Introduction
Recent improvements in living standards, changes in
dietary habits and ingredients and the increase in the aging
population have been associated with increases in the incidence and
mortality of colorectal cancer (CRC). Indeed, distant metastases
may be detected in a proportion of newly diagnosed patients, and
recurrent or distant metastases have been observed despite radical
surgery or postoperative adjuvant therapy. The overall survival
(OS) of patients with metastatic CRC (mCRC) is improved by
resection of the metastases, with 5-year survival rates of up to
50% following surgery (1), whereas
the survival rates remain low (<5%) among patients with
unresectable mCRC (2). However, the
majority of mCRC patients are considered to be unresectable at
diagnosis (3). Although previous
studies have demonstrated that preoperative chemotherapy, or
chemotherapy combined with targeted agents, may convert initially
unresectable CRC metastases to resectable (3,4), certain
patients remain unresectable due to extensive extrahepatic disease
or insufficient remaining liver parenchyma or multi-organ
metastases.
For unresectable mCRC patients, a combination of
chemotherapy and a molecular-targeted agent, either antiangiogenic
(i.e., bevacizumab) or a monoclonal antibody epidermal growth
factor receptor inhibitor (e.g., cetuximab and panitumumab), is
currently considered to be the standard therapy. The CALGB 80405
trial enrolled >1,100 wild-type KRAS cases who were randomly
assigned to group chemotherapy (either FOLFOX or FOLFIRI) plus
either cetuximab or bevacizumab. In the all-RAS wild-type
population, the median progression-free survival (PFS) was ~11
months in both arms [hazard ratio (HR)=1.1; P=0.31), with a median
OS of 31–32 months (HR=0.9; P=0.40) (5), which represented a significant
improvement. However, in China, these targeted drugs are not
included in the health insurance directory, and a proportion of the
patients harbor mutations in the RAS or BRAF genes, affecting the
application and efficacy of these treatments, as the OS was only
16–22 months for either FOLFOX or FOLFIRI without combination with
a molecular-targeted agent (6).
Consequently, the rational use of limited resources and the
appropriate combination of different treatment modalities to reduce
treatment cost, improve the quality of life and prolong survival,
is a major focus among Chinese oncologists. Recent oncology studies
have reported satisfactory results with local therapy, such as
microwave ablation, radioactive seed implantation and
intensity-modulated conformal radiotherapy. However, only a limited
number of studies have addressed the optimal administration of
systemic chemotherapy combined with these local treatments.
The aim of the present retrospective study was to
investigate the strategy of the combination of systemic
chemotherapy with local treatments to improve the OS of patients
with initial unresectable mCRC without using molecular-targeted
agents.
Patients and methods
Patient selection
Between April, 2007 and October, 2013, 273
consecutive patients with biopsy-proven stage IV CRC were treated
at the Third Affiliated Hospital of Soochow University (Changzhou,
China). Patients who only received systemic chemotherapy or local
treatments, or received molecular-targeted agents, were excluded.
Patients who exhibited progressive disease (PD) as defined by the
Response Evaluation Criteria in Solid Tumors (RECIST 1.1) (7) after two cycles of chemotherapy were
also excluded. Finally, 51 patients who received systemic
chemotherapy and local treatment were retrospectively analyzed. The
patients were aged 35–75 years, had a World Health Organization
performance status of ≤2, and were no longer considered candidates
for surgery. The characteristics of the patients are summarized in
Table I.
 | Table I.Patient characteristics (n=51). |
Table I.
Patient characteristics (n=51).
| Characteristics | n | (%) |
|---|
| Gender |
|
|
| Male | 40 | (78.4) |
|
Female | 11 | (21.6) |
| Median age, years
(range) | 62 (35–75) |
|
| Location of primary
cancer |
|
|
|
Rectum | 21 | (41.2) |
|
Colon | 30 | (59.8) |
| Resection of primary
cancer |
|
|
| Yes | 42 | (82.4) |
| No | 9 | (17.6) |
| Location of
metastasis |
|
|
|
Livera | 14 | (27.5) |
|
Lungs | 7 | (13.7) |
| >3
locations | 30 | (58.8) |
| Line of
chemotherapy |
|
|
|
First-line | 48 | (94.1) |
| Second-
and further line | 3 | (5.9) |
This retrospective study was conducted in accordance
with the Declaration of Helsinki including all amendments, and was
approved by the Independent Research Ethics Committee of the Third
Affiliated Hospital of Soochow University. Written informed consent
was obtained from all the patients prior to treatment.
