Introduction
Breast cancer, diagnosed in ~1.7 million patients
and being the cause of 521,900 mortalities in 2012, is the most
common diagnosed cancer and the most notable cause of cancer
mortality among females worldwide (1). Various factors have an impact in the
incidence of breast cancer, such as family history, gene
susceptibility, hormone, diet, lifestyle factors and environmental
exposures (2–6).
Increasing research has identified a significant
effect of reactive oxygen species (ROS) in breast cancer etiology
(7–12). ROS may induce oxidative stress,
resulting in DNA sequence changes and damage, such as mutations,
rearrangements and DNA strand breaks. ROS may also lead to damage
to lipids, proteins, membranes and mitochondria (8,13). In
humans, various antioxidant actions may balance the effect of ROS,
including antioxidant enzymes and antioxidant agents. Antioxidant
enzymes predominantly include glutathione peroxidase, catalase and,
most importantly, superoxide dismutase (SOD) (7,8).
There are three types of SOD, which are cytosolic
Cu-ZnSOD (SOD1), extracellular Cu-ZnSOD (SOD3) and mitochondrial
SOD (SOD2; MnSOD). SOD2 is mostly produced in mitochondria, having
a vital effect on balancing mitochondrial oxidant stress and
antioxidant defense (14). SOD2
gene, located on 6q25 of chromosome 6, encodes SOD2, whose
expression is highly regulated at transcription, translation and
posttranslational levels (15–17).
SOD2, a polymorphic enzyme, has several structural mutations and
single nucleotide polymorphisms (SNPs). The most common SNP is
rs4880 SNP, also called rs1799725 SNP, which is a T to C
substitution in exon 2, changing the amino acid codon at position
16 from valine to alanine, known as the SOD2 Val-16Ala genotype and
also as Ala-9Val as the SNP is 9 amino acids upstream of the
cleavage site (18–20).
According to the latest research, various studies
have demonstrated an association between the polymorphism of SOD2
and disease, particularly between SOD2 Val-16Ala and cancer.
Several meta-analyses have demonstrated that the SOD2 Val-16Ala SNP
polymorphism increases susceptibility to various types of cancer,
such as prostate cancer (21–23),
lung cancer, colorectal cancer and non-Hodgkin lymphoma (24). However, referring to breast cancer,
from 1999 to present, there have been many studies and cohorts to
investigate the relationship between SOD2 Val-16Ala polymorphism
and breast cancer; however, these studies cannot reach an agreement
and have drawn some conflicting conclusions (25–50). In
2008, the first meta-analysis to investigate the association
between Val-16Ala and breast cancer was conducted, with 13
publications including a total of 7,366 cases and 9,102 controls;
however, it indicated no overall association with the Val-16Ala
polymorphism (51). Subsequently,
from 2010–2012, four meta-analyses also demonstrated similar
negative effects (52–55). Presently, due to increased individual
studies and larger sample sizes, a more accurate estimation may be
obtained to judge this association. Thus, an updated meta-analysis
was performed to investigate whether SOD2 Val-16Ala polymorphism is
a risk factor and/or prognostic factor for breast cancer.
Materials and methods
Search strategy
A comprehensive strategy was used to search PubMed
(ncbi.nlm.nih.gov/pubmed) or MEDLINE
(medline.com) and EMBASE (embase.com) to
obtain the relevant publications about the association between
breast cancer risk and SOD2 gene polymorphism. The search terms
were ‘superoxide dismutase 2,’ ‘SOD2,’ ‘MnSOD,’ ‘ala9val,’
‘val16ala,’ ‘breast cancer,’ ‘breast carcinoma,’ ‘breast tumor,’
‘breast neoplasm,’ ‘mammary cancer,’ ‘polymorphism,’ ‘mutation’ and
‘variant,’ alone or in combination. The last updated data was
October 5, 2016, and with no restriction of the post time.
Additional publications listed in references were also retrieved by
a computer-aided manual search to gain more information about this
field. Furthermore, only publications in the English language were
included in the meta-analysis.
Inclusion and exclusion criteria
In the present meta-analysis, eligible publications
had to be randomized controlled trials, cohort studies or
case-control studies that investigated the association between
breast cancer and SOD2 gene polymorphism. The publications meeting
the following inclusion criteria were retained: i) The cases were
diagnosed with breast cancer that was pathologically confirmed and
the controls were free of breast cancer; ii) had sufficient data,
such as size of the sample, alleles and genotypes, to calculate the
odd ratios (ORs) or hazards ratios (HRs) with 95% confidence
intervals (95% CIs); and iii) preferably used subgroup analysis.
The exclusion criteria were as follows: i) Studies had no control
individuals; ii) studies were about the activity of the SOD2
enzyme; iii) the study was not about the rs4880 or rs1799725 SNP.
If there were some duplicated publications, the latest studies were
retained. If several different publications had the same patient
source, the studies with the largest number of individuals were
reserved. Furthermore, two cooperators reviewed the publications
independently to ensure that the appropriate studies were
chosen.
Data extraction
Available data were extracted and collected by two
investigators independently from all of the included publications,
following the same standard protocol. If there were any
inconsistencies between the data obtained by the two reviewers, the
problem was solved through a careful discussion. If an agreement
could not be reached, a third reviewer would take part in this to
make everyone satisfied. Data information from the publications
were about the first author, publish year, ethnicity, sources of
controls, genotyping methods, total number of cases and controls,
distribution of alleles and genotypes and the HR with 95%
confidence interval (CI) of relative polymorphism.
Statistical analysis
The crude ORs with 95% CIs for alleles and genotypes
were used to estimate the association between breast cancer risk
and SOD2 gene polymorphism, and HRs with 95% CIs were used for
survival analysis. The pooled ORs and HRs were calculated for the
genotypes of T vs. C, CT vs. CC, TT vs. CC, TT vs. CT + CC and CT +
TT vs. CC, which assumed the allele contrast model, two co-dominant
models, one recessive model and one dominant model of the SOD2
rs4880 variant, respectively. Subgroup analyses were also conducted
according to ethnicity and menopausal status using the ORs.
To assess the heterogeneity of the publications,
Chi-square (X2) tests were carried out. At first,
if I-squared ≤50%, the ORs with 95% CI were calculated using the
fixed effects model (Mantel-Haenszel) for meta-analysis (56). If I-squared >50%, the fixed
effects model could not be applied, and so the random effects model
(DerSimonian and Laird) was used (57). Conventionally, pooled OR ≠1 revealed
the existent association between breast cancer risk and SOD2 gene
polymorphism, and pooled HR ≠1 revealed association between cancer
survival and polymorphism. If 95% CI did not overlap 1, P<0.05
was considered to indicate a statistically significant difference.
The pooled ORs and HRs with 95% CI were presented in the form of
forest plots, using Stata version 12.0 (StataCorp LP, College
Station, TX, USA).
To assess potential publication bias, graphical
funnel plots were used, and Egger's and Begg's linear regression
methods were also utilized to estimate the funnel plot asymmetry.
An asymmetric funnel plot demonstrated possible publication bias
and P<0.05 was considered to indicate a statistically
significant publication bias.
