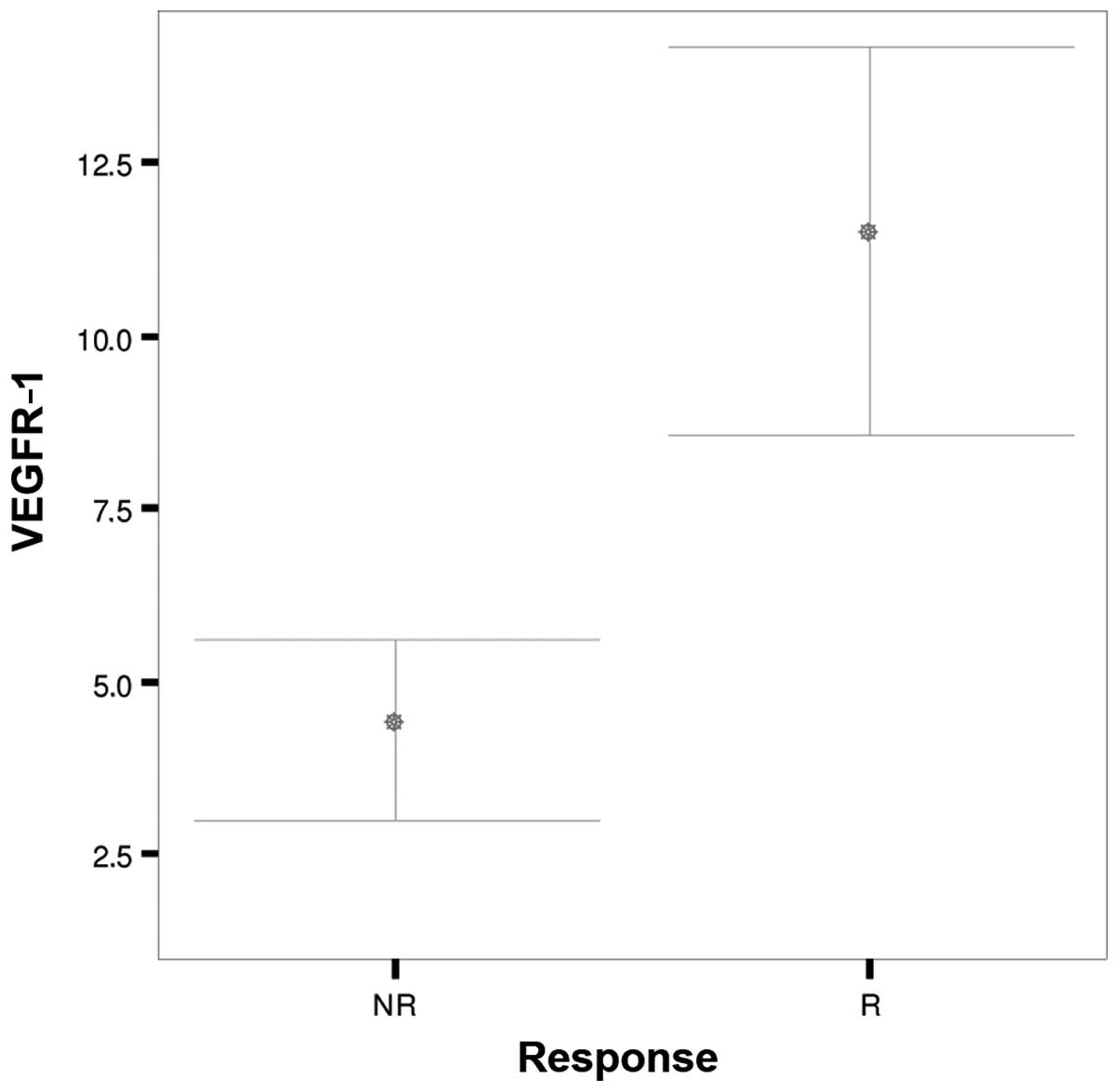
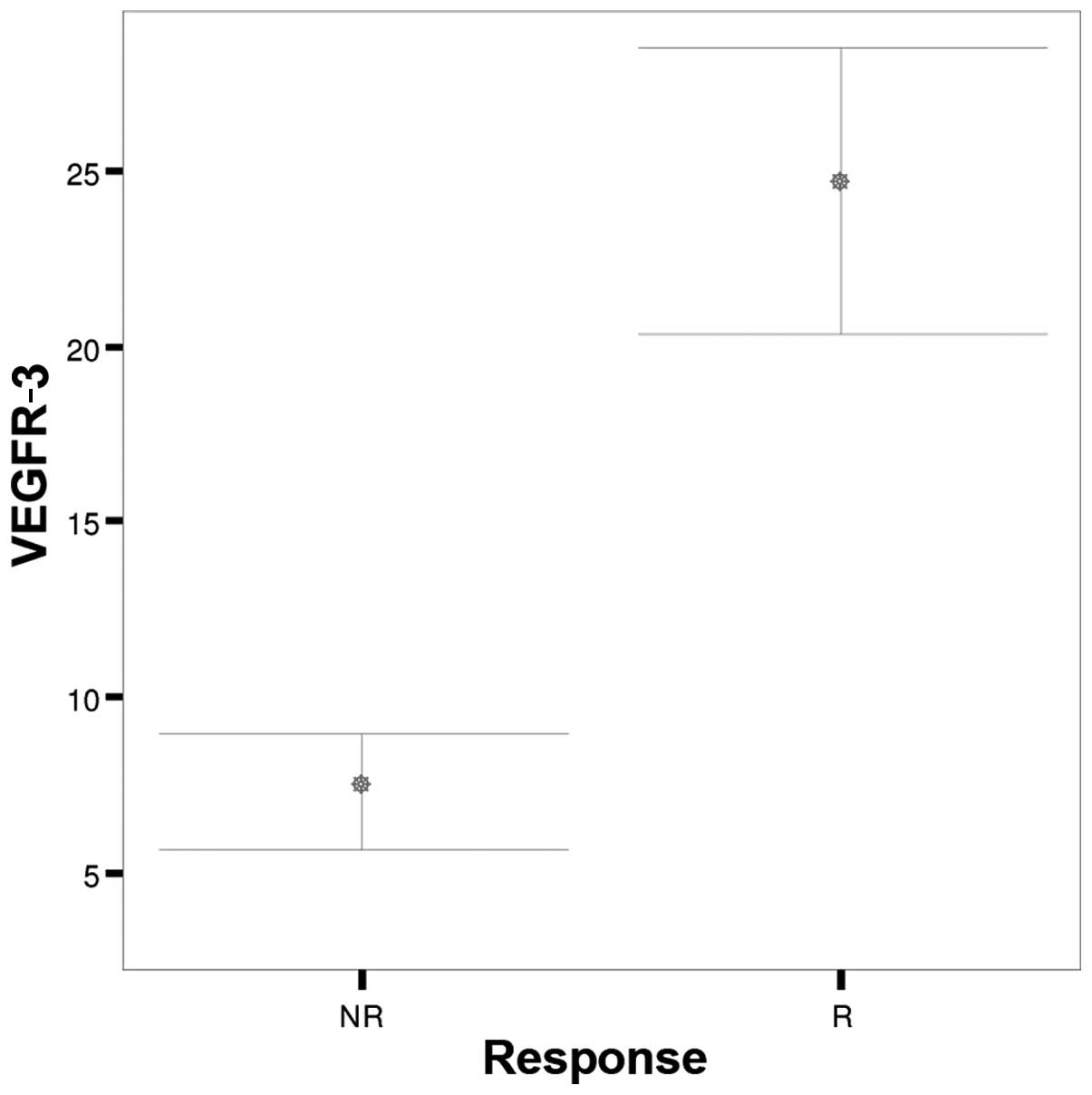
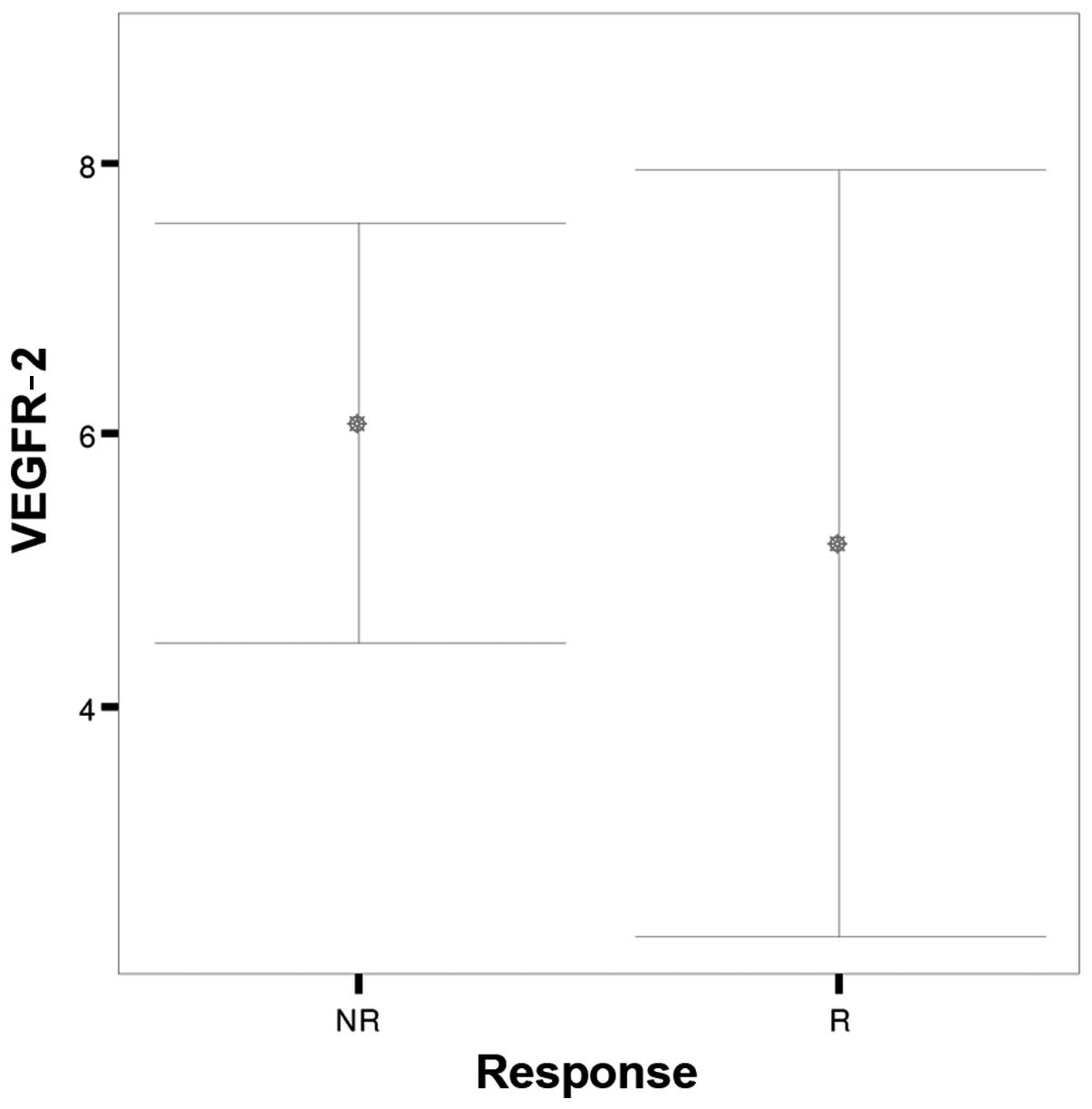
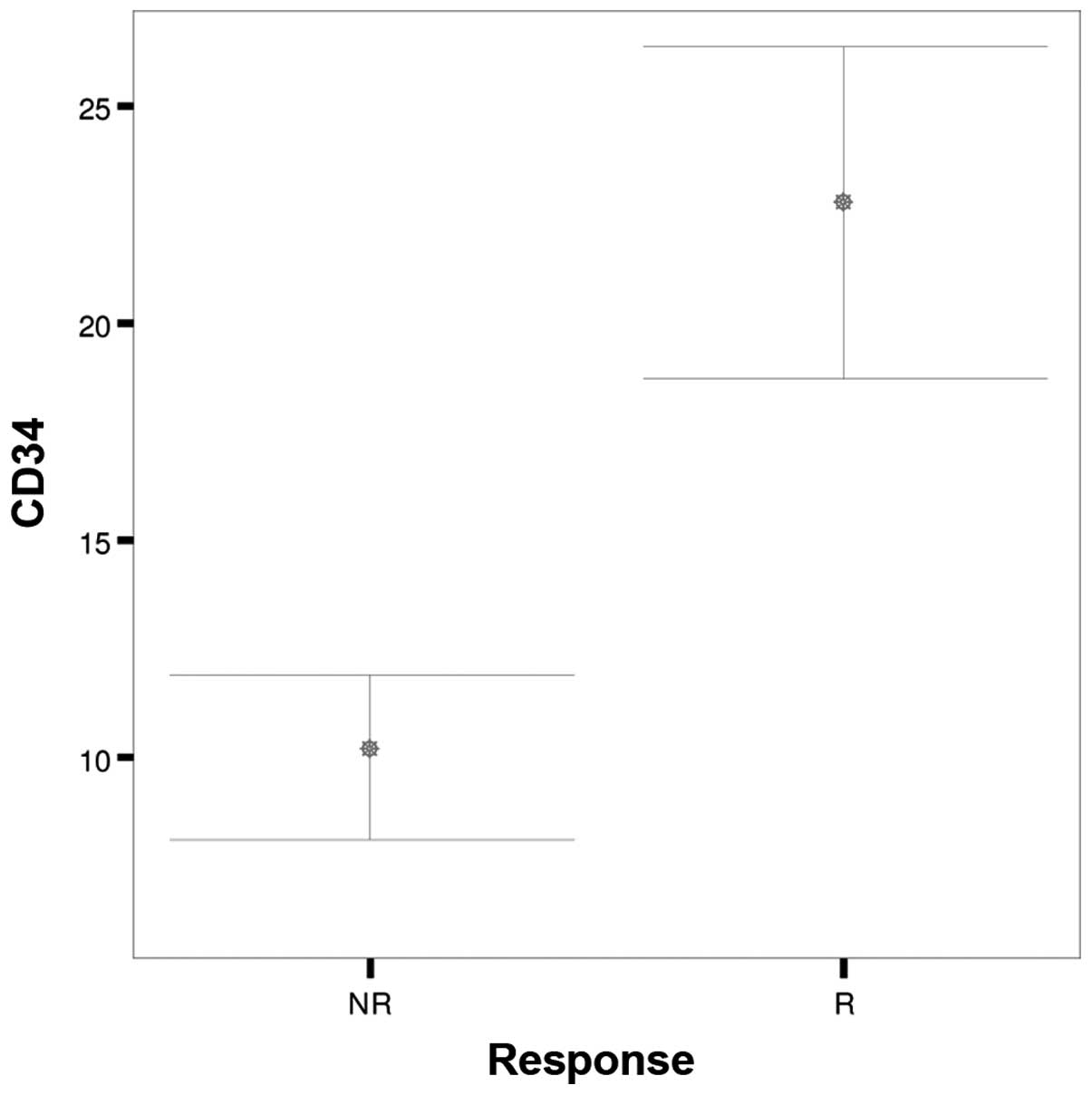
| Parameters | Response | N | Mean | SD | SEM | P-value |
|---|
| VEGFR-1 | NR | 35 |
4.29 |
3.862 | 0.653 | <0.001 |
|
| R | 35 | 11.37 |
8.153 | 1.378 |
|