Overall treatment principal
Systemic chemotherapy was applied as the initial
treatment. For patients with several (>3) or larger (>5 cm)
liver metastases, continuous infusion of floxuridine (FUDR) into
the hepatic artery was added. Two or three cycles after the initial
chemotherapy, the results of the enhanced spiral-computed
tomography (CT) were reviewed. If the disease was controlled and
the primary tumor was resectable, surgical removal of the primary
tumor was recommended. If the metastatic tumors were reduced to a
size of <5 cm, microwave or radiofrequency ablation were first
considered. If the metastases were not suitable for ablation,
radioactive seed implantation was recommended. In general, external
radiation therapy was administered for pelvic, bone and brain
metastases. Once the patients achieved tumor-free survival (TFS),
an additional four or six cycles of the original chemotherapy
regimen were administered to solidify the effect. Follow-up,
including a CT scan and assessment of tumor marker levels, was
performed every 2–3 months up to the time of progression. If the
patients did not achieve TFS, systemic chemotherapy was
administered.
Systemic chemotherapy
A total of 40 patients received an intravenous
infusion of 5-fluorouracil (5-FU) and leucovorin with oxaliplatin
(FOLFOX) as first-line treatment, 8 patients received capecitabine
with oxaliplatin (XELOX), 2 patients received irinotecan with
capecitabine (XELIRI), and 1 patient received an intravenous
infusion of 5-FU and leucovorin with irinotecan (FOLFIRI). The
FOLFOX regimen included i.v. leucovorin calcium at a dose of 200
mg/m2, bolus 5-FU at a dose of 400 mg/m2 and continuous i.v. 5-FU
at a dose of 600 mg/m2 on day 1, followed by 85 mg/m2 oxaliplatin
on day 2. The cycle was repeated every 2 weeks. XELOX consisted of
a 2-h intravenous infusion of oxaliplatin 130 mg/m2 on day 1 plus
oral administration of capecitabine 1,000 mg/m2 twice daily for 14
days and was repeated every 21 days. XELIRI consisted of a 2-h
intravenous infusion of irinotecan 180 mg/m2 on day 1 plus oral
administration of capecitabine 1,000 mg/m2 twice daily for 14 days,
and was repeated every 21 days. FOLFIRI included i.v. leucovorin
calcium at a dose of 200 mg/m2, bolus 5-FU at a dose of 400 mg/m2
and continuous i.v. 5-FU at a dose of 600 mg/m2 on day 1, followed
by 180 mg/m2 irinotecan on day 2, and was repeated every 2 weeks. A
total of 29 patients received FOLFIRI as a second-line regimen, 3
patients received FOLFOX, 3 patients did not undergo second-line
chemotherapy as they succumbed to the disease, and second-line
chemotherapy was not administered to 16 patients as they did not
exhibit disease progression after first-line chemotherapy and local
treatments at the last follow-up visit.
Local treatment
A total of 17 patients with unresectable liver
metastases underwent implantation using a hepatic artery kit and
received hepatic arterial infusion (HAI) therapy. FUDR was
delivered via a 14-day infusion at 0.15 mg/kg/day, and
dexamethasone (DXM) was delivered at 1 mg/m2/day using a pump with
FUDR heparin and saline. A total of 38 patients received microwave
ablation focused on the liver 49 times, the lung 15 times, and the
spleen once, if the metastases were sized <5 cm. A total of 29
patients received radioactive 125I seed implantation, in which the
liver was targeted 14 times, the lung 20 times, the pelvis 9 times,
the adrenal glands 4 times, the retroperitoneal lymph nodes once
and the abdomen once, when the metastases were not suitable for
microwave ablation. A total of 12 patients received external
radiation therapy focused on the pelvis 7 times, the bone 4 times,
the brain 3 times and the adrenal glands once. The required
instruments, materials and the protocols for local treatments were
as described in our previous articles (8–11).
Clinical evaluation and follow-up
According to RECIST 1.1, the response to
chemotherapy was classified as complete remission (CR), partial
remission (PR), progressive disease (PD), or stable disease (SD).
