Introduction
Patients with bone metastasis comprise the largest
group of patients receiving palliative radiation therapy (RT). RT
is a widely accepted and effective way to palliate pain caused by
bone metastases with few side effects (1). Local field RT has been used for
patients with symptomatic bone metastasis, and a variety of dose
fractionation regimens have been reported. Approximately 20% of
patients require additional treatments, including re-irradiation
(Re-RT) (2). Several studies have
demonstrated the beneficial effect of Re-RT on pain relief
(3–6); however, in cases of Re-RT, it is
important to consider the tolerance dose of the normal tissue. In
particular, the adverse events associated with Re-RT for bone
metastases have not yet been adequately evaluated (7). In addition, the overall survival (OS)
rate following Re-RT for bone metastasis remains unclear.
The present study aimed to assess the clinical
results in patients with spine and pelvic bone metastases following
Re-RT of the first irradiated site.
Patients and methods
A total of 103 patients with spine and pelvic bone
metastases who underwent Re-RT with intensity-modulated radiation
therapy (IMRT), three-dimensional conformal radiotherapy (3D-CRT),
or stereotactic body radiotherapy (SBRT) were identified in five
affiliated hospitals in Japan (Kyoto Prefectural University of
Medicine, Kyoto; Miyakojima iGRT Clinic, Osaka; Osaka Medical
Center for Cancer and Cardiovascular Diseases, Osaka; Uji Takeda
Hospital, Uji; Saiseikai Shigaken Hospital, Ritto) between April
2010 and March 2014. Of the 103 patients, five were excluded from
this study as they had skipped regular follow-up visits and their
data was missing. A retrospective analysis of the 98 patients who
underwent Re-RT for spine and pelvic bone metastases was conducted.
Re-RT was performed at the same location as the initial RT. In some
of these patients, the initial RT had been performed with
definitive intent or had not targeted the local relapse of spine or
pelvic bone lesions. The present study included those patients in
whom Re-RT was performed with curative intent (e.g.,
oligometastasis). All patients were informed regarding the risk of
Re-RT, particularly the risk of radiation-induced myelopathy, and
provided written informed consent. Patient characteristics are
summarized in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | No. of patients |
|---|
| Age, years |
|
| Median (range) | 61 (12–89) |
| Sex |
|
| Male | 68 |
|
Female | 30 |
| Performance
status |
|
| 0–1 | 56 |
| 2 | 29 |
| 3 | 13 |
| Primary tumor |
|
|
Lung/mediastinum | 17 |
|
Liver | 16 |
| Head and
neck | 8 |
|
Colorectal | 8 |
| Soft
tissue/bone | 7 |
|
Esophagus/stomach | 7 |
|
Uterus | 6 |
|
Breast | 5 |
|
Others | 27 |
| Follow-up period,
days |
|
| Median (range) | 256 (11–2,284) |
The choice of 3D-CRT, IMRT or SBRT was
non-randomized and primarily depended on the availability of the
Linac machines or the reference for the technique by the individual
physician. The time interval between RT episodes was defined as the
time between the end of the initial RT and the start of Re-RT. OS
was defined as the time from the end of Re-RT until the date of
mortality or final follow-up of the patient. The treatment
characteristics are summarized in Table
II.
 | Table II.Treatment characteristics. |
Table II.
Treatment characteristics.
| Characteristics | N |
|---|
| Site of
re-irradiation |
|
|
Cervical | 17 |
|
Thoracic | 43 |
|
Lumber | 22 |
|
Pelvis | 15 |
| Initial dose, Gy |
|
| Total
dose, median (range) | 39 (9–70) |
| Fraction,
median (range) | 13 (1–32) |
| BED2,
median (range) | 73 (18–174) |
| BED10,
median (range) | 48 (12–101) |
| Re-irradiated dose,
Gy |
|
| Total
dose, median (range) | 48.4 (4–65) |
| Fraction,
median (range) | 10 (1–25) |
| BED2,
median (range) | 150 (17–240) |
| BED10,
median (range) | 75 (10–89) |
| Cumulated dose,
Gy |
|
| BED2,
median (range) | 247 (57–354) |
| BED10,
median (range) | 117 (29–185) |
| Interval, days |
|
| Median
(range) | 439 (23–4,993) |
| Radiation
technique |
|
| IMRT | 82 |
|
3D-CRT | 15 |
|
SBRT | 1 |
Acute and late adverse events following Re-RT were
scored according to the Common Terminology Criteria of Adverse
Events, version 4.0 (8). Acute
adverse events were evaluated as those arising <2 months after
the first day of Re-RT, and late adverse events were evaluated as
those arising ≥2 months after the first day of Re-RT. Newly
developed neurological deficits following Re-RT were defined as
late adverse events in this study. The biologically effective dose
(BED) was calculated to assess the cumulative RT dose from the
initial RT and Re-RT, as the fractionation schemas used for the
initial RT and Re-RT were varied. The BED was calculated using the
following equation: BED = D × [1 + d/(α/β)], as
derived from the linear-quadratic model, where D = the total
dose and d = dose per fraction. We adopted 10 Gy as the α/β
ratio for the acute toxicities and 2 Gy for the late
toxicities.
