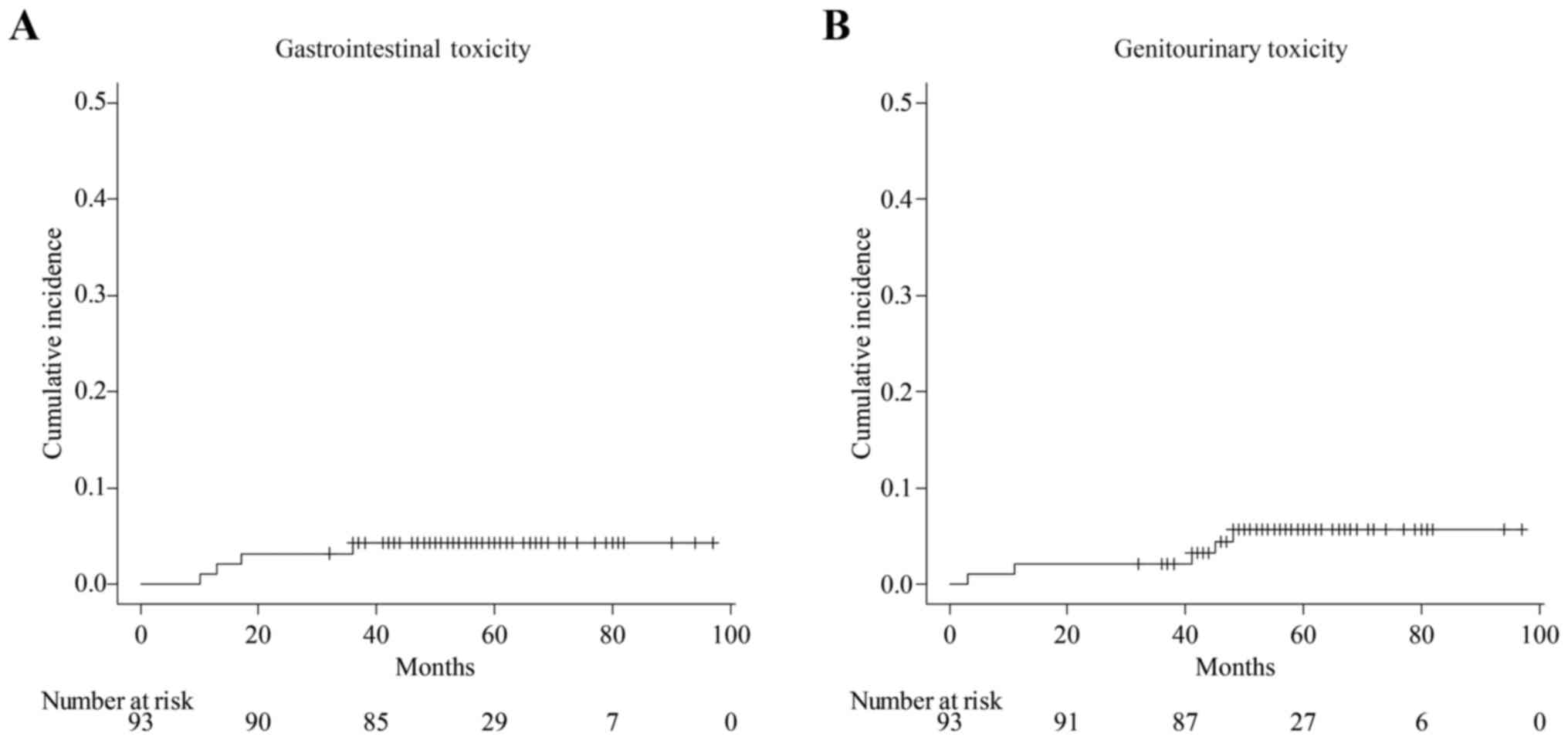
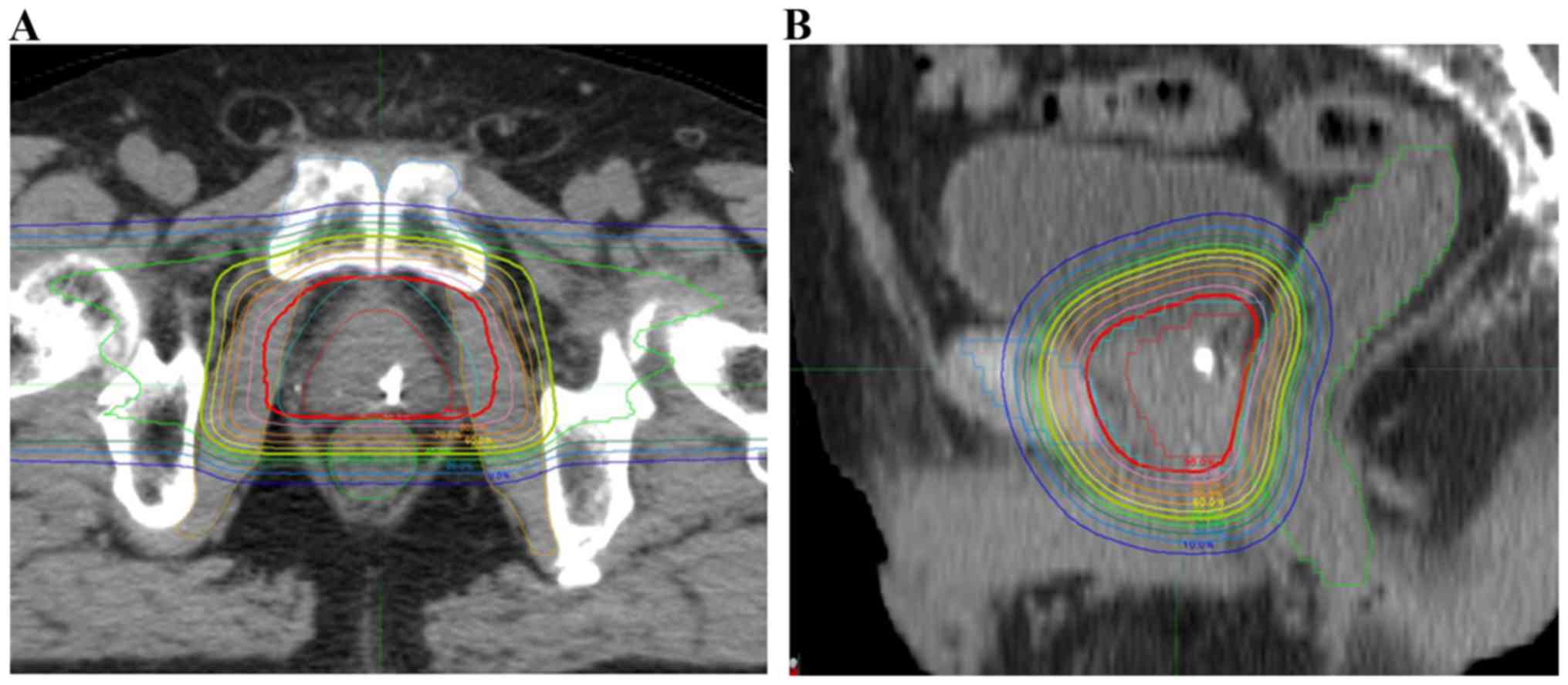
|
1
|
Fiorino C, Sanguineti G, Cozzarini C,
Fellin G, Foppiano F, Menegotti L, Piazzolla A, Vavassori V and
Valdagni R: Rectal dose-volume constraints in high-dose
radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol
Phy. 57:953–962. 2003. View Article : Google Scholar
|
|
2
|
Sheets NC, Goldin GH, Meyer AM, Wu Y,
Chang Y, Stürmer T, Holmes JA, Reeve BB, Godley PA, Carpenter WR
and Chen RC: Intensity-modulated radiation therapy, proton therapy,
or conformal radiation therapy and morbidity and disease control in
localized prostate cancer. JAMA. 307:1611–1620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishikawa H, Tsuji H, Kamada T, Hirasawa N,
Yanagi T, Mizoe JE, Akakura K, Suzuki H, Shimazaki J and Tsujii H:
Risk factors of late rectal bleeding after carbon ion therapy for
prostate cancer. Int J Radiat Oncol Biol Phys. 66:1084–1091. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michalski J, Winter K, Roach M, Markoe A,
Sandler HM, Ryu J, Parliament M, Purdy JA, Valicenti RK and Cox JD:
Clinical outcome of patients treated with 3D conformal radiation
therapy (3D-CRT) for prostate cancer on RTOG 9406. Int J Radiat
Oncol Biol Phys. 83:e363–e370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michalski JM, Bae K, Roach M, Markoe AM,
Sandler HM, Ryu J, Parliament MB, Straube W, Valicenti RK and Cox
JD: Long-term toxicity following 3D conformal radiation therapy for
prostate cancer from the RTOG 9406 phase I/II dose escalation
study. Int J Radiat Oncol Biol Phys. 76:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pollack A, Zagars GK, Starkschall G,
Antolak JA, Lee JJ, Huang E, von Eschenbach AC, Kuban DA and Rosen
I: Prostate cancer radiation dose response: Results of the M. D.
Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys.