Results
Characteristics of the studies
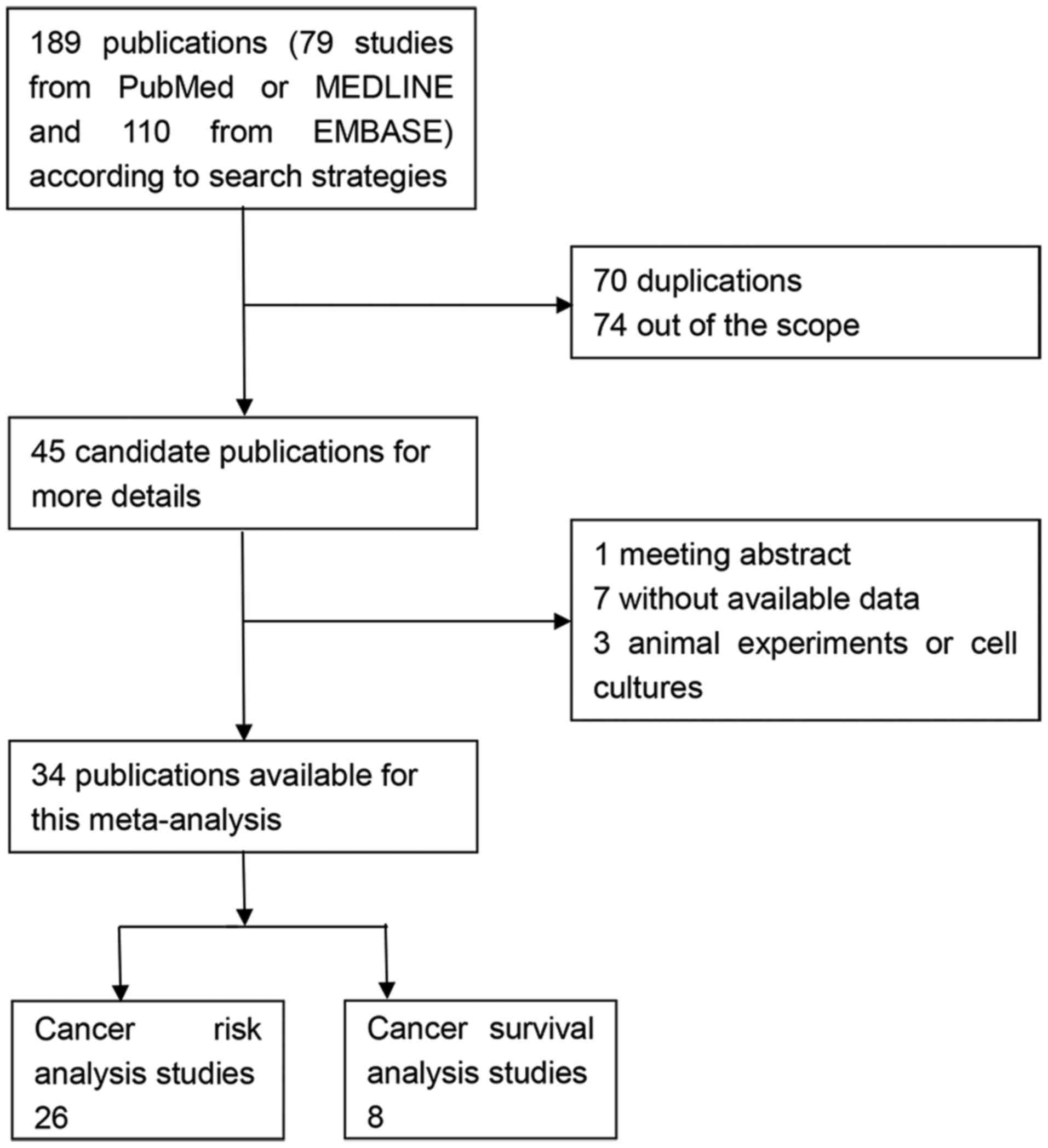
Through the primary search algorithm, 189
publications were acquired, which consisted of 79 studies from
PubMed or MEDLINE and 110 studies from EMBASE. Only 45 candidate
studies were retrieved for more detailed evaluation. By reading the
full texts, 11 studies were out of scope as they did not satisfy
the inclusion criteria (one meeting abstract, three animal
experiments or cell cultures and seven without available data).
Finally, a total of 34 publications were available for the present
meta-analysis, 26 for risk analysis (25–50) and
eight for survival analysis (58–65). The
flow chart of study search and inclusion was demonstrated in
Fig. 1.
For the risk analysis, the 26 included studies were
published between 1999 and 2015 and consisted of 18,481 cases and
19,527 controls. The total sample size of the patients was 38,008,
ranging from 187–9054 per cohort. As an article had two cohorts and
ethnicities (30), there were 27
cohorts. A total of 14 were conducted in Caucasian patients, five
in Asian patients, two in Danish patients, one in African American
patients and four in mixed races. All of the case patients were
confirmed by histological or pathological methods. The controls
were healthy or free of breast cancer, and matched for age,
ethnicity or area to cases. The majority of the cohorts examined
the blood sample using polymerase chain reaction (PCR) genotyping
methods. For the survival analysis, the included eight studies
contained 5746 participants, published between 2005 and 2014. The
primary characteristics of included cohorts were summarized in
Table I.
 | Table I.Characteristics of studies included
in analysis of association between superoxide dismutase 2 Val-16Ala
polymorphism (rs4880) and breast cancer risk or survival. |
Table I.
Characteristics of studies included
in analysis of association between superoxide dismutase 2 Val-16Ala
polymorphism (rs4880) and breast cancer risk or survival.