Statistical analysis
Data for continuous variables are presented as
median (range). All statistical analyses were performed using
StatView 5.0 statistical software (SAS Institute, Inc., Cary, NC,
USA). Survival data were estimated by using the Kaplan-Meier method
and examined for significance using the log-rank test. Cut-off
values were set as the average or median value of each variable
unless otherwise stated. Coxs proportional hazard model was used
for the multivariate analysis. For all analyses, P<0.05 was
considered to indicate a statistically significant difference.
Results
Follow-up and survival
The median follow-up period was 256 days (range,
11–2,284 days) for all eligible patients. At the final follow-up,
41 (42%) patients were alive and 57 (58%)patients had died. The
median interval from the initial RT to Re-RT was 439 days (range,
23–4,993 days). The Eastern Cooperative Oncology Group performance
status (PS) of 56 patients (57%) was 0 or 1. Overlapping sites of
the initial RT and Re-RT were as follows: Cervical spine in 16
patients, thoracic spine in 43 patients, lumbar spine in 22
patients and pelvic bone in 17 patients. The median BEDs for the
initial RT and Re-RT were 73 Gy2 (range, 18–174
Gy2; α/β = 2) and 150 Gy2 (range, 17–240
Gy2; α/β = 2), respectively. The median cumulative BED
from the initial RT and Re-RT was 247 Gy2 (range, 57–354
Gy2; α/β = 2). The fractionated schemas were determined
according to the condition of the patient, site of Re-RT and the
discretion of the radiation oncologists at their hospital. The
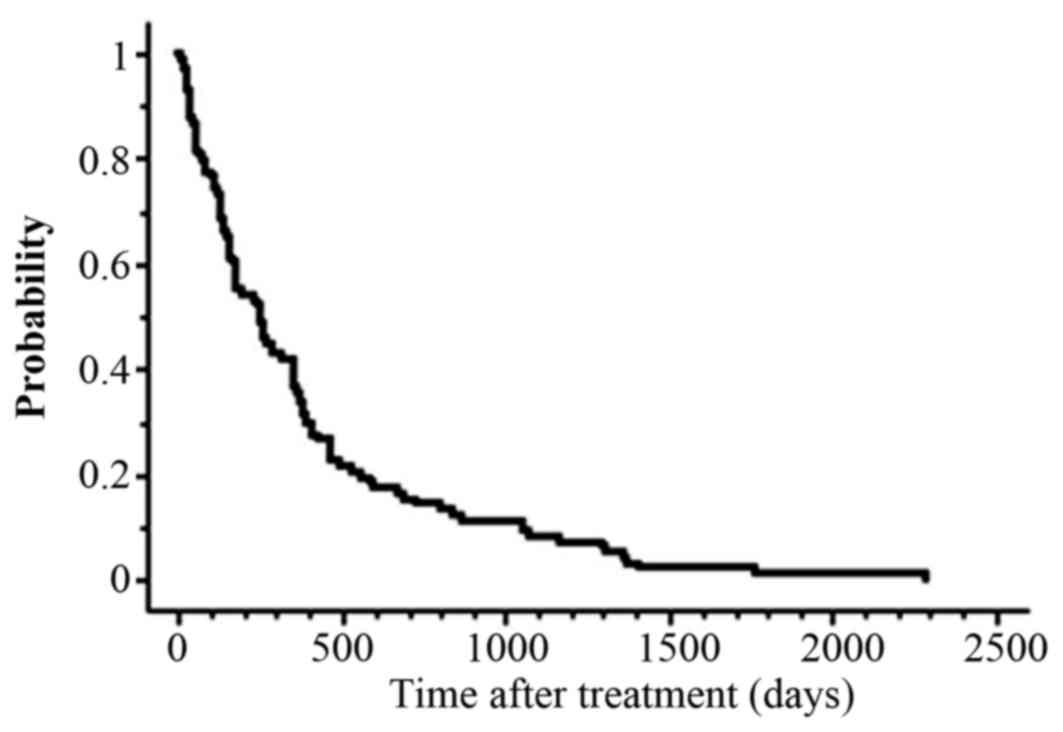
median survival time (MST) after Re-RT for the entire series was
255 days, and the actuarial OS rates at 1 year were 36% (Fig. 1).
