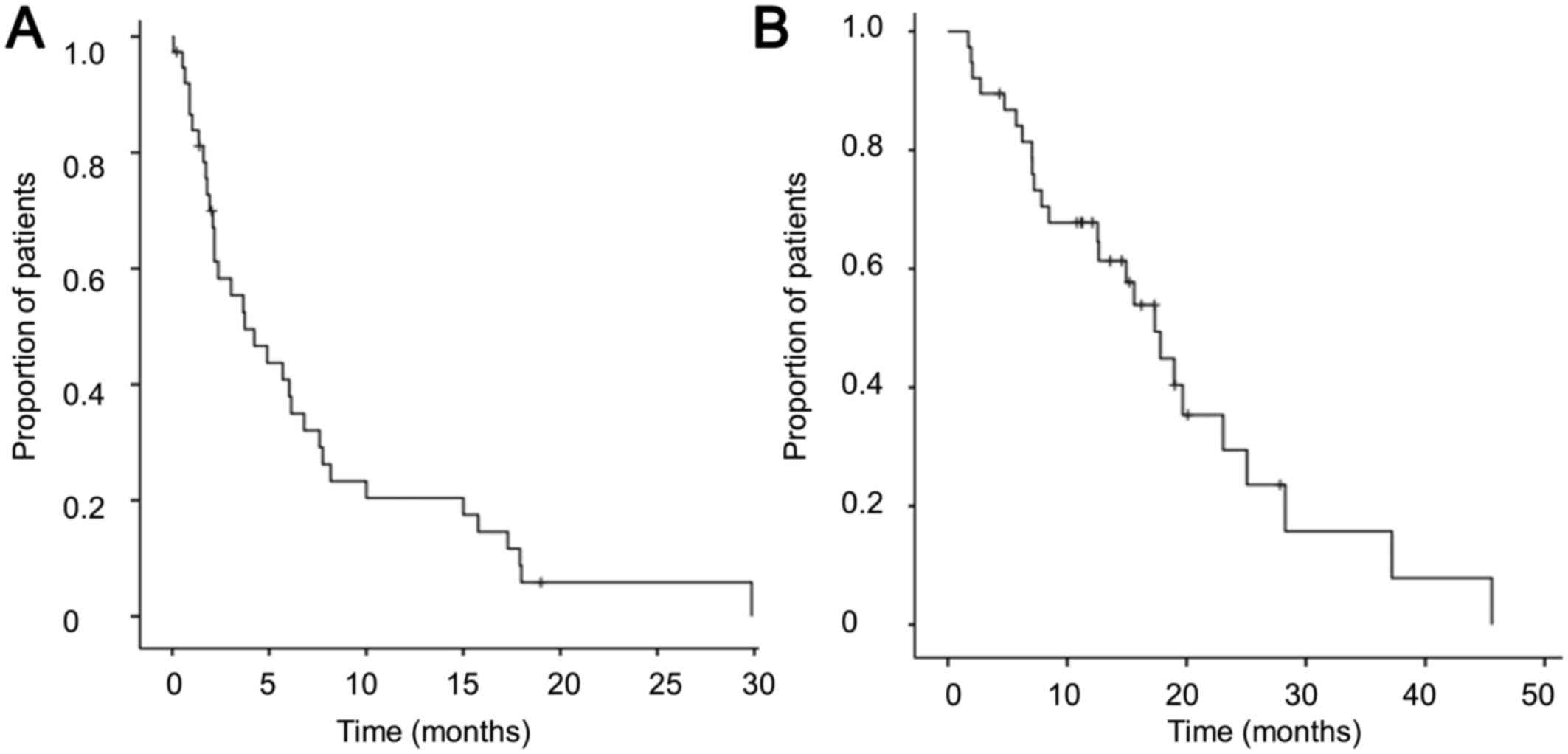
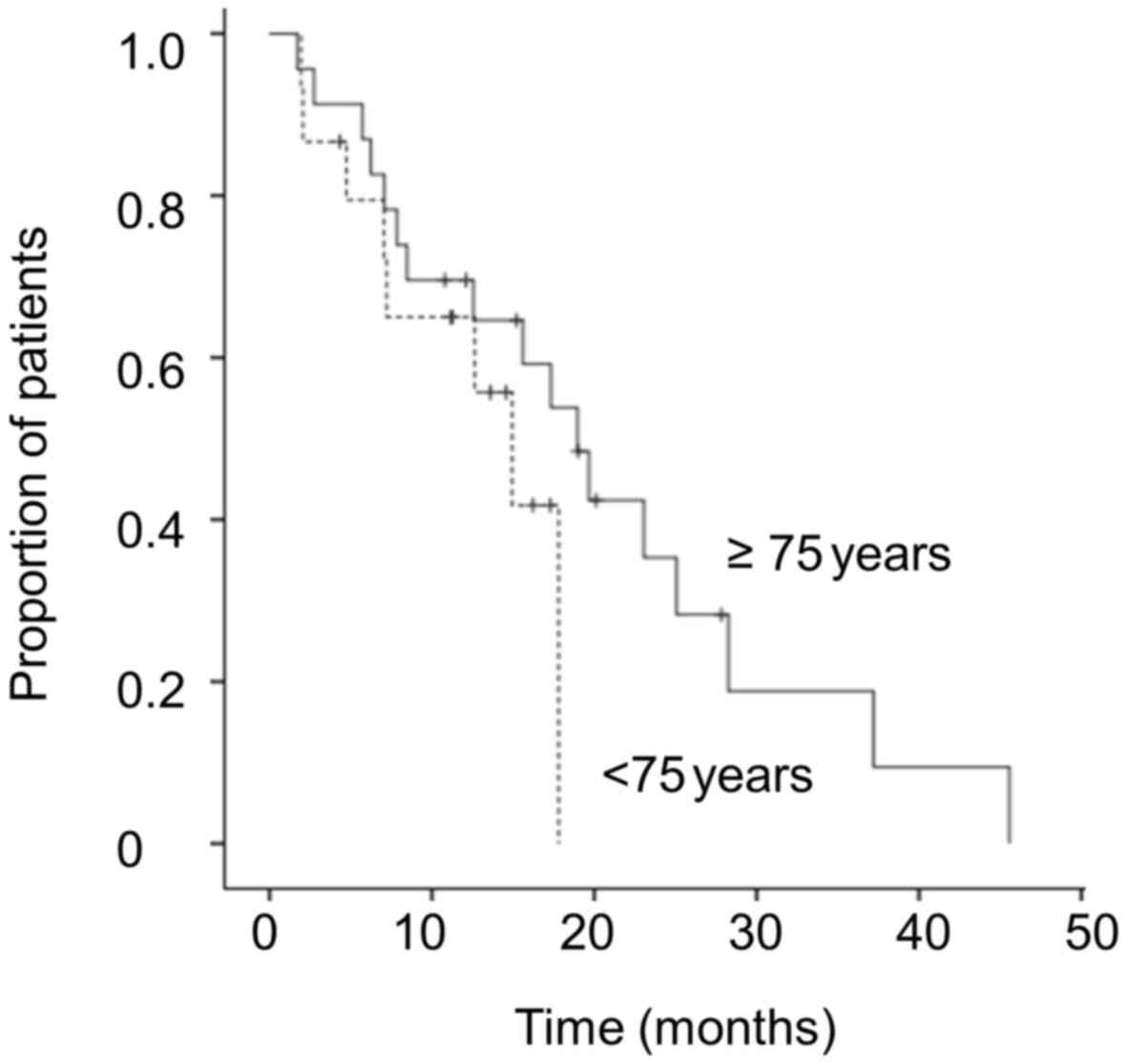
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shepherd FA, Pereira J Rodrigues, Ciuleanu
T, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D,
Maoleekoonpiroj S, Smylie M, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yasuda H, Park E, Yun CH, Sng NJ,
Lucena-Araujo AR, Yeo WL, Huberman MS, Cohen DW, Nakayama S,
Ishioka K, et al: Structural, biochemical and clinical
characterization of epidermal growth factor receptor (EGFR) exon 20
insertion mutations in lung cancer. Sci Transl Med. 5:216ra1772013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rossi D, Dennetta D, Ugolini M, Catalano
V, Alessandroni P, Giordani P, Baldelli AM, Casadei V, Graziano F
and Fedeli S Luzi: Activity and safety of erlotinib as second- and
third-line treatment in elderly patients with advanced non-small
cell lung cancer: A phase II trial. Target Oncol. 5:231–235. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kubota K, Nishiwaki Y, Tamura T, Nakagawa
K, Matsui K, Watanabe K, Hida T, Kawahara M, Katakami N, Takeda K,
et al: Efficacy and safety of erlotinib monotherapy for Japanese
patients with advanced non-small cell lung cancer: A phase II
study. J Thorac Oncol. 3:1439–1445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuura S, Inui N, Ozawa Y, Nakamura Y,
Toyoshima M, Yasuda K, Yamada T, Shirai T, Suganuma H, Yokomura K,