Two or three cycles after the initial chemotherapy, enhanced spiral
CT results were reviewed, followed by CT review every three cycles.
The final follow-up was conducted on April 1, 2016. Dose
adjustments were performed in the event of toxicity and assessed
according to the National Cancer Institute-Common Terminology
Criteria for Adverse Events (NCI-CTCAE), version 3.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf).
TFS was defined as the time from non-detection of tumors on CT
after the treatments, until the first discovery of local tumor
recurrence or distant metastasis, or until the date of the last
follow-up. Overall survival (OS) was defined as the time from the
diagnosis of unresectable mCRC to the date of death due to any
cause or the date of the last follow-up.
Statistical analysis
The survival rates were estimated using the life
table method. The survival analysis was performed by applying the
log-rank test. The associations of different potential predictive
factors with TFS were assessed using the Fisher's exact test for
categorical variables or the Wilcoxon rank-sum test for continuous
variables. The statistical analysis was performed using SPSS
software, version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Remission rates and survival time
According to RECIST 1.1, among the 51 patients who
were evaluated, after two or three cycles of initial chemotherapy,
0 patients achieved CR, 36 achieved PR (70.6%) and 15 achieved SD
(29.4%), with an objective response rate of 100%. Of the 9 patients
with newly diagnosed and unresectable primary tumors, 8 underwent
primary tumor resection after treatment, and the resection rate was
88.9%. At the last follow-up, 34 of the 51 patients had succumbed
to the disease and 17 remained alive. The median OS was 40 months
(range, 12–108 months). With the combination of minimally invasive
treatments with systemic chemotherapy, 39 patients obtained TFS.
Among those 39 patients, the TFS was 2–45 months, and the median
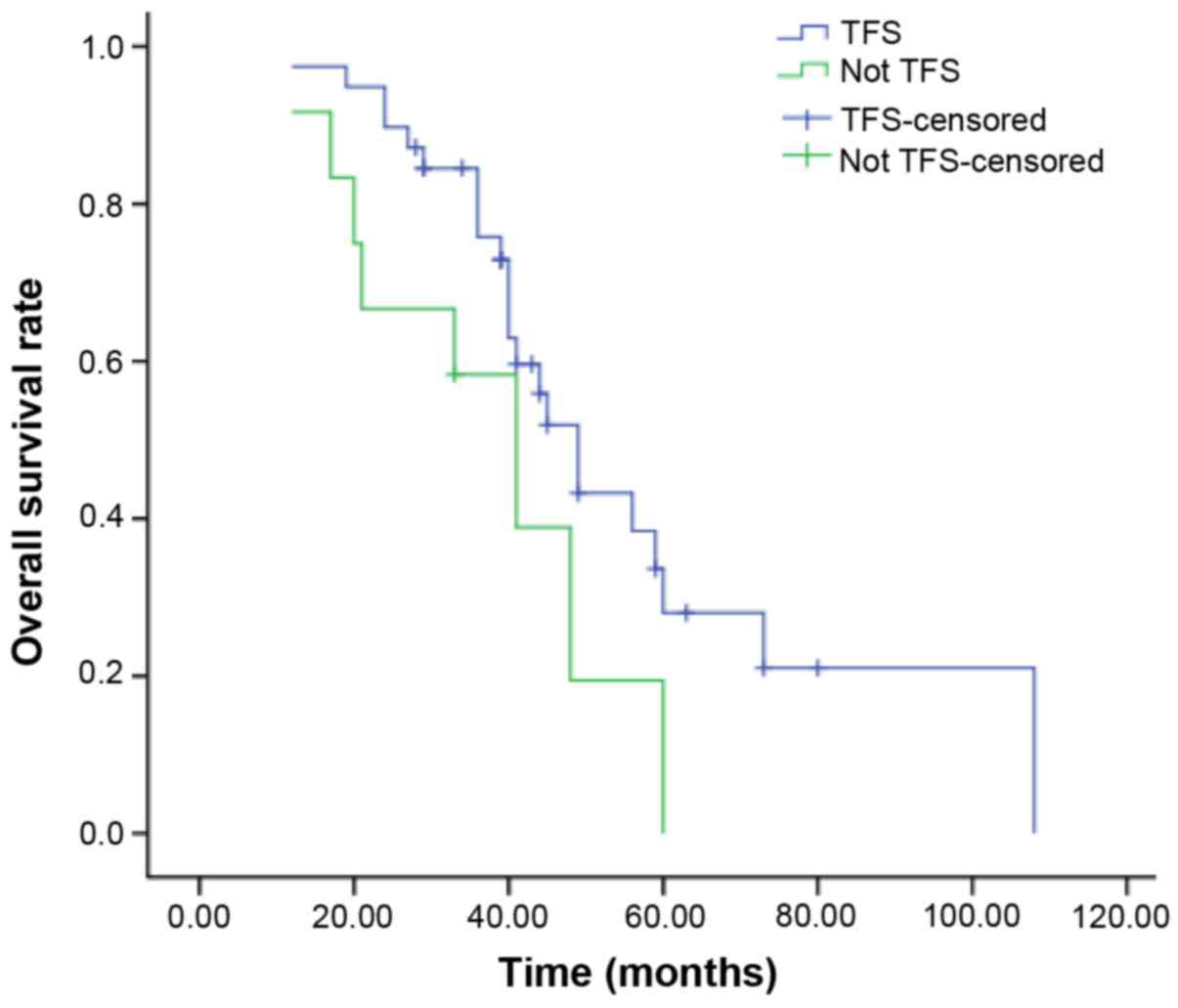
TFS was 9 months. The median OS of patients who achieved and those
who did not achieve TFS was 40 and 37 months, respectively. The
patients who obtained TFS had a significantly better OS compared
with those who did not (P=0.049, Fig.
1). The results of the univariate analysis demonstrated that