Associations between survival and
clinical factors
Table III presents
the univariate and multivariate analysis of various potential
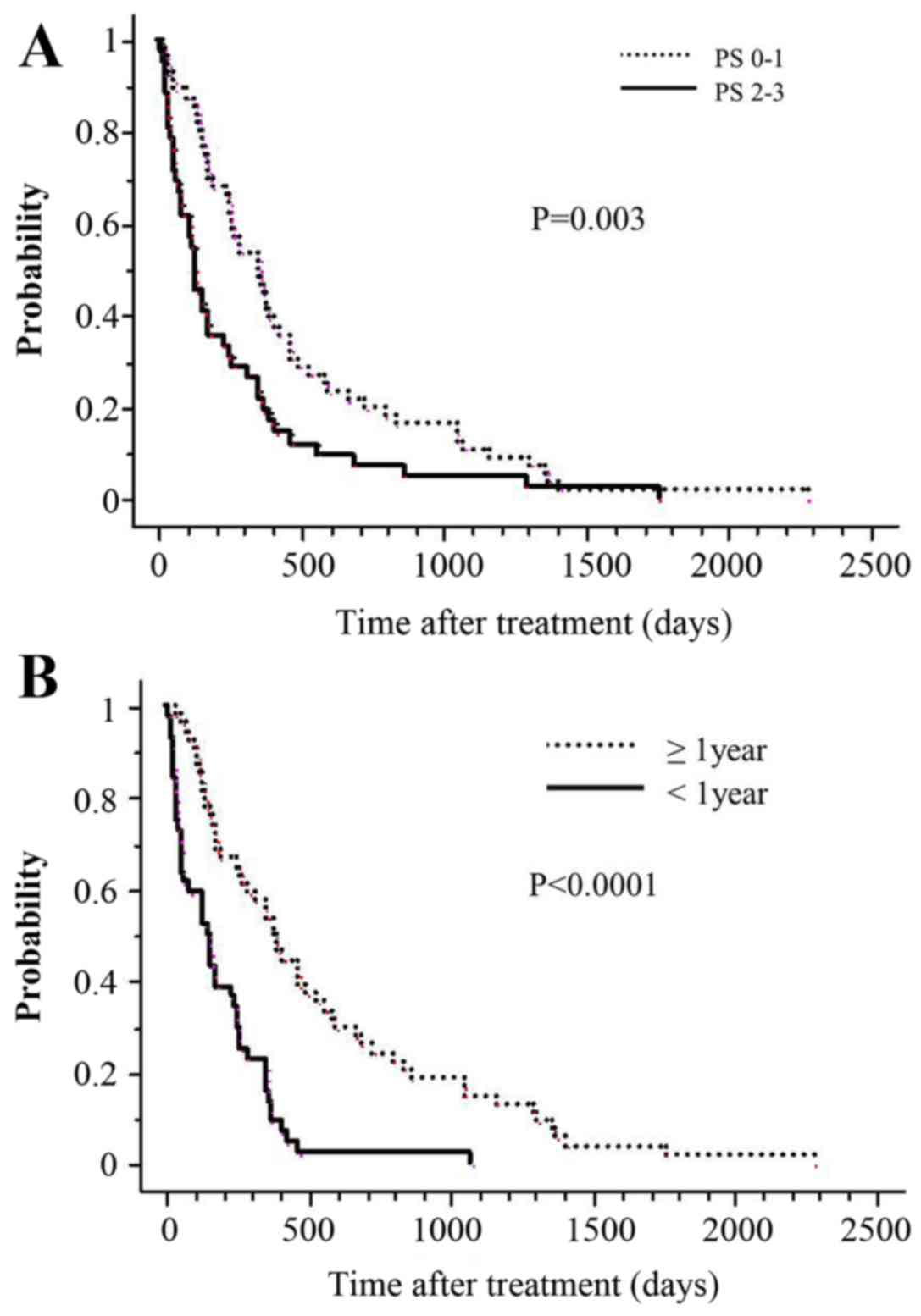
prognostic factors for OS rates. Univariate analysis revealed that
compared with patients with a PS of 2–3, patients with a PS of 0–1
had statistically significantly higher 1-year actuarial survival
rates (P=0.003; Fig. 2A). Similarly,
when the interval between the two irradiations was >1 year,
actuarial survival at 1 year was significantly higher (P<0.0001;
Fig. 2B). Additionally, PS (P=0.03)
and the interval between the two irradiations (P<0.0001)
remained significant independent prognostic factors for OS rates in
the multivariate analysis. A BED of 60 Gy10 means a
biologically equivalent dose of 50 Gy in 2 Gy fractions with an α/β
ratio of 10. A cut-off value of a BED at 60 Gy10 as the
curative irradiated dose was set; however, a BED of 60
Gy10 did not have a significant effect on OS in the
current study. In the multivariate analysis, PS (relative risk,
1.624; 95% confidence interval, 1.047–2.520; P=0.03) and the
interval between initial RT and Re-RT (relative risk, 0.341; 95%
confidence interval, 0.217–0.536; P<0.0001) remained significant
independent prognostic factors for OS.