et al: Phase II study of erlotinib as third-line monotherapy in
patients with advanced non-small-cell lung cancer without epidermal
growth factor receptor mutations. Jpn J Clin Oncol. 41:959–963.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Imielinski M, Berger AH, Hammerman PS,
Hernandez B, Pugh TJ, Hodis E, Cho J, Suh J, Capelletti M,
Sivachenko A, et al: Mapping the hallmarks of lung adenocarcinoma
with massively parallel sequencing. Cell. 150:1107–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Serizawa M, Koh Y, Kenmotsu H, Isaka M,
Murakami H, Akamatsu H, Mori K, Abe M, Hayashi I, Taira T, et al:
Assessment of mutational profile of Japanese lung adenocarcinoma
patients by multitarget assays: A prospective, single-institute
study. Cancer. 120:1471–1481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naoki K, Soejima K, Okamoto H, Hamamoto J,
Hida N, Nakachi I, Yasuda H, Nakayama S, Yoda S, Satomi R, et al:
The PCR-invader method (structure-specific 5′ nuclease-based
method), a sensitive method for detecting EGFR gene mutations in
lung cancer specimens; comparison with direct sequencing. Int J
Clin Oncol. 16:335–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thakkar JP, McCarthy BJ and Villano JL:
Age-specific cancer incidence rates increase through the oldest age
groups. Am J Med Sci. 348:65–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ershler WB and Longo DL: Aging and cancer:
Issues of basic and clinical science. J Natl Cancer Inst.