|
|
|
|
|
| Case, n | Control, n |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Analysis | Authors, year | Ethnicity | Source of
controls | Genotyping
method | CC | CT | TT | CC | CT | TT | (Refs.) |
|---|
| Risk analysis | Ambrosone et
al, 1999 | Caucasian | PB | PCR-RFLP | 90 | 137 | 39 | 63 | 169 | 63 | (25) |
|
| Mitrunen et
al, 2001 | Caucasian | PB | PCR-RFLP | 100 | 255 | 124 | 98 | 231 | 153 | (26) |
|
| Egan et al,
2003 | Caucasian | PB | PCR | 118 | 250 | 102 | 127 | 240 | 130 | (27) |
|
| Cai et al,
2004 | Asian | PB | PCR-RFLP | 28 | 266 | 831 | 23 | 290 | 884 | (28) |
|
| Knight et
al, 2004 | Mixed | PB | PCR | 105 | 187 | 107 | 87 | 195 | 90 | (29) |
|
| Millikan et
al (1), 2004 | African
American | PB | TaqMan | 129 | 372 | 259 | 124 | 357 | 196 | (30) |
|
| Millikan et
al (2), 2004 | Caucasian | PB | TaqMan | 311 | 681 | 273 | 283 | 586 | 266 | (30) |
|
| Tamimi et
al, 2004 | Mixed | HB | PCR | 245 | 468 | 255 | 296 | 612 | 297 | (31) |
|
| Bergman et
al, 2005 | Caucasian | NA | PCR | 12 | 73 | 33 | 43 | 88 | 43 | (32) |
|
| Cheng et al,
2005 | Asian | HB | PCR | 11 | 115 | 343 | 11 | 183 | 545 | (33) |
|
| Gaudet et
al, 2005 | Mixed | PB | PCR | 270 | 511 | 253 | 281 | 539 | 264 | (34) |
|
| Kocabaş et
al, 2005 | Caucasian | NA | PCR-RFLP | 18 | 38 | 28 | 38 | 39 | 26 | (35) |
|
| Cebrian et
al, 2006 | Caucasian | PB | TaqMan and PCR | 1,297 | 2,237 | 940 | 1,328 | 2,290 | 962 | (36) |
|
| Silva et al,
2006 | Caucasian | HB | PCR-RFLP | 59 | 146 | 36 | 99 | 276 | 82 | (37) |
|
| Slanger et
al, 2006 | Caucasian | PB | TaqMan and PCR | 152 | 318 | 144 | 289 | 528 | 263 | (38) |
|
| Justenhoven et
al, 2008 | Caucasian | PB | MALDI-TOF MS | 133 | 312 | 159 | 145 | 313 | 163 | (39) |
|
| Bica et al,
2009 | Mixed | PB | PCR | 14 | 51 | 24 | 26 | 252 | 94 | (40) |
|
| Eras-Erdogan et
al, 2009 | Caucasian | PB | PCR-RFLP | 30 | 113 | 107 | 39 | 141 | 150 | (41) |
|
| Kostrykina et
al, 2009 | Caucasian | HB | TaqMan and PCR | 119 | 233 | 123 | 90 | 183 | 103 | (42) |
|
| Ermolenko et
al, 2010 | Asian | HB | PCR | 239 | 454 | 228 | 104 | 235 | 121 | (43) |
|
| Kim et al,
2010 | Asian | HB | TaqMan and PCR | 4 | 66 | 234 | 7 | 90 | 279 | (44) |
|
| Cerne et al,
2011 | Caucasian | HB | PCR-RFLP | 143 | 269 | 118 | 71 | 134 | 65 | (45) |
|
| Tsai et al,
2012 | Asian | HB | PCR |
| 68 | 192 |
| 86 | 138 | (46) |
|
| Attatippaholkun and
Wikainapakul, 2013 | Asian | NA | PCR | 5 | 54 | 82 | 3 | 48 | 84 | (47) |
|
| Méplan et
al, 2013 | Danish | PB | PCR | 226 | 485 | 228 | 227 | 494 | 237 | (48) |
|
| Jablonska et
al, 2015 | Caucasian | HB | TaqMan and PCR | 29 | 75 | 32 | 50 | 92 | 41 | (49) |
|
| Kakkoura et
al, 2015 | Danish | PB | TaqMan | 212 | 512 | 342 | 252 | 550 | 343 | (50) |
| Survival
analysis | Ambrosone et
al, 2005 | Caucasian | NA | PCR-RFLP | 267 |
|
| – |
|
| (58) |
|
| Udler et al,
2007 | Caucasian | NA | TaqMan | 4,181 |
|
| – |
|
| (59) |
|
| Bewick et
al, 2008 | Caucasian | NA | DNA MiniKit | 95 |
|
| – |
|
| (60) |
|
| Glynn et al,
2009 | Caucasian,
mixed | NA | TaqMan and PCR | 322 |
|
| – |
|
| (61) |
|
| Yao et al,
2010 | Mixed | NA | MALDI-TOF | 432 |
|
| – |
|
| (62) |
|
| Hubackova et
al, 2012 | Caucasian | NA | TaqMan | 59 |
|
| – |
|
| (63) |
|
| Cronin-Fenton et
al, 2014 | Dan | NA | TaqMan | 326 |
|
| – |
|
| (64) |
|
| Tengström et
al, 2014 | Caucasian | NA | PCR-RFLP | 64 |
|
| – |
|
| (65) |
Data synthesis
SOD2 polymorphism and risk analysis
