Introduction
The discovery of epidermal growth factor receptor
(EGFR)-activating mutations in non-small cell lung cancer (NSCLC)
and EGFR tyrosine kinase inhibitors (TKIs) have changed the
strategy of NSCLC therapy. Recently, EGFR-TKIs, including
gefitinib, erlotinib and afatinib, have become the standard therapy
for patients with advanced EGFR-mutated NSCLC. However, the
majority of the patients progress and second-line therapy is
required.
The EGFR T790M point mutation (T790M) is the most
common mechanism underlying drug resistance to EGFR-TKIs in EGFR
mutation-positive NSCLC patients. Osimertinib (AZD9291) is a
third-generation EGFR-TKI approved for the treatment of
EGFR-T790M-positive NSCLC (1).
Furthermore, immune checkpoint modulation with programmed death-1
(PD-1) or programmed death-ligand 1 (PD-L1) inhibition has also
shown promise in changing the strategy of NSCLC therapy. Nivolumab,
an anti-PD-1 inhibitor, is a novel drug used in the second-line
treatment of NSCLC (2). In both
EGFR-TKI and immune checkpoint inhibitors therapy, interstitial
lung disease (ILD) is recognized as one of most severe adverse
events. These two therapies may be sequentially applied in the same
patient, but the effects of the interaction between these two drugs
on the risk of ILD remains unclear. A case of ILD that occurred
during osimertinib treatment in a patient with a history of
previous nivolumab treatment is reported herein.
Case report
A 59-year-old female was diagnosed with stage IV
(cT3N2M1a) lung adenocarcinoma harboring a deletion in exon 19 of
the EGFR gene. The patient was treated with platinum-pemetrexed
with bevacizumab, followed by maintenance therapy for 6 months.
Subsequently, the patient received third-line treatment with
afatinib for 6 months, fourth-line S-1 for 5 months, fifth-line
docetaxel for 2 months and sixth-line nivolumab for 1 month
(administered twice). Eventually, 2.5 years after the initial
diagnosis, the patient's disease progressed and a computed
tomography (CT)-guided percutaneous needle biopsy for chest wall
invasion revealed an EGFR-T790M-encoding mutation in EGFR exon 20.
A total of 37 days after the last initiation of nivolumab, oral
osimertinib therapy was initiated at a dose of 80 mg per day. Two
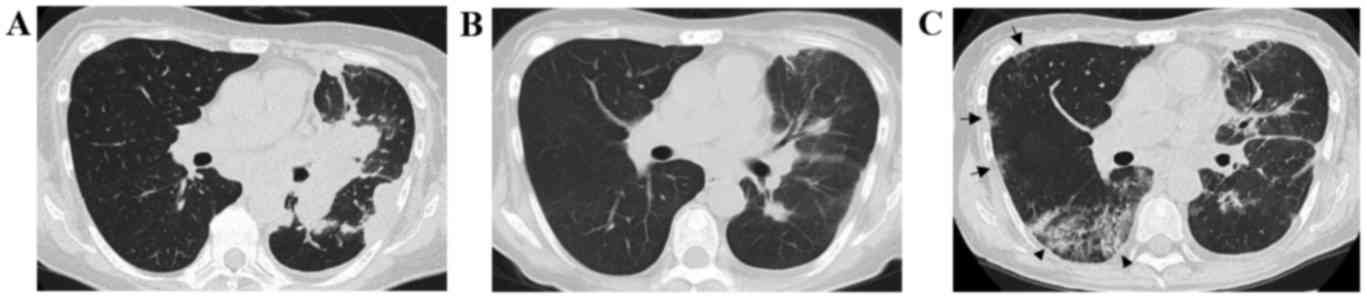
months later, a chest CT revealed a partial response (Fig. 1A and B) and the patient's performance
status (PS) improved from 3 to 1. However, on the 63rd day after
osimertinib initiation, the patient presented to the hospital with
shortness of breath. The patient had a light cough, without sputum
or fever. A chest X-ray revealed ground-glass shadows in the lungs
bilaterally and a chest CT showed the emergence of ground-glass
opacities and consolidation with bronchiectasis in the right middle
and lower lung lobes (Fig. 1C). The
patient's peripheral capillary oxygen saturation was 96%, which was
lower compared with that at the previous visit, and the laboratory
findings revealed increased levels of lactate dehydrogenase (427
IU/l; normal range, 119-229 IU/l) and C-reactive protein (1.43
mg/dl; normal range, <0.30 mg/dl). The patient was hospitalized
with a suspected diagnosis of ILD induced by osimertinib, and was
treated with steroid pulse therapy, antibiotics (levofloxacin) and
discontinuation of osimertinib. Following treatment with steroid
pulse therapy, the patient's chest findings and respiratory
condition improved and the steroid therapy was switched to
prednisolone and the dose was reduced. The serum laboratory tests,
including tests for antibodies against atypical pneumonia and
immune rheumatic disease, β-D glucan and cytomegalovirus antigen,
were all negative. After 10 days of hospitalization, the patient
was discharged from the hospital with continuous steroid therapy of
10 mg prednisolone. Subsequently, the ILD remained in remission but
the cancer rapidly progressed. Re-treatment with osimertinib and
other chemotherapy was not administered; instead, opioid therapy
was initiated. Twenty days following hospitalization for ILD, the
patient succumbed to cancer progression.
Informed consent was obtained from the patient
regarding the publication of the case details and associated
images.