| VEGFR-2 | NR | 35 |
6.00 |
4.505 | 0.761 | 0.06 |
|
| R | 35 |
5.11 |
8.231 | 1.391 |
|
| VEGFR-3 | NR | 35 |
7.29 |
4.902 | 0.829 | <0.001 |
|
| R | 35 | 24.43 | 11.805 | 1.995 |
|
| CD34 | NR | 35 | 10.00 |
5.557 | 0.939 | <0.001 |
|
| R | 35 | 22.57 | 11.073 | 1.872 |
|
Patients who responded to chemotherapy had
significantly higher pre-treatment immunostaining scores for
VEGFR-1 and VEGFR-3 compared with non-responders (P<0.001)
(Figs. 1 and 2). No significant difference was noted in
VEGFR-2 (P=0.06) immunostaining scores between the two patient
groups (Fig. 3). The CD34
immunostaining score was significantly higher in those who
responded compared with those who did not respond to treatment
(P<0.001) (Fig. 4). There was no
significant difference in the distribution of the CD105 expression
between responders and non-responders (P=0.06) (Table III). The median survival for the 70
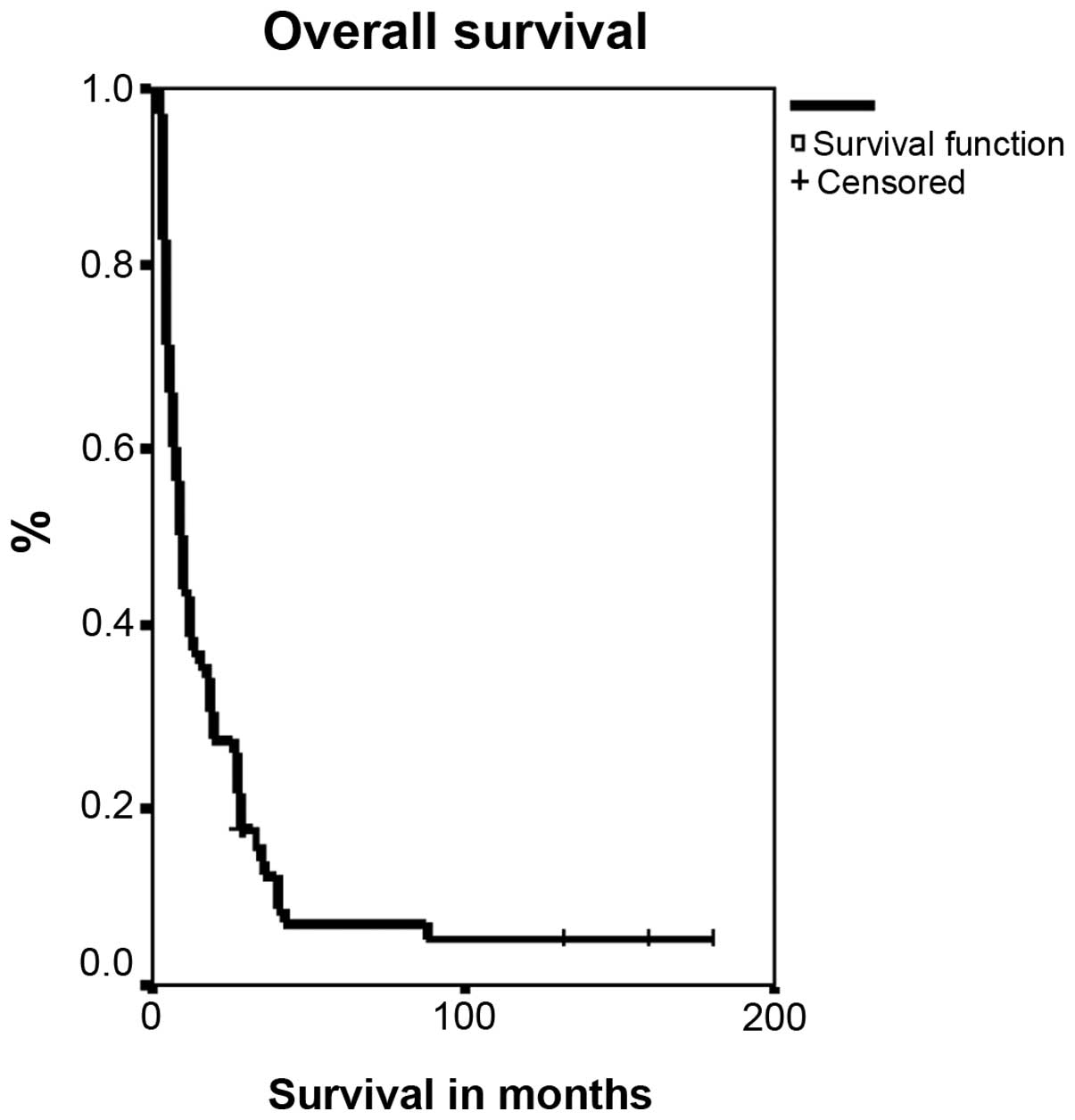
patients was 8.5 months (Fig. 5).