To estimate the association between breast cancer
risk and SOD2 gene polymorphism, the ORs and their corresponding
95% CIs were reconstructed from the 27 cohorts. As demonstrated in
Table II and Fig. 2, in all of the cohorts included in
the present analysis, there were no significant relationships found
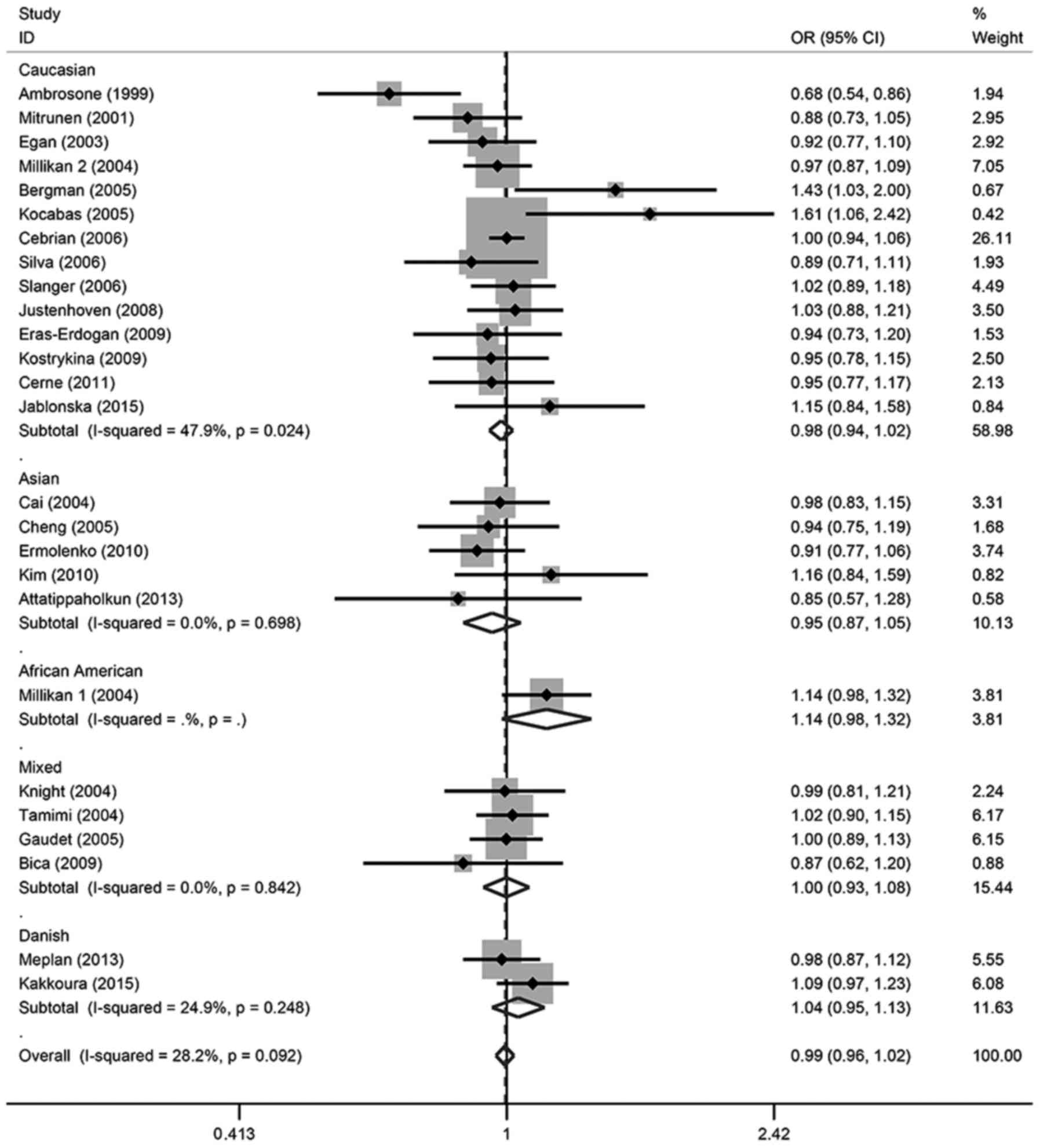
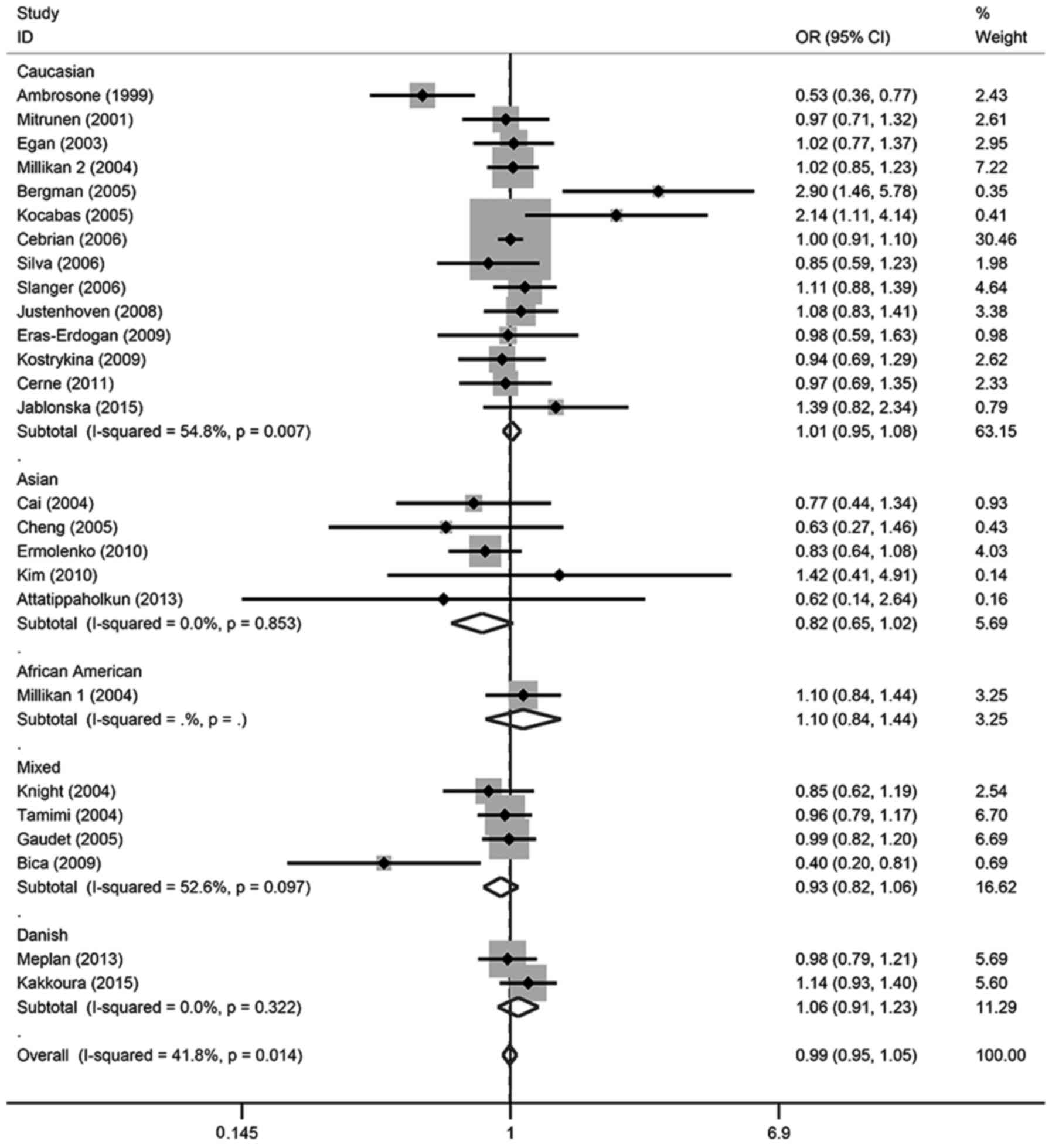
in any of the genetic models. For the allele contrast, the
wild-type allele did not increase or decrease the risk of breast
cancer compared with the variant allele (T vs. C: OR, 0.99; 95% CI,
0.96–1.02). There was no association between breast cancer risk and
SOD2 gene polymorphism in two co-dominant models (CT vs. CC: OR,
1.00; 95% CI, 0.95–1.05; TT vs. CC: OR, 0.98; 95% CI, 0.92–1.05),
one recessive model (TT vs. CT + CC: OR, 1.07; 95% CI, 0.94–1.21)
or one dominant model (CT + TT vs. CC: OR, 0.99; 95% CI,
0.95–1.05).
 | Table II.Summary of the included studies in
the risk analysis regarding the ethnicity of patients. |
Table II.
Summary of the included studies in
the risk analysis regarding the ethnicity of patients.
| Polymorphisms | Ethnicity | Studies, n | Participants,
n | Odds ratio | 95% confidence
interval | P-value | Egger's value | Begg's value |
|---|
| T vs. C | Caucasian | 14 | 20,589 | 0.98 | 0.94–1.02 | 0.357 |
|
|
|
| Asian | 5 | 5,867 | 0.95 | 0.87–1.05 | 0.319 |
|
|
|
| Danish | 2 | 4,108 | 1.04 | 0.95–1.13 | 0.388 |
|
|
|
| African
American | 1 | 1,437 | 1.14 | 0.98–1.32 | 0.080 |
|
|
|
| Mixed | 4 | 55,23 | 1.00 | 0.93–1.08 | 0.971 |
|
|
|
| Total | 26 | 37,524 | 0.99 | 0.96–1.02 | 0.702 | 0.612 | 0.865 |
| CT vs. CC | Caucasian | 14 | 15,821 | 1.03 | 0.97–1.10 | 0.351 |
|
|
|
| Asian | 5 | 2,236 | 0.82 | 0.65–1.03 | 0.089 |
|
|
|
| Danish | 2 | 2,958 | 1.05 | 0.89–1.22 | 0.574 |
|
|
|
| African
American | 1 | 982 | 1.00 | 0.75–1.33 | 0.991 |
|
|
|
| Mixed | 4 | 4,139 | 0.90 | 0.79–1.03 | 0.136 |
|
|
|
| Total | 26 | 26,136 | 1.00 | 0.95–1.05 | 0.961 | 0.523 | 0.810 |
| TT vs. CC | Caucasian | 14 | 10,142 | 0.97 | 0.89- 1.05 | 0.409 |
|
|
|
| Asian | 5 | 4,066 | 0.80 | 0.62–1.03 | 0.090 |
|
|
|
| Danish | 2 | 2,067 | 1.08 | 0.91–1.29 | 0.386 |
|
|
|
| African
American | 1 | 708 | 1.27 | 0.93–1.73 | 0.129 |
|
|
|
| Mixed | 4 | 2,708 | 0.99 | 0.85–1.15 | 0.850 |
|
|
|
| Total | 26 | 19,691 | 0.98 | 0.92–1.05 | 0.601 | 0.440 | 0.514 |
| TT vs. CT + CC | Caucasian | 14 | 20,589 | 0.94 | 0.88–1.00 | 0.059 |
|
|
|
| Asian | 6 | 6,351 | 1.03 | 0.92–1.16 | 0.558 |
|
|
|
| Danish | 2 | 4,108 | 1.05 | 0.91–1.20 | 0.505 |
|
|
|
| African
American | 1 | 1,437 | 1.27 | 1.01–1.59 | 0.037 |
|
|
|
| Mixed | 4 | 5,523 | 1.07 | 0.94–1.21 | 0.312 |
|
|
|
| Total | 27 | 38,008 | 1.00 | 0.95–1.05 | 0.954 | 0.755 | 0.904 |
| CT + TT vs. CC | Caucasian | 14 | 21,068 | 1.01 | 0.95–1.08 | 0.742 |
|
|
|
| Asian | 5 | 5,867 | 0.82 | 0.65–1.02 | 0.073 |
|
|
|
| Danish | 2 | 4,108 | 1.06 | 0.91–1.23 | 0.456 |
|
|
|
| African
American | 1 | 1,437 | 1.10 | 0.84–1.44 | 0.505 |
|
|
|
| Mixed | 4 | 5,523 | 0.93 | 0.82–1.06 | 0.280 |
|
|
|
| Total | 26 | 37,524 | 0.99 | 0.95–1.05 | 0.839 | 0.737 | 0.261 |
In the subgroup analysis, there was also no
significant association detected in different ethnicities or
menopausal status, except for TT vs. CT + CC in Caucasian patients,
which demonstrated a marginal association (OR, 0.94; 95% CI,
0.88–1.00); however, this association was not significant
(P>0.05). These details were listed in Tables II and III, and Figs.