certain characteristics, such as the number of lesions and maximum
tumor diameter, were associated with the achievement of TFS
(Table II).
 | Table II.Univariate analysis of the predictors
of TFS. |
Table II.
Univariate analysis of the predictors
of TFS.
| Characteristics | TFS (n=39) | No TFS (n=12) | P-value |
|---|
| Gender |
|
| 0.262 |
| Male | 32 | 8 |
|
|
Female | 7 | 4 |
|
| Site of primary
cancer |
|
| 0.739 |
|
Colon | 22 | 8 |
|
|
Rectum | 17 | 4 |
|
| Location of
metastasis |
|
| 0.134 |
|
Liver | 13 | 1 |
|
|
Lungs | 6 | 1 |
|
| >3
locations | 20 | 10 |
|
| Number of
lesions |
|
| 0.002 |
| ≤5 | 28 | 2 |
|
|
>5 | 11 | 10 |
|
| Maximum diameter |
|
| 0.001 |
| of the tumor, cm |
| ≤5 | 33 | 5 |
|
|
>5 | 6 | 7 |
|
| Age, years |
|
|
|
|
Median | 63 | 60 | 0.820 |
|
Range | 42–75 | 35–78 |
|
Chemotherapy-related toxicity
Grade 3–4 myelosuppression occurred in 32 patients
(63.2%), mainly manifesting as neutropenia. Grade 1–2 liver
toxicity developed in 37 patients (72.4%), grade 1–2 neurotoxicity
occurred in 10 patients (19.7%), whereas no allergic reactions were
observed as a consequence of oxaliplatin. Grade 1–2 nausea or
vomiting was documented in 27 patients (53.9%). Of the 17 patients
who underwent a procedure with a hepatic artery kit with continuous
FUDR perfusion, 7 (41.2%) experienced grade 3–4 diarrhea, 15
(88.2%) had grade 1–2 abdominal pain and 2 (11.8%) experienced a
local infection and required debridement. All the side effects were
restored following drug withdrawal and symptomatic treatment, and
no deaths were reported (Table
III).
 | Table III.Frequency of chemotherapy-related
toxicities. |
Table III.
Frequency of chemotherapy-related
toxicities.
| Toxicities | n | (%) |
|---|
| Myelosuppression | 32 | (63.2) |
| Liver damage | 37 | (72.4) |
| Neuropathy | 10 | (19.7) |
| Diarrhea | 7 | (41.2) |
| Allergy | 0 | (0.0) |
| Anorexia/nausea | 27 | (53.9) |
| Fatigue | 6 | (11.8) |
| Hand-foot
syndrome | 2 | (3.9) |
| Other | 2 | (3.9) |
| Any grade ≥3 | 9 | (17.6) |
Severe local treatment-related complications were
not observed in any of the patients. Common complications included
puncture pain, vomiting, fever, bone marrow suppression, transient
liver damage and pneumothorax, all of which responded to
symptomatic treatment.