Discussion
Angiogenesis is one of hallmarks of cancer
evolution, as laid out by Hanahan and Weinberg (1). VEGFRs play a significant biological role
in this process; however, there is no solid evidence regarding the
prognostic effect of VEGFR expression on disease outcome.
A number of earlier studies have assessed the
prognostic significance of VEGFRs in NSCLC, with controversial
findings (14,15). Particularly, in a meta-analysis, the
VEGF expression was found to be associated with dismal outcome in
patients with NSCLC (16). In line
with this finding were the results of another study, which
demonstrated a significant association of strong VEGFR-1 and
VEGFR-2 expression with worse survival, whereas the expression of
VEGFR-3 was associated with a favorable outcome in the study
population (4).
In a recent meta-analysis of 5,386 NSCLC and SCLC
patients, VEGF overexpression indicated poor survival for those
with NSCLC histology, whereas VEGFR-3 expression did not affect
prognosis (17).
Although the majority of these studies identified a
correlation between VEGFR-1 and VEGFR-2 expression with poor
prognosis, the data regarding the association of VEGFR3 expression
with survival are conflicting. For example, in a study of 180 NSCLC
patients, the cases who stained positive for VEGF-C and VEGFR-3
exhibited worse survival rates compared with those with weak to no
staining (P=0.003 and 0.001 respectively) (18).
The diversity of the patient cohorts in terms of
stage, histology, median follow-up and size between the various
studies may, to some extent, account for these disparities.
Interestingly, in a previous study, angiogenic
factor expression was strongly correlated with lower risk of
progression only for patients with early-stage squamous cell lung
cancer, but not for those with adenocarcinoma, which underlined the
diverse biological role of angiogenesis between different
histological subtypes of NSCLC (19).
Several studies have assessed an eventual
association between MVD and prognosis in NSCLC patient. Macchiarini
et al (20) demonstrated that
an increased MVD count predicted the aggressive behaviour of the
disease. In particular, NSCLC cases with increased MVD exhibited a
higher metastatic potential, tumour size and proliferative
activity. A meta-analysis published by Meert et al concluded
that a high MVD assessed by CD34, factor VIII and CD31, is a poor
prognostic factor of survival for surgically treated NSCLC patients
(13).
However, in a meta-analysis of 2,719 NSCLC patients,
MVD was not proven to be a prognostic marker of survival (33). It was therefore suggested that the
apparent inconsistency may be attributed to methodological
differences between studies, such as the antibody/marker used,
sample selection and counting methods.
Regarding NSCLC management, induction chemotherapy,
with or without the addition of radiotherapy, has been considered
the gold standard treatment for stage IIIA NSCLC (34). However, in the currently available
literature, no angiogenesis-related predictive factors of response
to chemotherapy have been validated in large studies in this
setting.
In the BATTLE trial (21), 255 heavily pretreated NSCLC patients
were randomly allocated to receive erlotinib, vandetanib, erlotinib
plus bexarotene, or sorafenib, based on molecular biomarkers
assessed in fresh core needle biopsy specimens. Patients with high
tumour VEGFR-2 expression exhibited improved 8-week disease control
rates following treatment with vandetanib, compared with those
exhibiting low VEGFR-2 expression (P=0.05). However, despite the
improved control rate, VEGFR-2 expression failed to confer any
statistically significant overall survival benefit in this phase II
study.
Interestingly, several immunocytochemical markers,
including angiogenesis markers, were evaluated in 515 cases of
stage I NSCLC in relation to the clinical course of the disease. No
sufficient evidence supporting any change in clinical practice
emerged from that study (22).
Surprisingly, in a previous study, a high MVD index
was predictive of disease response in NSCLC patients who received
chemotherapy with the addition of bevacizumab, a monoclonal
anti-VEGF antibody (23). In
particular, a strong correlation was observed between the largest
percentage of tumour shrinkage and the MVD of undifferentiated
vessels (P=0.019). However, this finding has yet to be validated in
large studies.