2–6.
 | Table III.Summary of the included studies in
the risk analysis regarding the menopausal status of patients. |
Table III.
Summary of the included studies in
the risk analysis regarding the menopausal status of patients.
| Polymorphisms | Menopausal
status | Studies, n | Participants,
n | Odds ratio | 95% confidence
interval | P-value | Egger's value | Begg's value |
|---|
| T vs. C | Premenopausal | 8 | 3,962 | 0.93 | 0.85–1.01 | 0.095 | 0.138 | 0.101 |
|
| Postmenopausal | 9 | 6,182 | 0.97 | 0.90–1.04 | 0.417 | 0.404 | 0.341 |
| CT vs. CC | Premenopausal | 8 | 2,924 | 0.94 | 0.80–1.10 | 0.419 | 0.621 | 0.566 |
|
| Postmenopausal | 9 | 4,604 | 0.99 | 0.87–1.12 | 0.846 | 0.404 | 0.225 |
| TT vs. CC | Premenopausal | 8 | 1,993 | 0.87 | 0.72–1.04 | 0.136 | 0.458 | 0.204 |
|
| Postmenopausal | 9 | 3,110 | 0.94 | 0.82–1.09 | 0.433 | 0.144 | 0.128 |
| TT vs. CT + CC | Premenopausal | 8 | 3,962 | 0.89 | 0.77–1.04 | 0.135 | 0.138 | 0.227 |
|
| Postmenopausal | 9 | 6,182 | 0.95 | 0.85–1.07 | 0.389 | 0.095 | 0.029 |
| CT + TT vs. CC | Premenopausal | 9 | 5,475 | 0.89 | 0.77–1.03 | 0.121 | 0.144 | 0.242 |
|
| Postmenopausal | 10 | 6,982 | 0.98 | 0.87–1.10 | 0.706 | 0.929 | 0.381 |
SOD2 polymorphism and survival analysis
No significant relationship was detected from the
present meta-analysis, which included eight studies that
investigated the association between SOD2 polymorphism and breast
cancer overall survival (OS). There were no significant differences
between patients with the T carrier and CC genotype (CT + TT vs.
CC: HR, 0.67; 95% CI, 0.29–1.06), or between TT and CC + CT
genotype (TT vs. CT + CC: HR, 1.00; 95% CI, 0.10–1.90). When
compared with the CC genotype, it was not demonstrated that TT or
CT genotypes had a better outcome (TT vs. CC: HR, 1.06; 95% CI,
0.45–1.68; CT vs. CC: HR, 1.21; 95% CI, 0.96–1.45). In addition,
due to the limited number of included studies, only one cohort
compared the T allele with C allele, so the OS could not be
evaluated (Table IV).
 | Table IV.Summary of the included studies in
the survival analysis. |
Table IV.
Summary of the included studies in
the survival analysis.
| Model | Variables | Studies, n | Hazard ratio (95%
confidence interval) | P-value | I-squared, % |
|---|
| Dominant | CC | 3 | Reference |
0.001 | 51.6 |
|
| CT/TT |
| 0.67
(0.29–1.06) |
|
|
| Recessive | CC/CT | 2 | Reference | 0.03 | 81.4 |
|
| TT |
| 1.00
(0.10–1.90) |
|
|
| Homozygote | CC | 4 | Reference |
0.001 | 86.5 |
|
| TT |
| 1.06
(0.45–1.68) |
|
|
| Heterozygote | CC | 2 | Reference | <0.001 | 0.0 |
|
| CT |
| 1.21
(0.96–1.45) |
|
|
| Allelic | C | 1 | Reference | – | – |
|
| T |
| 1.06
(0.94–1.20) |
|
|
Sensitivity analysis
Sensitivity analyses were carried out to determine
whether there was association between breast cancer risk and SOD2
gene polymorphism. The inclusion criteria were altered to fit the
Hardy-Weinberg equilibrium (HWE) and nine cohorts were excluded due
to not meeting the HWE in the distribution of the genotype among
the controls. However, all the ORs and corresponding 95% CIs were
not substantially altered, suggesting that the results were
statistically robust (data not shown).
Bias analysis
To assess the potential publication bias, Egger's
and Begg's linear regression methods were applied for this
analysis. As demonstrated in Tables
II and III, there was no
evidence of statistically significant differences among the whole
analysis, except for the TT vs. CT + CC in the subgroup of
postmenopausal status, which had a Begg's value of 0.029 but an
Egger's value of 0.095 (Table
III). However, this result had little impact on the present
analysis.
Discussion
Due to the electron transport chain and environment
exposure in mitochondrion, many ROS are formed as a by-product
during cell metabolic or oxidative phosphorylation processes, such
as hydrogen peroxide, superoxide anion radical and hydroxyl radical
(66). As a very important part of
the antioxidant defense system, SOD2 has a crucial role in
balancing oxidant stress and ROS in mitochondria (67). In an animal experiment, a murine
model with SOD2 gene deficiency demonstrated neurodegeneration,
myocardial injury and perinatal death due to the impaired SOD2
activity (68). As a polymorphic
enzyme, the SOD2 gene has a series of SNPs, such as Val-16Ala and
Ile58Thr polymorphisms (20,69), and the SNPs in the oxidative
stress-related genes have some links with cancer risk (70). In a review, several studies
demonstrated that, in cancer cells, the activity and expression of
SOD2 was usually lower compared with the normal cells (71). Thus, various studies have focused on
illuminating the association between SOD2 polymorphism and breast
cancer; however, the results have remained controversial and
uncertain.