 | Table III.Univariate and multivariate analysis
of various potential prognostic factors for overall survival. |
Table III.
Univariate and multivariate analysis
of various potential prognostic factors for overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | No. of
patients | OS, 1-year rate
(%) | P-value | P-value |
|---|
| Age, years |
|
|
|
|
|
<60 | 48 | 42 |
|
|
|
≥60 | 50 | 28 |
0.23 | n.s. |
| Sex |
|
|
|
|
|
Male | 65 | 33 |
|
|
|
Female | 33 | 33 |
0.66 | n.s. |
| Performance
status |
|
|
|
|
|
0–1 | 56 | 46 |
|
|
|
2–3 | 42 | 19 | 0.003 | 0.03 |
| RT technique |
|
|
|
|
|
IMRT/SBRT | 83 | 41 |
|
|
|
3D-CRT | 15 | 0 |
0.08 | n.s. |
| BED10 |
|
|
|
|
| <60
Gy10 | 39 | 26 |
|
|
| ≥60
Gy10 | 59 | 41 |
0.29 | n.s. |
| Interval between
two RT |
|
|
|
|
|
<1-year | 44 | 14 |
|
|
|
≥1-year | 54 | 50 | <0.0001 | <0.0001 |
Toxicities
There were no cases with acute adverse events of
grade ≥3. The incidence and severity of late adverse events were
evaluated in the 82 patients who survived for >2 months after
the onset of Re-RT. At the time of analysis, two cases developed
grade 3–4 late adverse events, and the incidence of grade ≥3 events
was 2% (2/82).
Representative cases
Here, two representative cases in the current cohort
are discussed. The first case was a 45-year-old female with lumbar
spine metastasis from cervical cancer, who received 3D-CRT with 6
Gy in a single fraction and suffered from grade 3 ileus, 6 months
after Re-RT. The Re-RT BED was 24 Gy2, and the
cumulative BED was 120 Gy2. The interval between initial
RT and Re-RT was 6 months. The causal association between Re-RT and
the occurrence of ileus was unclear, as this case also had
peritoneal dissemination. The second case was a 67-year-old male
with a bulky sacral metastasis from hepatocellular carcinoma, who
received 3D-CRT of 20 Gy in four fractions once daily. He suffered
from grade 4 injury to the cauda equina 2 months after the Re-RT.
The Re-RT BED was 70 Gy2, and the cumulative BED was 168
Gy2. The interval between the initial RT and Re-RT was 7
months. None of the cases treated with IMRT experienced grade ≥3
toxicity.
Discussion
The current study performed a retrospective
multi-institutional analysis of patients treated with Re-RT for
metastatic disease in the spine or pelvic bone. The results
demonstrated that Re-RT performed for spine or pelvic bone
metastases was associated with a comparatively acceptable rate of
severe adverse events. The most important adverse effect of Re-RT
was radiation-induced myelopathy or radiation-induced injury to the
cauda equina. In the present study, one patient treated with 3D-CRT
was suspected to have injury to the cauda equina; however, it is
not completely certain that this particular case developed
radiation-induced injury to the cauda equinal as there are no
clinical features that are able to differentiate between
radiation-induced injury and compressive injury with certainty.
Furthermore, in this case, follow-up studies (e.g., MRI, PET or CT)
were not able to be conducted due to the patients extremely poor
general condition.