89:1489–1497. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gridelli C and Shepherd FA: Chemotherapy
for elderly patients with non-small cell lung cancer: A review of
the evidence. Chest. 128:947–957. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jackman DM, Yeap BY, Lindeman NI, Fidias
P, Rabin MS, Temel J, Skarin AT, Meyerson M, Holmes AJ, Borras AM,
et al: Phase II clinical trial of chemotherapy-naive patients >
or = 70 years of age treated with erlotinib for advanced
non-small-cell lung cancer. J Clin Oncol. 25:760–766. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maruyama R, Nishiwaki Y, Tamura T,
Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F,
Eguchi K, et al: Phase III study, V-15–32, of gefitinib versus
docetaxel in previously treated Japanese patients with
non-small-cell lung cancer. J Clin Oncol. 26:4244–4252. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wheatley-Price P, Ding K, Seymour L, Clark
GM and Shepherd FA: Erlotinib for advanced non-small-cell lung
cancer in the elderly: An analysis of the national cancer institute
of canada clinical trials group study BR.21. J Clin Oncol.
26:2350–2357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takahashi T, Yamamoto N, Nukiwa T, Mori K,
Tsuboi M, Horai T, Masuda N, Eguchi K, Mitsudomi T, Yokota S, et
al: Phase II study of erlotinib in Japanese patients with advanced
non-small cell lung cancer. Anticancer Res. 30:557–563.
2010.PubMed/NCBI
|
|
22
|
Kurishima K, Satoh H, Kaburagi T,
Nishimura Y, Shinohara Y, Inagaki M, Endo T, Saito T, Hayashihara
K, Hizawa N, et al: Erlotinib for elderly patients with
non-small-cell lung cancer: Subset analysis from a population-based
observational study by the Ibaraki Thoracic Integrative (POSITIVE)
Research Group. Mol Clin Oncol. 1:828–832. 2013.PubMed/NCBI
|
|
23
|
Yoshioka H, Komuta K, Imamura F, Kudoh S,
Seki A and Fukuoka M: Efficacy and safety of erlotinib in elderly
patients in the phase IV POLARSTAR surveillance study of Japanese
patients with non-small-cell lung cancer. Lung Cancer. 86:201–206.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inoue Y, Inui N, Asada K, Karayama M,
Matsuda H, Yokomura K, Koshimizu N, Imokawa S, Yamada T, Shirai T,
et al: Phase II study of erlotinib in elderly patients with
non-small cell lung cancer harboring epidermal growth factor
receptor mutations. Cancer Chemother Pharmacol. 76:155–161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minemura H, Yokouchi H, Azuma K, Hirai K,
Sekine S, Oshima K, Kanazawa K, Tanino Y, Inokoshi Y, Ishii T, et
al: A phase II trial of erlotinib monotherapy for pretreated
elderly patients with advanced EGFR wild-type non-small cell lung
cancer. BMC Res Notes. 8:2202015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawaguchi T, Ando M, Asami K, Okano Y,
Fukuda M, Nakagawa H, Ibata H, Kozuki T, Endo T, Tamura A, et al:
Randomized phase III trial of erlotinib versus docetaxel as second-
or third-line therapy in patients with advanced non-small-cell lung
cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin
Oncol. 32:1902–1908. 2014. View Article : Google Scholar : PubMed/NCBI
|