In 1999, the first research reporting the
relationship between SOD2 Val-16Ala polymorphism and breast cancer
in a Caucasian population was conducted by Ambrosone et al
(25), which drew a conclusion that
SOD2 had a significant role in breast cancer risk, particularly in
premenopausal women. More specifically, premenopausal women with
homozygous CC demonstrated a 4-fold higher risk of developing
breast cancer compared with heterozygote CT or homozygous TT (OR,
4.3; 95% CI, 1.7–10.8). In 2005, Bergman et al (32) conducted a case-control study that
included 118 women with early onset breast cancer and 174
age-matched controls, which indicated that SOD2 TT and CT genotype
could increase the prevalence rate of breast cancer (OR, 2.7; 95%
CI, 2.2–5.5; OR, 3.0; 95% CI, 1.4–6.5). In the same year, Kocabaş
et al (35) also carried out
another case-control study, including 103 patients and 84 controls,
demonstrating a similar result to Bergman et al (32); however, no significant difference
about the risk of allele T and C was observed. Furthermore, the
majority of other publications suggested that the SOD2 Val-16Ala
could not increase or decrease breast cancer risk and survival
(26–31,33,34,36–50).
It is widely accepted that meta-analyses have been
the gold standard to judge the association between risk factors and
diseases (72). In 2008, Bag and Bag
(51) conducted the first
meta-analysis investigating the association between Val-16Ala and
breast cancer, with 13 publications that included 7,366 cases and
9,102 controls. Their findings indicated no overall association
with the Val-16Ala polymorphism (25). Two years later, Ma et al
(53) and Qiu et al (55) conducted two independent
meta-analyses, respectively, and obtained the same negative
conclusion. Although the analysis of Qiu et al (55) involved 58,448 subjects of 26,022
cases and 32,426 controls, the patient resources of several
case-control studies were mixed and unavailable at present.
Subsequently, two relative meta-analyses were published in 2011 and
2012, but all of the included studies and cohorts were carried out
before 2009 (52,54). From 2010–2016, there were another
eight studies published, and so the present meta-analysis was
conducted (43–50).
The present updated meta-analysis consisted of
18,481 cases and 19,527 controls from 27 cohorts or case-control
studies for risk analysis and 5,746 cancer patients from eight
studies for survival analysis. It aimed to more accurately estimate
and investigate the association between the SOD2 Val-16Ala
polymorphism and breast cancer risk or survival. From the present
analysis, a marginal association between breast cancer risk and SOD
polymorphism was demonstrated in terms of TT vs. CT + CC genotype
in Caucasian patients, which means the TT genotype may slightly
decrease the risk of breast cancer compared with CT + CC. However,
no other positive results were observed in the risk and survival
analysis of breast cancer, which demonstrated no direct
relationship between SOD2 polymorphism and breast cancer.
There were some limitations in the present
meta-analysis. Given these, it is necessary to carefully analyze
some considerable issues that may affect the study conclusion, to
obtain a more cautious result. First, the quantity of the included
studies cannot satisfy the condition of meta-analysis for the
survival analysis, although every effort was made to search
carefully on the PubMed and EMBASE databases with the various
combinations of search terms by a computer-aided bibliographic
technology. Therefore, the power about the SOD2 polymorphism and
breast cancer survival may not be sufficient to make a statistical
statement, and the conclusion is also limited, which requires more
trails and larger sample sizes to clarify the relationship. Second,
the included participants coming from hospital or population may
have had some underlying diseases, which may influence the health
of participants and the conclusion of the present study. For
example, three studies had unclear expression about the underlying
diseases in the controls (29,35,43).
There was no evidence of statistically significant publication bias
according to the graphical funnel plots, and Egger's and Begg's
linear regression methods; however, the potential bias cannot be
ignored, and this may have affected the final conclusion. Only
English publications were available and included in this
meta-analysis and the rest were out of scope because the
investigators could not understand the language. Last but not
least, several different genotyping methods were applied in the
studies used, such as PCR-restriction fragment length polymorphism,
TaqMan and matrix-assisted laser desorption/ionization-time of
flight mass spectrometry, which maybe make a difference to the
present conclusion.
In conclusion, the present meta-analysis indicated
that there was no significant relationship between SOD2 Val-16Ala
polymorphism and breast cancer risk or survival, although in
Caucasian patients, the SOD2 TT genotype may marginally decrease
the risk of breast cancer in comparison to the CT + CC genotype.
Given this conclusion, more multicenter high-quality
epidemiological studies or randomized controlled trials with a
larger sample size should be conducted to clarify the association
between the SOD2 Val-16Ala polymorphism and breast cancer risk or
survival.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81171320).
Glossary
Abbreviations
Abbreviations:
|
SOD
|
superoxide dismutase
|
|
OR
|
odds ratio
|
|
HR
|
hazards ratio
|
|
ROS
|
reactive oxygen species
|
|
CI
|
confidence interval
|
|
SNP
|
single nucleotide polymorphism
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hulka BS and Stark AT: Breast cancer:
Cause and prevention. Lancet. 346:883–887. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adami HO, Signorello LB and Trichopoulos
D: Towards an understanding of breast cancer etiology. Semin Cancer
Biol. 8:255–262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeBruin LS and Josephy PD: Perspectives on
the chemical etiology of breast cancer. Environ Health Perspect.
110 Suppl 1:S119–S128. 2002. View Article : Google Scholar
|
|
5
|
Lichtenstein P, Holm NV, Verkasalo PK,
Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A and Hemminki
K: Environmental and heritable factors in the causation of
cancer-analyses of cohorts of twins from Sweden, Denmark, and
Finland. N Engl J Med. 343:78–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao Z, Shi A, Lu C, Song T, Zhang Z and
Zhao J: Breast Cancer: Epidemiology and Etiology. Cell Biochem
Biophys. 72:333–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becuwe P, Ennen M, Klotz R, Barbieux C and
Grandemange S: Manganese superoxide dismutase in breast cancer:
From molecular mechanisms of gene regulation to biological and
clinical significance. Free Radic Biol Med. 77:139–151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blokhina O, Virolainen E and Fagerstedt
KV: Antioxidants, oxidative damage and oxygen deprivation stress: A
review. Ann Bot. 91:179–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diao QX, Zhang JZ, Zhao T, Xue F, Gao F,
Ma SM and Wang Y: Vitamin E promotes breast cancer cell
proliferation by reducing ROS production and p53 expression. Eur
Rev Med Pharmacol Sci. 20:2710–2717. 2016.PubMed/NCBI
|
|
10
|
Paul S, Sengupta S, Bandyopadhyay TK and
Bhattacharyya A: Stevioside induced ROS-mediated apoptosis through
mitochondrial pathway in human breast cancer cell line MCF-7. Nutr
Cancer. 64:1087–1094. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren G, Luo W, Sun W, Niu Y, Ma DL, Leung
CH, Wang Y, Lu JJ and Chen X: Psoralidin induced reactive oxygen
species (ROS)-dependent DNA damage and protective autophagy
mediated by NOX4 in breast cancer cells. Phytomedicine. 23:939–947.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Shu F, Liang X, Chang H, Shi L,
Peng X, Zhu J and Mi M: Ampelopsin induces cell growth inhibition
and apoptosis in breast cancer cells through ROS generation and
endoplasmic reticulum stress pathway. PLoS One. 9:e890212014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matés JM, Segura JA, Alonso FJ and Marquez
J: Oxidative stress in apoptosis and cancer: An update. Arch
Toxicol. 86:1649–1665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oberley TD and Oberley LW: Antioxidant
enzyme levels in cancer. Histol Histopathol. 12:525–535.