Regarding late toxicities associated with Re-RT for
spine metastases, the criteria of Nieder et al (9,10) prove
to be useful indices for reference in evaluating the safety of
Re-RT. These indices suggested that the risk of radiation
myelopathy caused by Re-RT was low when the cumulative BED from the
initial RT and Re-RT was <135.5 Gy2 and the interval
between the initial RT and Re-RT was not <6 months. The risk was
low when the BED of each course was not >98 Gy2
(9,10). Although the patient was suspected to
have radiation-induced injury to the cauda equina, the cumulative
BED2 for both treatments was 124 Gy2, and the interval
between the initial RT and Re-RT was 6 months. The initial BED was
75 Gy2, and the second was 49 Gy2. The onset
of signs and symptoms was seen 2 months after Re-RT. During the
follow-up, no severe toxicities, including radiation-induced
myelopathy, occurred for any of the patients treated with IMRT;
therefore, it was inferred that the cumulative doses to the spinal
cord and cauda equina were within the permissible range as given in
published reports (9,10). IMRT can be relatively more flexible
in designing a dose distribution fitting a lesion with a concave
shape than 3D-CRT. This distinction may be useful in the
irradiation of spine or pelvic bone metastases, particularly for
Re-RT. Kawashiro et al (11)
reported clinical IMRT results of Re-RT for spinal metastasis. They
concluded that spinal metastasis Re-RT using IMRT was safe and that
pain relief and paresis improvement and/or prevention may be
expected along with a reduced risk of radiation-induced toxicity,
especially in the spinal cord (11).
In addition, Jereczek-Fossa et al (12) reported that the low toxicity of Re-RT
with IMRT or SBRT should allow for delivery of higher doses and
lead to improvement in Re-RT outcomes.
The present study found a favorable 1-year OS rate
of 36%, and the MST following Re-RT for the entire series was 255
days (range, 7–2,285 days). Hayashi et al (3) found that the MST after Re-RT was of 4
months. Similarly, Hernanz et al (13) identified that the MST following Re-RT
was of 3 months. Additionally, they noted that the survival was
significantly longer when the interval between initial RT and Re-RT
was >1 year (13). It must be
noted that the results of the present study are consistent with
those of this previous report. Other factors, such as Karnofsky
Performance Status prior to treatment or the administered BED were
not significantly associated with survival in their study (13). In the present study, multivariate
analysis revealed that not only the intervals between initial RT
and Re-RT but also PS at the time of Re-RT were observed as
statistically significant prognostic factors. The finding of PS as
a prognostic factor in the current study is consistent with certain
previous reports (14,15).
Regarding pain improvement, the present study was
not able to quantitatively determine the degree of improvement, as
the pain was not objectively recorded prior to and following Re-RT
in the majority of the patients. Furthermore, details of medication
were not reliably collected to determine whether the subjectively
documented pain response was confounded by the use of analgesics
and varying supportive care. For the same reason, mild toxicities
of grade ≤2 were also difficult to evaluate in the current study.
These issues are considered to be limitations of the study.
Documentation of severe toxicities is more reliable despite the
retrospective nature of the study given the permanent nature of the
event. Most importantly, we experienced two cases with severe late
toxicities of grade ≥3: One case of grade 3 ileus and one case of
radiation-induced injury to the cauda equina, both of which were
treated with 3D-CRT.
In recent years, as a direct result of progress in
anticancer therapy, the survival rate of patients with distant
metastatic disease has been improving. With this increasing rate of
survival, there is an increasing requirement for Re-RT in these
patients. Wong et al (16)
reported that latent times for myelopathy following a single course
of treatment (mean, 18.5 months; range, 7–57 months) were
significantly longer than those following Re-RT (mean, 11.4 months;
range, 4–25 months). Re-RT using IMRT or SBRT, which is able to
achieve a greater dose prescription than 3D-CRT, for metastatic
spine or pelvic bone may provide the possibility of lasting tumor
control, as well as prevention of radiation myelopathy and
radiation-induced injury to the cauda equina.