53:1097–1105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zapatero A, Guerrero A, Maldonado X,
Alvarez A, Segundo Gonzalez San C, Rodríguez Cabeza MA, Macias V,
Olive Pedro A, Casas F, Boladeras A, et al: High-dose radiotherapy
with short-term or long-term androgen deprivation in localised
prostate cancer (DART01/05 GICOR): A randomised, controlled, phase
3 trial. Lancet Oncol. 16:320–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pedroni E, Bacher R, Blattmann H,
Böhringer T, Coray A, Lomax A, Lin S, Munkel G, Scheib S, Schneider
U, et al: The 200-MeV proton therapy project at the Paul Scherrer
Institute: Conceptual design and practical realization. Med Phys.
22:37–53. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zietman AL, DeSilvio ML, Slater JD, Rossi
CJ Jr, Miller DW, Adams JA and Shipley WU: Comparison of
conventional-dose vs high-dose, conformal radiation therapy in
clinically localized adenocarcinoma of the prostate: A randomized
controlled trial. JAMA. 294:1233–1239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horwitz EM and Hanks GE: External beam
radiation therapy for prostate cancer. CA Cancer J Clin.
50:349–375; quiz 376–379. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher
GJ, Fleshner NE, Venkatramen ES, Reuter VE, Fair WR, Ling CC and
Fuks Z: Dose escalation with three-dimensional conformal radiation
therapy affects the outcome in prostate cancer. Int J Radiat Oncol
Biol Phys. 41:491–500. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akimoto T, Katoh H, Kitamoto Y, Tamaki T,
Harada K, Shirai K and Nakano T: Rectal bleeding after
high-dose-rate brachytherapy combined with hypofractionated
external-beam radiotherapy for localized prostate cancer: Impact of
rectal dose in high-dose-rate brachytherapy on occurrence of grade
2 or worse rectal bleeding. Int J Radiat Oncol Biol Phys.
65:364–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fenwick JD, Khoo VS, Nahum AE,
Sanchez-Nieto B and Dearnaley DP: Correlations between dose-surface
histograms and the incidence of long-term rectal bleeding following
conformal or conventional radiotherapy treatment of prostate
cancer. Int J Radiat Oncol Biol Phys. 49:473–480. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schaly B, Bauman GS, Song W, Battista JJ
and Van Dyk J: Dosimetric impact of image-guided 3D conformal
radiation therapy of prostate cancer. Phys Med Biol. 50:3083–3101.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zelefsky MJ, Kollmeier M, Cox B, Fidaleo
A, Sperling D, Pei X, Carver B, Coleman J, Lovelock M and Hunt M:
Improved clinical outcomes with high-dose image guided radiotherapy
compared with non-IGRT for the treatment of clinically localized
prostate cancer. Int J Radiat Oncol Biol Phys. 84:125–129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inada T, Hayakawa Y, Tada J, Takada Y and
Maruhashi A: Characteristics of proton beams after field shaping at
PMRC. Med Biol Eng Comput. 31:Suppl. S44–S48. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jackson A, Skwarchuk MW, Zelefsky MJ,
Cowen DM, Venkatraman ES, Levegrun S, Burman CM, Kutcher GJ, Fuks
Z, Liebel SA and Ling CC: Late rectal bleeding after conformal
radiotherapy of prostate cancer. II. Volume effects and dose-volume
histograms. Int J Radiat Oncol Biol Phys. 49:685–698. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pederson AW, Fricano J, Correa D,
Pelizzari CA and Liauw SL: Late toxicity after intensity-modulated
radiation therapy for localized prostate cancer: An exploration of
dose-volume histogram parameters to limit genitourinary and
gastrointestinal toxicity. Int J Radiat Oncol Biol Phys.
82:235–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
D'Amico AV, Whittington R, Malkowicz SB,
Cote K, Loffredo M, Schultz D, Chen MH, Tomaszewski JE, Renshaw AA,
Wein A and Richie JP: Biochemical outcome after radical
prostatectomy or external beam radiation therapy for patients with
clinically localized prostate carcinoma in the prostate specific
antigen era. Cancer. 95:281–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dearnaley DP, Sydes MR, Graham JD, Aird
EG, Bottomley D, Cowan RA, Huddart RA, Jose CC, Matthews JH, Millar
J, et al: Escalated-dose versus standard-dose conformal
radiotherapy in prostate cancer: First results from the MRC RT01
randomised controlled trial. Lancet Oncol. 8:475–487. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vora SA, Wong WW, Schild SE, Ezzell GA and
Halyard MY: Analysis of biochemical control and prognostic factors
in patients treated with either low-dose three-dimensional
conformal radiation therapy or high-dose intensity-modulated
radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol
Phys. 68:1053–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zelefsky MJ, Chan H, Hunt M, Yamada Y,
Shippy AM and Amols H: Long-term outcome of high dose intensity
modulated radiation therapy for patients with clinically localized
prostate cancer. J Urol. 176:1415–1419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kupelian PA, Willoughby TR, Reddy CA,
Klein EA and Mahadevan A: Hypofractionated intensity-modulated
radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate
cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys.