In a recently published study, the predictive value
of several angiogenic biomarkers, including angiopoietin-2, bone
morphogenetic protein-9, epidermal growth factor (EGF), endoglin,
endothelin-1, fibroblast growth factor (FGF)-1, FGF-2, follistatin,
granulocyte-colony stimulating factor, heparin-binding EGF,
hepatocyte growth factor (HGF), interleukin-8, leptin, placental
growth factor, VEGF-A, VEGF-C, and VEGF-D, was assessed in 41
patients with stage IV non-squamous NSCLC, treated with either
chemotherapy alone or with the addition of bevacizumab. Serum was
collected before and after treatment initiation. An increased
VEGF-A level after the first cycle of chemotherapy was correlated
with worse progression-free survival (PFS) in patients who received
chemotherapy with bevacizumab. By contrast, increased leptin levels
were associated with improved survival in the group that received
the antibody. Increased angiopoietin-2, HGF, follistatin, VEGF-C
and VEGF-D levels were associated with poor survival, whereas
increased FGF-1 and endothelin-1 levels predicted improved survival
(25). Another study evaluated the
same panel of angiogenic factors in 68 patients with stage IV
non-squamous NSCLC, treated with either chemotherapy alone or with
the addition of bevacizumab. Serum was collected immediately prior
to chemotherapy. High levels of endothelin-1, follistatin and
VEGF-C were associated with worse PFS, regardless of the type of
chemotherapy. High HGF levels conferred worse PFS and overall
survival in patients who received chemotherapy with bevacizumab
compared with those who received chemotherapy alone. Similarly,
high endoglin levels were correlated with worse PFS in patients who
received the antibody (24).
In our study, the expression of certain angiogenic
factors in relation to the response to induction TIP chemotherapy
was retrospectively assessed. The unexpectedly high response rate
(64%; 95% confidence interval: 50.7–77.3%) observed with this
regimen in a previous study by our group led us to retrospectively
focus on possible biomarkers affecting prognosis and chemotherapy
effectiveness in the study population (27).
Although there is no direct evidence that TIP
chemotherapy or its individual components target angiogenesis, the
association between high pretreatment expression of VEGFR-1 and
VEGFR-3 and the response to chemotherapy in our study may advocate
a possible connection. In support of this hypothesis, data
presented by Linderholm et al demonstrated that a decrease
of circulating VEGF level affects time-to-progression after 12
weeks of therapy with weekly paclitaxel in metastatic breast cancer
patients, supporting a possible role for angiogenic factors in
monitoring the treatment efficacy of non-VEGF-targeted therapies
(35).
Furthermore, there is strong evidence of crosstalk
between several diverse molecular pathways driving tumour invasion
and metastasis in the cancer cell and its microenvironment
(36). For example, agents targeting
certain gene aberrations may also induce antiangiogenic responses.
This may occur due to the downregulation of proangiogenic factors
(37).
We therefore hypothesized there may be crosstalk
between angiogenesis and molecular events that repair DNA damage,
such as nucleotide excision repair and base-mismatch repair
pathways. This hypothesis may explain the high effectiveness of the
TIP regimen, which inhibits the repair of DNA lesions, in the
presence of a high expression of VEGFR-1, VEGFR-3 and CD34.
Tumour stroma, which is composed of fibroblastic,
inflammatory and immune cells, is an additional source of
angiogenic factors (38). In
particular, there is a network of paracrine and autocrine signaling
pathways within the tumour cell and its microenvironment. This
network may be an appealing therapeutic target in NSCLC (39). Tumour-inducible hypoxia in the stroma
may impede the activity of common chemotherapy regimens. The
synergism between paclitaxel and alkylating agents, such as
cisplatin and ifosfamide, has been extensively investigated
(40). This phenomenon may explain
the effectiveness of the TIP regimen as an induction or even rescue
treatment in several diverse malignancies (41–43).
Whether the TIP regimen may overcome the tumour hypoxia and exert
its action in the tumour microenvironment requires further
investigation.
In our study, CD34 expression was evident in all the
samples examined and significantly higher in responders, whereas
only one-fifth of the patients exhibited immunostaining for the
marker CD105, without any effect on treatment outcome. A likely
reason for this variability is the inherent capacity of the
pan-endothelial marker CD34 to react well with endothelial cells in
all blood vessels (11), as opposed
to CD105, which appears to bind preferentially to activated
endothelial cells in tissues participating in angiogenesis
(12).
The retrospective nature and the small population of
our study limit the impact of the results. However, given the
scarcity of the literature in translational research studies and
the lack of predictive factors in the treatment of non-metastatic
NSCLC, we suggest that the results of this study merit further
investigation. In line with this, we plan to conduct a study in
order to assess angiogenic factor expression and its effect on
response to chemotherapy regimens with antiangiogenic activity,
such as bevacizumab and metronomic vinorelbine, for stage IV NSCLC
patients.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benelli R, Lorusso G, Albini A and Noonan
DM: Cytokines and chemokines as regulators of angiogenesis in
health and disease. Curr Pharm Des. 12:3101–3115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bremnes RM, Camps C and Sirera R:
Angiogenesis in non-small cell lung cancer: The prognostic impact
of neoangiogenesis and the cytokines VEGF and bFGF in tumours and
blood. Lung Cancer. 51:143–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Santa Pau E Carrillo, Arias FC, Caso
Peláez E, Muñoz Molina GM, Sánchez Hernández I, Muguruza Trueba I,
Moreno Balsalobre R, Sacristán López S, Gómez Pinillos A and del
Val Toledo Lobo M: Prognostic significance of the expression of
vascular endothelial growth factors A, B, C and D and their
receptors R1, R2 and R3 in patients with nonsmall cell lung cancer.