1997.PubMed/NCBI
|
|
15
|
Church SL, Grant JW, Meese EU and Trent
JM: Sublocalization of the gene encoding manganese superoxide
dismutase (MnSOD/SOD2) to 6q25 by fluorescence in situ
hybridization and somatic cell hybrid mapping. Genomics.
14:823–825. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Creagan R, Tischfield J, Ricciuti F and
Ruddle FH: Chromosome assignments of genes in man using mouse-human
somatic cell hybrids: Mitochondrial superoxide dismutase
(indophenol oxidase-B, tetrameric) to chromosome 6. Humangenetik.
20:203–209. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhar SK and St Clair DK: Manganese
superoxide dismutase regulation and cancer. Free Radic Biol Med.
52:2209–2222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosenblum JS, Gilula NB and Lerner RA: On
signal sequence polymorphisms and diseases of distribution. Proc
Natl Acad Sci USA. 93:4471–4473. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crawford A, Fassett RG, Geraghty DP, Kunde
DA, Ball MJ, Robertson IK and Coombes JS: Relationships between
single nucleotide polymorphisms of antioxidant enzymes and disease.
Gene. 501:89–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimoda-Matsubayashi S, Matsumine H,
Kobayashi T, Nakagawa-Hattori Y, Shimizu Y and Mizuno Y: Structural
dimorphism in the mitochondrial targeting sequence in the human
manganese superoxide dismutase gene. A predictive evidence for
conformational change to influence mitochondrial transport and a
study of allelic association in Parkinson's disease. Biochem
Biophys Res Commun. 226:561–565. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Shen M, Cai H, Liu K, Liu Y, Huang
Z, Liang C, Deng X, Ye J, Zou Q and Li J: Association between
manganese superoxide dismutase (MnSOD) polymorphism and prostate
cancer susceptibility: A meta-analysis. Int J Biol Markers.
31:e422–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao C, Qiu LX, Zhan P, Xue K, Ding H, Du
FB, Li J and Chen Q: MnSOD Val16Ala polymorphism and prostate
cancer susceptibility: A meta-analysis involving 8,962 subjects. J
Cancer Res Clin Oncol. 136:975–979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Wang F, Shi X, Dai J, Peng Y, Guo
X, Wang X, Shen H and Hu Z: Association between manganese
superoxide dismutase (MnSOD) Val-9Ala polymorphism and cancer
risk-A meta-analysis. Eur J Cancer. 45:2874–2881. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang SW: Superoxide dismutase 2 gene and
cancer risk: Evidence from an updated meta-analysis. Int J Clin Exp
Med. 8:14647–14655. 2015.PubMed/NCBI
|
|
25
|
Ambrosone CB, Freudenheim JL, Thompson PA,
Bowman E, Vena JE, Marshall JR, Graham S, Laughlin R, Nemoto T and
Shields PG: Manganese superoxide dismutase (MnSOD) genetic
polymorphisms, dietary antioxidants and risk of breast cancer.
Cancer Res. 59:602–606. 1999.PubMed/NCBI
|
|
26
|
Mitrunen K, Sillanpää P, Kataja V,
Eskelinen M, Kosma VM, Benhamou S, Uusitupa M and Hirvonen A:
Association between manganese superoxide dismutase (MnSOD) gene
polymorphism and breast cancer risk. Carcinogenesis. 22:827–829.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egan KM, Thompson PA, Titus-Ernstoff L,
Moore JH and Ambrosone CB: MnSOD polymorphism and breast cancer in
a population-based case-control study. Cancer Lett. 199:27–33.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai Q, Shu XO, Wen W, Cheng JR, Dai Q, Gao
YT and Zheng W: Genetic polymorphism in the manganese superoxide
dismutase gene, antioxidant intake, and breast cancer risk: Results
from the Shanghai Breast Cancer Study. Breast Cancer Res.
6:R647–R655. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Knight JA, Onay UV, Wells S, Li H, Shi EJ,
Andrulis IL and Ozcelik H: Genetic Variants of GPX1 and SOD2 and
breast cancer risk at the ontario site of the breast cancer family
registry. Cancer Epidemiol Biomarkers Prev. 13:146–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Millikan RC, Player J, de Cotret AR,
Moorman P, Pittman G, Vannappagari V, Tse CK and Keku T: Manganese
superoxide dismutase Ala-9Val polymorphism and risk of breast
cancer in a population-based case-control study of African
Americans and whites. Breast Cancer Res. 6:R264–R274. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tamimi RM, Hankinson SE, Spiegelman D,
Colditz GA and Hunter DJ: Manganese superoxide dismutase
polymorphism, plasma antioxidants, cigarette smoking and risk of
breast cancer. Cancer Epidemiol Biomarkers Prev. 13:989–996.
2004.PubMed/NCBI
|
|
32
|
Bergman M, Ahnström M, Wegman Palmebäck P
and Wingren S: Polymorphism in the manganese superoxide dismutase
(MnSOD) gene and risk of breast cancer in young women. J Cancer Res
Clin Oncol. 131:439–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng TC, Chen ST, Huang CS, Fu YP, Yu JC,
Cheng CW, Wu PE and Shen CY: Breast cancer risk associated with
genotype polymorphism of the catechol estrogen-metabolizing genes:
A multigenic study on cancer susceptibility. Int J Cancer.
113:345–353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaudet MM, Gammon MD, Santella RM, Britton
JA, Teitelbaum SL, Eng SM, Terry MB, Bensen JT, Schroeder J, Olshan
AF, et al: MnSOD Val-9Ala genotype, pro- and anti-oxidant
environmental modifiers and breast cancer among women on Long
Island, New York. Cancer Causes Control. 16:1225–1234. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kocabaş NA, Şardaş S, Cholerton S, Daly
AK, Elhan AH and Karakaya AE: Genetic polymorphism of manganese
superoxide dismutase (MnSOD) and breast cancer susceptibility. Cell
Biochem Funct. 23:73–76. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cebrian A, Pharoah PD, Ahmed S, Smith PL,
Luccarini C, Luben R, Redman K, Munday H, Easton DF, Dunning AM and
Ponder BA: Tagging single-nucleotide polymorphisms in antioxidant
defense enzymes and susceptibility to breast cancer. Cancer Res.
66:1225–1233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Silva SN, Cabral MN, de Castro Bezerra G,
Pires M, Azevedo AP, Manita I, Pina JE, Rueff J and Gaspar J:
Breast cancer risk and polymorphisms in genes involved in
metabolism of estrogens (CYP17, HSD17beta1, COMT and MnSOD):
Possible protective role of MnSOD gene polymorphism Val/Ala and
Ala/Ala in women that never breast fed. Oncol Rep. 16:781–788.