68:1424–1430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cahlon O, Zelefsky MJ, Shippy A, Chan H,
Fuks Z, Yamada Y, Hunt M, Greenstein S and Amols H: Ultra-high dose
(86.4 Gy) IMRT for localized prostate cancer: Toxicity and
biochemical outcomes. Int J Radiat Oncol Biol Phys. 71:330–337.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin JM, Bayley A, Bristow R, Chung P,
Gospodarowicz M, Menard C, Milosevic M, Rosewall T, Warde PR and
Catton CN: Image guided dose escalated prostate radiotherapy: Still
room to improve. Radiat Oncol. 4:502009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guckenberger M, Lawrenz I and Flentje M:
Moderately hypofractionated radiotherapy for localized prostate
cancer: Long-term outcome using IMRT and volumetric IGRT.
Strahlenther Onkol. 190:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schulte RW, Slater JD, Rossi CJ Jr and
Slater JM: Value and perspectives of proton radiation therapy for
limited stage prostate cancer. Strahlenther Onkol. 176:3–8. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mendenhall NP, Hoppe BS, Nichols RC,
Mendenhall WM, Morris CG, Li Z, Su Z, Williams CR, Costa J and
Henderson RH: Five-year outcomes from 3 prospective trials of
image-guided proton therapy for prostate cancer. Int J Radiat Oncol
Biol Phys. 88:596–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takagi M, Mima M, Terashima K, Fujii O,
Demizu Y, Nagano F, Jin D, Okimoto T, Waki T, Murakami M and Fuwa
N: Long-term outcomes in patients treated with proton therapy for
localized prostate cancer. Int J Radiat Oncol Biol Phys.
93:E186–E187. 2015. View Article : Google Scholar
|
|
30
|
Bryant C, Smith TL, Henderson RH, Hoppe
BS, Mendenhall WM, Nichols RC, Morris CG, Williams CR, Su Z, Li Z,
et al: Five-year biochemical results, toxicity and patient-reported
quality of life after delivery of dose-escalated image guided
proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys.
95:422–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vargas C, Yan D, Kestin LL, Krauss D,
Lockman DM, Brabbins DS and Martinez AA: Phase II dose escalation
study of image-guided adaptive radiotherapy for prostate cancer:
Use of dose-volume constraints to achieve rectal isotoxicity. Int J
Radiat Oncol Biol Phys. 63:141–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rana S, Cheng C, Zheng Y, Risalvato D,
Cersonsky N, Ramirej E, Zhao L, Larson G and Vargas C: Proton
therapy vs. VMAT for prostate cancer: A treatment planning study.
International Journal of Particle Therapy. 1:22–33. 2014.
View Article : Google Scholar
|
|
33
|
Rana S, Cheng C, Zhao L, Park S, Larson G,
Vargas C, Dunn M and Zheng Y: Dosimetric and radiobiological impact
of intensity modulated proton therapy and RapidArc planning for
high-risk prostate cancer with seminal vesicles. J Med Radiat Sci.
64:18–24. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
McDonald AM, Baker CB, Popple RA, Cardan
RA and Fiveash JB: Increased radiation dose heterogeneity within
the prostate predisposes to urethral strictures in patients
receiving moderately hypofractionated prostate radiation therapy.
Pract Radiat Oncol. 5:338–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tran A, Zhang J, Woods K, Yu V, Nguyen D,
Gustafson G, Rosen L and Sheng K: Treatment planning comparison of
IMPT, VMAT and 4π radiotherapy for prostate cases. Radiat Oncol.
12:102017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krauss DJ, Ye H, Martinez AA, Mitchell B,
Sebastian E, Limbacher A and Gustafson GS: Favorable preliminary
outcomes for men with low- and intermediate-risk prostate cancer
treated with 19-Gy single-fraction high-dose-rate brachytherapy.
Int J Radiat Oncol Biol Phys. 97:98–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kasuya G, Ishikawa H, Tsuji H, Nomiya T,
Makishima H, Kamada T, Akakura K, Suzuki H, Shimazaki J, Haruyama
Y, et al: Significant impact of biochemical recurrence on overall
mortality in patients with high-risk prostate cancer after
carbon-ion radiotherapy combined with androgen deprivation therapy.
Cancer. 122:3225–3231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paganetti H, Niemierko A, Ancukiewicz M,
Gerweck LE, Goitein M, Loeffler JS and Suit HD: Relative biological
effectiveness (RBE) values for proton beam therapy. Int J Radiat
Oncol Biol Phys. 53:407–421. 2002. View Article : Google Scholar : PubMed/NCBI
|