Cancer. 115:1701–1712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cébe-Suarez S, Zehnder-Fjällman A and
Ballmer-Hofer K: The role of VEGF receptors in angiogenesis;
complex partnerships. Cell Mol Life Sci. 63:601–615. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rapisarda A and Melillo G: Role of the
VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res.
114:237–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith NR, Baker D, James NH, Ratcliffe K,
Jenkins M, Ashton SE, Sproat G, Swann R, Gray N, Ryan A, et al:
Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3
are localized primarily to the vasculature in human primary solid
cancers. Clin Cancer Res. 16:3548–3561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hiratsuka S, Nakamura K, Iwai S, Murakami
M, Itoh T, Kijima H, Shipley JM, Senior RM and Shibuya M: MMP9
induction by vascular endothelial growth factor receptor-1 is
involved in lung-specific metastasis. Cancer Cell. 2:289–300. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lenton K: VEGFR-2 (KDR/Flk-1). J Biol
Regul Homeost Agents. 16:227–232. 2002.PubMed/NCBI
|
|
10
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fontanini G, Bigini D, Vignati S, Basolo
F, Mussi A, Lucchi M, Chine S, Angeletti CA, Harris AL and
Bevilacqua G: Microvessel count predicts metastatic disease and
survival in non-small cell lung cancer. J Pathol. 177:57–63. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar P, Wang JM and Bernabeu C: CD 105
and angiogenesis. J Pathol. 178:363–366. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meert AP, Paesmans M, Martin B, Delmotte
P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C and Sculier JP:
The role of microvessel density on the survival of patients with
lung cancer: A systematic review of the literature with
meta-analysis. Br J Cancer. 87:694–701. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Volm M, Koomägi R and Mattern J:
Prognostic value of vascular endothelial growth factor and its
receptor Flt-1 in squamous cell lung cancer. Int J Cancer.
74:64–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Decaussin M, Sartelet H, Robert C, Moro D,
Claraz C, Brambilla C and Brambilla E: Expression of vascular
endothelial growth factor (VEGF) and its two receptors
(VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung
carcinomas (NSCLCs): Correlation with angiogenesis and survival. J
Pathol. 188:369–377. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Delmotte P, Martin B, Paesmans M,
Berghmans T, Mascaux C, Meert AP, Steels E, Verdebout JM, Lafitte
JJ and Sculier JP: VEGF and survival of patients with lung cancer:
A systematic literature review and meta-analysis. Rev Mal Respir.
19:577–584. 2002.(In French). PubMed/NCBI
|
|
17
|
Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, Lin
XQ, Yu LK and Song Y: Prognostic value of vascular endothelial
growth factor expression in patients with lung cancer: A systematic
review with meta-analysis. J Thorac Oncol. 4:1094–1103. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arinaga M, Noguchi T, Takeno S, Chujo M,
Miura T and Uchida Y: Clinical significance of vascular endothelial
growth factor C and vascular endothelial growth factor receptor 3
in patients with nonsmall cell lung carcinoma. Cancer. 97:457–464.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pajares MJ, Agorreta J, Larrayoz M, Vesin
A, Ezponda T, Zudaire I, Torre W, Lozano MD, Brambilla E, Brambilla
C, et al: Expression of tumor-derived vascular endothelial growth
factor and its receptors is associated with outcome in early
squamous cell carcinoma of the lung. J Clin Oncol. 30:1129–1136.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Macchiarini P, Fontanini G, Hardin MJ,
Squartini F and Angeletti CA: Relation of neovascularisation to
metastasis of non-small-cell lung cancer. Lancet. 340:145–146.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim ES, Herbst RS, Wistuba II, Lee JJ,
Blumenschein GR Jr, Tsao A, Stewart DJ, Hicks ME, Erasmus J Jr,
Gupta S, et al: The BATTLE trial: Personalizing therapy for lung
cancer. Cancer Discov. 1:44–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pastorino U, Andreola S, Tagliabue E,
Pezzella F, Incarbone M, Sozzi G, Buyse M, Menard S, Pierotti M and
Rilke F: Immunocytochemical markers in stage I lung cancer:
Relevance to prognosis. J Clin Oncol. 15:2858–2865. 1997.PubMed/NCBI
|
|
23
|
Zhao YY, Xue C, Jiang W, Zhao HY, Huang Y,
Feenstra K, Resau JH, Qian CN and Zhang L: Predictive value of
intratumoral microvascular density in patients with advanced
non-small cell lung cancer receiving chemotherapy plus bevacizumab.