Improvement of clinical symptoms
Among the 15 patients experiencing pain, 11 achieved
symptom relief; the remission rate was 73.3%. Chest tightness and
shortness of breath were improved in 3 patients with lung
metastases; the remission rate was 100%. Of the 51 patients, 18
(35.3%) experienced weight gain.
Discussion
CRC is the third most common malignant tumor
worldwide and has the second highest mortality among malignant
tumors (12). Metastasis occurs in
~30% patients after the initial diagnosis. Systemic chemotherapy is
the principal therapeutic modality in patients with unresectable
mCRC. In recent years, palliative chemotherapy for unresectable
mCRC has progressed considerably, particularly with the application
of irinotecan and oxaliplatin, resulting in an increase in the
median survival time from 16 to 22 months (6). In addition, the application of targeted
drugs has further improved patient survival (13). However, targeted drugs are still not
widely used in China, and RAS or BRAF mutations in patients limit
the application and efficacy of molecular-targeted treatments.
Thus, there is a need for better understanding the variety of
available treatment modalities in order to improve survival and
quality of life without significantly increasing the total
treatment cost. However, strategies to achieve such goals remain
underinvestigated.
Local treatments, such as microwave ablation and
radioactive seed implantation, have been shown to be effective
(14,15).
In the present study, for patients with several
(>3) or larger (>5 cm) liver metastases, continuous infusion
of FUDR into the hepatic artery was added. When doxifluridine was
infused into the hepatic artery, the drug uptake rate by the liver
was 95%, and the drug concentration in the tumors was 16 times
higher compared with that assessed using conventional intravenous
chemotherapy (16). Continuous
infusion using a hepatic arterial kit may be effective in
maintaining drug concentrations and extending the delivery time,
thereby increasing the drug sensitivity. Shi et al (11) reported that CRC patients with liver
metastasis who were treated with HAI FUDR and systemic XELOX
experienced a high resection rate for asymptomatic CRC with
unresectable liver metastases, as well as a low rate of
complications associated with unresectable primary cancer.
Metastasis resection may significantly improve the
prognosis of patients with mCRC; however, certain challenges remain
for patients with unresectable metastasis but no symptoms from the
primary lesions. Controversial retrospective studies have been
conducted to determine whether it is appropriate to resect the
primary tumor in patients with mCRC (17). In the present study, if the disease
was controlled and the primary tumor was considered to be
resectable, it was recommended that patients undergo resection of
the primary tumor. Systemic chemotherapy combined with resection of
the primary tumor provided a proportion of the patients in this
group with TFS and improved their quality of life. Thus, it is
crucial for clinicians to identify the appropriate opportunities
for this treatment.
Following systemic chemotherapy, the metastatic
tumors regressed in a proportion of the patients. A number of
clinical studies (18,19) support the use of capecitabine as
maintenance therapy, as it has been shown to prolong PFS, but
provides no OS benefit. It is recommended that such patients
receive a local, minimally invasive treatment to achieve TFS. Some
residual cancer cells may persist after complete radiographic
remission. After a few cycles of chemotherapy, these remaining
tumor cells may become resistant to chemotherapeutic drugs; they
may not only become resistant to the same type of drug, but may
also develop cross-resistance to other, previously unused drugs.
Thus, the effect may not be optimal, even in the presence of other
chemotherapeutic drugs. In addition, several cycles of chemotherapy
may impair the immune system of the patient and result in
unendurable toxic adverse effects. These phenomena all promote
tumor progression and, in such cases, chemotherapy was terminated
and other methods were used to achieve TFS, such as microwave or
radiofrequency ablation, radioactive seed implantation, or
intensity-modulated conformal radiotherapy, depending on the
locations of the metastases in each patient. Petre et al
(20) used radiofrequency ablation
to treat 45 patients with 69 lung metastases resulting from CRC.
The 3-year local control rate was 89%, and the 1-, 2- and 3-year OS
rates were 95, 72 and 50%, respectively. Zhou et al
(21) reported that microwave
treatment may also enhance local and systemic immune function. Wang
et al (22) reported a local
control rate of 87% using CT-guided 125I seed
implantation to treat recurrent colorectal cancer, with 1- and
2-year survival rates of 93 and 50%, respectively. In the present
study, 29 patients underwent radioactive 125I seed
implantation, including 14 times in the liver, 20 times in the
lung, 9 times in the pelvis, 4 times in the adrenal glands, once in
the retroperitoneal lymph nodes and once in the abdominal wall.