Discussion
For patients with advanced EGFR-mutated NSCLC,
treatment with first- or second-generation EGFR-TKIs is associated
with response rates of 56-74% (3–6), but the
majority of the patients develop disease progression within 1-2
years following treatment initiation (3–6). In ~60%
of the patients, the mechanism underlying the acquired resistance
is the development of an additional EGFR mutation, encoding EGFR
T790M (7).
Osimertinib treatment has demonstrated a response
rate of >60%, with durable progression-free survival for NSCLC
patients with the EGFR-T790M-resistance mutation (1). The incidence of interstitial pneumonia
during osimertinib treatment was reported to be 3%, and grade 5 was
observed in 1% of the cases (1). As
osimertinib is used in pretreated patients, greater attention must
be paid to the incidence of interstitial pneumonia induced by
osimertinib compared with other EGFR-TKIs.
It is known that risk factors of ILD in EGFR-TKI
treatment include a history of smoking, concomitant interstitial
pneumonia and a poor PS (8). In the
present study, a poor PS may have been the reason for the incidence
of ILD. On the other hand, Ahn et al (9) reported that ILD was observed in 38% of
cases receiving combination treatment with osimertinib and
durvalumab, a PD-L1 inhibitor, which suggests that treatment with
such a drug combination may dramatically increase the risk of ILD.
Notably, a similar increased risk of ILD has not been reported for
combination treatment with durvalumab and gefitinib (10). These studies suggest that previous
treatment with nivolumab may affect the onset of ILD during
osimertinib treatment. Durability of the response following
discontinuation of nivolumab has been observed (11). As previous treatment with nivolumab
may be a critical risk factor for ILD during osimertinib treatment,
further verification of this possibility is required.
In sub-group analysis in a previous phase III study
(2), it was reported that nivolumab
was relatively inferior to docetaxel in patients harboring an EGFR
mutation. Furthermore, a meta-analysis reported that
immune-checkpoint inhibitors do not improve overall survival over
docetaxel in EGFR-mutated advanced NSCLC (12). We consider that nivolumab treatment
prior to osimertinib treatment should be considered with caution
when devising a treatment strategy for patients with EGFR-mutated
advanced NSCLC. The presence of PD-L1 expression has been reported
to be a predictive biomarker of the efficacy of anti-PD-1/PD-L1
antibodies, including nivolumab (13). Assessment of tumor PD-L1 expression
may aid with decision-making regarding the adoption of nivolumab
treatment in patients with EGFR-mutated advanced NSCLC.
In conclusion, the present study reports a case of
ILD during osimertinib treatment following treatment with
nivolumab. Careful assessment is required prior to commencing
osimertinib treatment in patients previously treated with
nivolumab. Further studies investigating the risk of ILD during
EGFR-TKI treatment following nivolumab pre-treatment are required
to reach more definitive conclusions.
Acknowledgements
Tetsuya Oguri and Akio Niimi have received personal
fees from ONO PHARMACEUTICAL Co., Ltd. (Osaka, Japan), and
AstraZeneca (Osaka, Japan). Ken Maeno has received grants from
AstraZeneca. Masaya Takemura has received grants and personal fees
from AstraZeneca and personal fees from ONO PHARMACEUTICAL Co.,
Ltd.
References
|
1
|
Jänne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sequist LV, Yang JC, Yamamoto N, O'Byrne
K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al:
Phase III study of afatinib or cisplatin plus pemetrexed in
patients with metastatic lung adenocarcinoma with EGFR mutations. J
Clin Oncol. 31:3327–3334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotta K, Kiura K, Takigawa N, Yoshioka H,
Harita S, Kuyama S, Yonei T, Fujiwara K, Maeda T, Aoe K, et al:
Comparison of the incidence and pattern of interstitial lung
disease during erlotinib and gefitinib treatment in Japanese
Patients with non-small cell lung cancer: The Okayama Lung Cancer
Study Group experience. J Thorac Oncol. 5:179–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahn MJ, Yang J, Yu H, Saka H, Ramalingam
S, Goto K, Kim SW, Yang L, Walding A and Oxnard GR: 136O:
Osimertinib combined with durvalumab in EGFR-mutant non-small cell
lung cancer: Results from the TATTON phase Ib trial. J Thorac
Oncol. 11:S1152016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibbons DL, Chow LQ, Kim DW, Kim SW, Yeh
T, Song X, Jiang H, Taylor R, Karakunnel J and Creelan B: 570
Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a
human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody,
combined with gefitinib (G): A phase I expansion in TKI-naïve
patients (pts) with EGFR mutant NSCLC. J Thorac Oncol. 11:S792016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gettinger SN, Horn L, Gandhi L, Spigel DR,
Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman
DM, et al: Overall survival and long-term safety of nivolumab
(anti-programmed death 1 Antibody, BMS-936558, ONO-4538) in
patients with previously treated advanced non-small-cell lung
cancer. J Clin Oncol. 33:2004–2012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CK, Man J, Lord S, Links M, Gebski V,
Mok T and Yang JC: Checkpoint inhibitors in metastatic EGFR-mutated
non-small cell lung cancer-A meta-analysis. J Thorac Oncol.
12:403–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang T, Xie J, Arai S, Wang L, Shi X, Shi
N, Ma F, Chen S, Huang L, Yang L, et al: The efficacy and safety of
anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory
cancers: A meta-analysis. Oncotarget. 7:73068–73079.
2016.PubMed/NCBI
|