In conclusion, the present data suggest that Re-RT
using IMRT or SBRT is safer than 3D-CRT. In particular, patients
with a long interval from initial radiation and good PS (0–1) may
survive long enough to benefit from local intensive RT, such as
IMRT or SBRT.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Glossary
Abbreviations
Abbreviations:
|
BED
|
biologically effective dose
|
|
MST
|
median survival time
|
|
OS
|
overall survival
|
|
PS
|
performance status
|
|
RT
|
radiation therapy
|
|
Re-RT
|
re-irradiation
|
|
IMRT
|
intensity-modulated radiation
therapy
|
|
SBRT
|
stereotactic body radiotherapy
|
|
3D-CRT
|
three-dimensional conformal
radiotherapy
|
References
|
1
|
Lutz S, Berk L, Chang E, Chow E, Hahn C,
Hoskin P, Howell D, Konski A, Kachnic L, Lo S, et al: American
Society for Radiation Oncology (ASTRO): Palliative radiotherapy for
bone metastases: an ASTRO evidence-based guideline. Int J Radiat
Oncol Biol Phys. 79:965–976. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huisman M, van den Bosch MA, Wijlemans JW,
van Vulpen M, van der Linden YM and Verkooijen HM: Effectiveness of
reirradiation for painful bone metastases: a systematic review and
meta-analysis. Int J Radiat Oncol Biol Phys. 84:8–14. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayashi S, Hoshi H and Iida T:
Reirradiation with local-field radiotherapy for painful bone
metastases. Radiat Med. 20:231–236. 2002.PubMed/NCBI
|
|
4
|
Jeremic B, Shibamoto Y and Igrutinovic I:
Single 4 Gy re-irradiation for painful bone metastasis following
single fraction radiotherapy. Radiother Oncol. 52:123–127. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mithal NP, Needham PR and Hoskin PJ:
Retreatment with radiotherapy for painful bone metastases. Int J
Radiat Oncol Biol Phys. 29:1011–1014. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Linden YM, Lok JJ, Steenland E,
Martijn H, van Houwelingen H, Marijnen CA and Leer JW; Dutch Bone
Metastasis Study Group, : Single fraction radiotherapy is
efficacious: a further analysis of the Dutch Bone Metastasis Study
controlling for the influence of retreatment. Int J Radiat Oncol
Biol Phys. 59:528–537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong E, Hoskin P, Bedard G, Poon M, Zeng
L, Lam H, Vulpe H, Tsao M, Pulenzas N and Chow E: Re-irradiation
for painful bone metastases - a systematic review. Radiother Oncol.
110:61–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events (CTCAE) version 4.0.
https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40January
8–2016
|
|
9
|
Nieder C, Grosu AL, Andratschke NH and
Molls M: Proposal of human spinal cord reirradiation dose based on
collection of data from 40 patients. Int J Radiat Oncol Biol Phys.
61:851–855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nieder C, Grosu AL, Andratschke NH and
Molls M: Update of human spinal cord reirradiation tolerance based
on additional data from 38 patients. Int J Radiat Oncol Biol Phys.
66:1446–1449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawashiro S, Harada H, Katagiri H, Asakura
H, Ogawa H, Onoe T, Sumita K, Murayama S, Murata H, Nemoto K, et
al: Reirradiation of spinal metastases with intensity-modulated
radiation therapy: an analysis of 23 patients. J Radiat Res
(Tokyo). 57:150–156. 2016. View Article : Google Scholar
|
|
12
|
Jereczek-Fossa BA, Kowalczyk A, DOnofrio
A, Catalano G, Garibaldi C, Boboc G, Vitolo V, Leonardi MC, Cambria
R and Orecchia R: Three-dimensional conformal or stereotactic
reirradiation of recurrent, metastatic or new primary tumors.
Analysis of 108 patients. Strahlenther Onkol. 184:36–40. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernanz R, Montero A, Fernandez-Lizarbe E,
Polo A and Ramos A: Retreatment with radiotherapy for symptomatic
bone, brain or visceral metastases. Clin Transl Oncol. 15:72–78.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hashmi A, Guckenberger M, Kersh R,
Gerszten PC, Mantel F, Grills IS, Flickinger JC, Shin JH, Fahim DK,
Winey B, et al: Re-irradiation stereotactic body radiotherapy for
spinal metastases: a multi-institutional outcome analysis. J
Neurosurg Spine. 25:646–653. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Damast S, Wright J, Bilsky M, Hsu M, Zhang
Z, Lovelock M, Cox B, Zatcky J and Yamada Y: Impact of dose on
local failure rates after image-guided reirradiation of recurrent
paraspinal metastases. Int J Radiat Oncol Biol Phys. 81:819–826.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong CS, van Dyk J, Milosevic M and
Laperriere NJ: Radiation myelopathy following single courses of
radiotherapy and retreatment. Int J Radiat Oncol Biol Phys.
30:575–581. 1994. View Article : Google Scholar : PubMed/NCBI
|