J Thorac Oncol. 7:71–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borgia JA, Pithadia R, Ibrahem Z, Fhied C,
Basu S, Lie WR, Fidler MJ, Batus M and Bonomi PD: Potential
predictive value of hepatocyte growth factor (HGF) in advanced
non-small cell lung cancer (NSCLC) treated with a platinum doublet
and bevacizumab. J Clin Oncol. 32(Suppl): e220002014.
|
|
25
|
Batus M, Pithadia R, Kubasiak J, Fhied C,
Ibrahem Z, Melinamani S, Fughhi I, Lie WR, Basu S, Fidler MJ,
Bonomi PD and Borgia JA: Differences in circulating angiogenic
biomarkers as prognosticator for outcome in bevacizumab-treated
nonsquamous non-small cell lung cancer (NSCLC) patients. J Clin
Oncol. 32(5s Suppl): S110372014.
|
|
26
|
Pohl G, Krajnik G, Malayeri R, Müller RM,
Klepetko W, Eckersberger F, Schafer-Prokop C, Pokrajac B, Schmeikal
S, Maier A, et al: Induction chemotherapy with the TIP regimen
(paclitaxel/ifosfamide/cisplatin) in stage III non-small cell lung
cancer. Lung Cancer. 54:63–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kosmas C, Tsavaris NB, Polyzos A,
Kalofonos HP, Sepsas E, Malamos NA, Vadiaka M, Dosios T and
Antonopoulos MJ: A phase II study of
paclitaxel-ifosfamide-cisplatin combination in advanced nonsmall
cell lung carcinoma. Cancer. 89:774–782. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ripley RT and Rusch VW: Role of induction
therapy: Surgical resection of non-small cell lung cancer after
induction therapy. Thorac Surg Clin. 23:273–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Greene FL, Page DC, Fleming ID, et al:
AJCC Cancer staging manual (6th). Springer-Verlag. New York:
p4352002.
|
|
30
|
Common Terminology Criteria for Adverse
Events (CTCAE). Version 4.0. 2009.http://evs.nci.nih.gov/ftpl/CTCAE/CTCAE
_4.03_2010-06-14_QuickReference_8.5x11.pdfAccessed. May
28–2009
|
|
31
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trivella M, Pezzella F, Pastorino U,
Harris AL and Altman DG: Prognosis In Lung Cancer (PILC)
Collaborative Study Group: Microvessel density as a prognostic
factor in non-small-cell lung carcinoma: A meta-analysis of
individual patient data. Lancet Oncol. 8:488–499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horita N, Miyazawa N, Morita S, Kojima R,
Kimura N, Kaneko T and Ishigatsubo Y: Preoperative chemotherapy is
effective for stage III resectable non-small-cell lung cancer:
Metaanalysis of 16 trials. Clin Lung Cancer. 14:488–494. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Linderholm BK, Lidbrink E, Tallroth E,
Einbeigi Z, Svensson H, von Wachenfeldt A, Norberg B, Carlsson L,
Olsson ME, Bergh J, et al: Angiogenic factors in relation to
clinical effect in a phase II trial of weekly paclitaxel. Breast.
22:1142–1147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma J and Waxman DJ: Combination of
antiangiogenesis with chemotherapy for more effective cancer
treatment. Mol Cancer Ther. 7:3670–3684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amin DN, Hida K, Bielenberg DR and
Klagsbrun M: Tumor endothelial cells express epidermal growth
factor receptor (EGFR) but not ErbB3 and are responsive to EGF and
to EGFR kinase inhibitors. Cancer Res. 66:2173–2180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Crinò L and Metro G: Therapeutic options
targeting angiogenesis in nonsmall cell lung cancer. Eur Respir
Rev. 23:79–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chou TC, Motzer RJ, Tong Y and Bosl GJ:
Computerized quantitation of synergism and antagonism of taxol,
topotecan and cisplatin against human teratocarcinoma cell growth:
A rational approach to clinical protocol design. J Natl Cancer
Inst. 86:1517–1524. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kondagunta GV, Bacik J, Donadio A, Bajorin
D, Marion S, Sheinfeld J, Bosl GJ and Motzer RJ: Combination of
paclitaxel, ifosfamide and cisplatin is an effective second-line
therapy for patients with relapsed testicular germ cell tumors. J
Clin Oncol. 23:6549–6555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lissoni AA, Colombo N, Pellegrino A, Parma
G, Zola P, Katsaros D, Chiari S, Buda A, Landoni F, Peiretti M, et
al: A phase II, randomized trial of neo-adjuvant chemotherapy
comparing a three-drug combination of paclitaxel, ifosfamide and
cisplatin (TIP) versus paclitaxel and cisplatin (TP) followed by
radical surgery in patients with locally advanced squamous cell
cervical carcinoma: The Snap-02 Italian Collaborative Study. Ann
Oncol. 20:660–665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mountzios G, Dimopoulos MA, Bamias A,
Vourli G, Kalofonos H, Aravantinos G, Fountzilas G and
Papadimitriou CA: Randomized multicenter phase II trial of
cisplatin and ifosfamide with or without paclitaxel in recurrent or
metastatic carcinoma of the uterine cervix: A Hellenic Cooperative
Oncology Group (HeCOG) study. Ann Oncol. 20:1362–1368. 2009.
View Article : Google Scholar : PubMed/NCBI
|