Compared with other treatment methods, radioactive 125I
seed implantation was simple, had a reduced impact on the
surrounding normal tissue, was reliable and resulted in fewer
complications, particularly in patients with local recurrence or
metastases in the adrenal glands, retroperitoneal lymph nodes and
abdominal wall. Furthermore, this approach effectively controlled
local recurrence and tumor growth and improved patient survival. Of
note, the successful implementation of this technique depends on
the experience of the surgeon. Our sample included a 75-year-old
male patient with lung metastasis after ascending colon resection:
The lung metastasis was isolated, but due to the poor
cardiopulmonary function of the patient, surgical resection was not
possible. The patient received initial systemic chemotherapy and
radioactive 125I seed implantation, and there were no
new metastases after >3 years of follow-up. The patient
experienced a significant improvement in his quality of life and
prolonged survival. In the present study, certain characteristics,
such as the number of lesions and the maximum diameter of the
tumor, were associated with patients who achieved TFS. However, if
local treatment was successfully performed, its effects were also
associated with those characteristics.
The OPTIMOXl study (23) demonstrated that six cycles of
oxaliplatin using a simplified LV/5-FU solution to maintain the
chemotherapeutic effect exerted the same effect on the response
rate, PFS and OS, compared with continuous use of oxaliplatin until
the patients could no longer tolerate the side effects or
experienced disease progression; furthermore, with this treatment,
patients were able to avoid grade 3–4 toxicities after six cycles.
In centers in which >50% of the patients received a second
treatment with the FOLFOX7 program, the OS was >25 months. These
results indicate that intense chemotherapy after maintenance
therapy may prolong the survival of patients. We also observed that
oxaliplatin was effective after a period of inactivity. In the
present study, the majority of the patients tolerated FOLFOX or
XELOX and FOLFIRI as first-line and second-line chemotherapy,
respectively. When the patients achieved TFS, the chemotherapeutic
regimen was suspended until the tumors relapsed. Thus, the majority
of the patients tolerated the applied treatments, including
oxaliplatin as third-line therapy, which was effective in some
patients and may prolong OS.
In summary, the results of the present study
indicate that, in patients with unresectable mCRC who are not
suitable to receive molecular-targeted agents, particularly
patients with <5 metastatic lesions or a maximum tumor diameter
of <5 cm, systemic chemotherapy may be administered combined
with local treatment to achieve TFS, and the TFS benefits are
subsequently converted to a prolongation of OS. To the best of our
knowledge, this strategy has not been previously reported. However,
this was a retrospective study, and the analyzed cohort was small.
Additionally, there were some indications for minimally invasive
treatments, resulting in a selection bias. Thus, future prospective
randomized studies are required to confirm our results. The use of
drugs such as capecitabine as maintenance therapy or combined with
molecular-targeted agents to prolong TFS and OS should also be
assessed in future investigations.
References
|
1
|
Mayo SC and Pawlik TM: Current management
of colorectal hepatic metastasis. Expert Rev Gastroenterol Hepatol.
3:131–144. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai
SY, Ye QH, Yu Y, Xu B, Qin XY and Xu J: Randomized controlled trial
of cetuximab plus chemotherapy for patients with KRAS wild- type
unresectable colorectal liver-limited metastases. J Clin Oncol.
31:1931–1938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morris EJ, Forman D, Thomas JD, Quirke P,
Taylor EF, Fairley L, Cottier B and Poston G: Surgical management
and outcomes of colorectal cancer liver metastases. Br J Surg.
97:1110–1118. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folprecht G, Gruenberger T, Bechstein W,
Raab HR, Weitz J, Lordick F, Hartmann JT, Stoehlmacher-Williams J,
Lang H, Trarbach T, et al: Survival of patients with initially
unresectable colorectal liver metastases treated with
FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary
concept (CELIM study). Ann Oncol. 25:1018–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Formica V and Roselli M: Targeted therapy
in first line treatment of RAS wild type colorectal cancer. World J
Gastroenterol. 21:2871–2874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi L, Wu C, Wu J, Zhou W, Ji M, Zhang H,
Zhao J, Huang Y, Pei H, Li Z, et al: Computed tomography-guided
permanent brachytherapy for locoregional recurrent gastric cancer.
Radiat Oncol. 7:1142012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi L, Li X, Pei H, Zhao J, Qiang W, Wang
J, Xu B, Chen L, Wu J, Ji M, et al: Phase II study of computed
tomography-guided (125)I-seed implantation plus chemotherapy for
locally recurrent rectal cancer. Radiother Oncol. 118:375–381.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X,
Sun M, Wen W, Dai X, Yang M, et al: PD-1 blockade boosts
radiofrequency ablation-elicited adaptive immune responses against
tumor. Clin Cancer Res. 22:1173–1184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi L, Zhao J, Lu Q, Chen X, Wang H, Jiang
Y, Wu J, Ji M, Xu B, Chen L, et al: Initial hepatic artery infusion
and systemic chemotherapy for asymptomatic colorectal cancer with
un-resectable liver metastasis. Int J Clin Exp Med. 8:1000–1008.
2015.PubMed/NCBI
|
|
12
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun M: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Skitzki JJ and Chang AE: Hepatic artery
chemotherapy for colorectal liver metastases: Technical
considerations and review of clinical trials. Surg Oncol.
11:123–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eng OS, Tsang AT, Moore D, Chen C,
Narayanan S, Gannon CJ, August DA, Carpizo DR and Melstrom LG:
Outcomes of microwave ablation for colorectal cancer liver
metastases: A single center experience. J Surg Oncol. 111:410–413.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Lu J, Liu L, Liu T, Chen K, Liu F
and Huang G: Clinical application of CT-guided (125)I seed
interstitial implantation for local recurrent rectal carcinoma.
Radiat Oncol. 6:1382011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Loon K and Venook AP: Curable patient
with metastatic colorectal cancer: Balancing effective therapies
and toxicities. J Clin Oncol. 32:991–996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venderbosch S, de Wilt JH, Teerenstra S,
Loosveld OJ, van Bochove A, Sinnige HA, Creemers GJ, Tesselaar ME,
Mol L, Punt CJ and Koopman M: Prognostic value of resection of
primary tumor in patients with stage IV colorectal cancer:
Retrospective analysis of two randomized studies and a review of
the literature. Ann Surg Oncol. 18:3252–3260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tournigand C, Samson B, Scheithauer W,
Lledo G, Viret F, Andre T, Ramée JF, Tubiana-Mathieu N, Dauba J,
Dupuis O, et al: Bevacizumab (Bev) with or without erlotinib as
maintenance therapy, following induction first-line chemotherapy
plus Bev, in patients with metastatic colorectal cancer (mCRC):
Efficacy and safety results of the international GERCOR DREAM phase
III trial. J Clin Oncol. 30(suppl): S35002012. View Article : Google Scholar
|
|
19
|
Yalcin S, Uslu R, Dane F, Yilmaz U, Zengin
N, Buyukunal E, Buyukberber S, Camci C, Sencan O, Kilickap S, et
al: Bevacizumab+ capecitabine as maintenance therapy after initial
bevacizumab+ XELOX treatment in previously untreated patients with
metastatic colorectal cancer: Phase III ‘stop and go’ study
results-a Turkish oncology group trial. Oncology. 85:328–335. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petre EN, Jia X, Thornton RH, Sofocleous
CT, Alago W, Kemeny NE and Solomon SB: Treatment of pulmonary
colorectal metastases by radiofrequency ablation. Clin Colorectal
Cancer. 12:37–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou P, Liang P, Dong B, Yu X, Han Z and
Xu Y: Phase I clinical study of combination therapy with microwave
ablation and cellular immunotherapy in hepatocellular carcinoma.
Cancer Biol Ther. 11:450–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Yuan H and Liu J: The effects of
CT guided interstitial 125I seed implantation in the treatment of
recurrent colorectal cancer. J Chin Radiation Oncol. 15:319–322.
2006.
|
|
23
|
Tournigand C, Cervantes A, Figer A, Lledo
G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, et
al: OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with
oxaliplatin in a stop-and-Go fashion in advanced colorectal
cancer-a GERCOR study. J Clin Oncol. 24:394–400. 2006. View Article : Google Scholar : PubMed/NCBI
|