2006.PubMed/NCBI
|
|
38
|
Slanger TE, Chang-Claude J and Wang-Gohrke
S: Manganese superoxide dismutase Ala-9Val polymorphism,
environmental modifiers, and risk of breast cancer in a German
population. Cancer Causes Control. 17:1025–1031. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Justenhoven C, Hamann U, Schubert F,
Zapatka M, Pierl CB, Rabstein S, Selinski S, Mueller T, Ickstadt K,
Gilbert M, et al: Breast cancer: A candidate gene approach across
the estrogen metabolic pathway. Breast Cancer Res Treat.
108:137–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bica CG, De Moura Da Silva LL, Toscani NV,
da Cruz IB, Sá G, Graudenz MS and Zettler CG: MnSOD gene
polymorphism association with steroid-dependent cancer. Pathol
Oncol Res. 15:19–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eras-Erdogan N, Akbas E, Senli H, Kul S
and Çolak T: Relationship between polymorphism in the manganese
superoxide dismutase gene and breast cancer. Mutat Res. 680:7–11.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kostrykina NA, Pechkovskiy EA, Boyarskikh
UA, Sushko AG, Voronina EN, Lazarev AF, Petrova VD, Zarubina NA,
Selezneva IA, Sinkina TV, et al: Associations of polymorphic
variant of MnSOD gene with breast cancer in residents of the Altai
Region. Bull Exp Biol Med. 147:84–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kostrykina Ermolenko NA, Boyarskih UA,
Voronina E, Sushko AG, Selezneva IA and Lazarew AF: Associations of
polymorphisms in genes antioxidant enzymes and detoxification
enzymes and breast cancer risk in residents of the west Siberian
region. J Clinical Oncol. 28:2010.
|
|
44
|
Kim MK, Ahn SH, Son BH and Sung MK: Plasma
antioxidant concentration, not superoxide dismutase polymorphism,
is associated with breast cancer risk in Korean women. Nutr Res.
30:705–713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cerne JZ, Pohar-Perme M, Novakovic S,
Frkovic-Grazio S, Stegel V and Gersak K: Combined effect of CYP1B1,
COMT, GSTP1, and MnSOD genotypes and risk of postmenopausal breast
cancer. J Gynecol Oncol. 22:110–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsai SM, Wu SH, Hou MF, Chen YL, Ma H and
Tsai LY: Oxidative stress-related enzyme gene polymorphisms and
susceptibility to breast cancer in non-smoking,
non-alcohol-consuming Taiwanese women: A case-control study. Ann
Clin Biochem. 49:152–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Attatippaholkun W and Wikainapakul K:
Predominant genotypes and alleles of two functional polymorphisms
in the manganese superoxide dismutase gene are not associated with
thai cervical or breast cancer. Asian Pac J Cancer Prev.
14:3955–3961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meplan C, Dragsted LO, Ravn-Haren G,
Tjønneland A, Vogel U and Hesketh J: Association between
polymorphisms in glutathione peroxidase and selenoprotein P genes,
glutathione peroxidase activity, HRT use and breast cancer risk.
PLoS One. 8:e733162013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jablonska E, Gromadzinska J, Peplonska B,
Fendler W, Reszka E, Krol MB, Wieczorek E, Bukowska A, Gresner P,
Galicki M, et al: Lipid peroxidation and glutathione peroxidase
activity relationship in breast cancer depends on functional
polymorphism of GPX1. BMC Cancer. 15:6572015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kakkoura MG, Demetriou CA, Loizidou MA,
Loucaides G, Neophytou I, Malas S, Kyriacou K and Hadjisavvas A:
MnSOD and CAT polymorphisms modulate the effect of the
Mediterranean diet on breast cancer risk among Greek-Cypriot women.
Eur J Nutr. 55:1535–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bag A and Bag N: Target sequence
polymorphism of human manganese superoxide dismutase gene and its
association with cancer risk: A review. Cancer Epidemiol Biomarkers
Prev. 17:3298–3305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu G, Sun G, Wang Y, Wang D, Hu W and
Zhang J: Association between manganese superoxide dismutase gene
polymorphism and breast cancer risk: A meta-analysis of 17,842
subjects. Mol Med Rep. 6:797–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma X, Chen C, Xiong H, Fan J, Li Y, Lin H,
Xu R, Huang G and Xu B: No association between SOD2 Val16Ala
polymorphism and breast cancer susceptibility: A meta-analysis
based on 9,710 cases and 11,041 controls. Breast Cancer Res Treat.
122:509–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen Y and Pei J: Possible risk
modifications in the association between MnSOD Ala-9Val
polymorphism and breast cancer risk: Subgroup analysis and
evidence-based sample size calculation for a future trial. Breast
Cancer Res Treat. 125:495–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qiu LX, Yao L, Mao C, Chen B, Zhan P, Yuan
H, Xue K, Zhang J and Hu XC: Lack of association between MnSOD
Val16Ala polymorphism and breast cancer risk: A meta-analysis
involving 58,448 subjects. Breast Cancer Res Treat. 123:543–547.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
57
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials revisited. Contemp Clin Trials. 45:139–145.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ambrosone CB, Ahn J, Singh KK, Rezaishiraz
H, Furberg H, Sweeney C, Coles B and Trovato A: Polymorphisms in
genes related to oxidative stress (MPO, MnSOD, CAT) and survival
after treatment for breast cancer. Cancer Res. 65:1105–1111.
2005.PubMed/NCBI
|
|
59
|
Udler M, Maia AT, Cebrian A, Brown C,
Greenberg D, Shah M, Caldas C, Dunning A, Easton D, Ponder B and
Pharoah P: Common germline genetic variation in antioxidant defense
genes and survival after diagnosis of breast cancer. J Clin Oncol.
25:3015–3023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bewick MA, Conlon MSC and Lafrenie RM:
Polymorphisms in manganese superoxide dismutase, myeloperoxidase
and glutathione-S-transferase and survival after treatment for
metastatic breast cancer. Breast Cancer Res Treat. 111:93–101.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Glynn SA, Boersma BJ, Howe TM, Edvardsen
H, Geisler SB, Goodman JE, Ridnour LA, Lønning PE, Børresen-Dale
AL, Naume B, et al: A mitochondrial target sequence polymorphism in
manganese superoxide dismutase predicts inferior survival in breast
cancer patients treated with cyclophosphamide. Clin Cancer Res.
15:4165–4173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yao S, Barlow WE, Albain KS, Choi JY, Zhao
H, Livingston RB, Davis W, Rae JM, Yeh IT, Hutchins LF, et al:
Manganese superoxide dismutase polymorphism, treatment-related
toxicity and disease-free survival in SWOG 8897 clinical trial for
breast cancer. Breast Cancer Res Treat. 124:433–439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hubackova M, Vaclavikova R, Ehrlichova M,
Mrhalova M, Kodet R, Kubackova K, Vrána D, Gut I and Soucek P:
Association of superoxide dismutases and NAD(P)H quinone
oxidoreductases with prognosis of patients with breast carcinomas.
Int J Cancer. 130:338–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cronin-Fenton DP, Christensen M, Lash TL,
Ahern TP, Pedersen L, Garne JP, Ewertz M, Autrup H, Sørensen HT and
Hamilton-Dutoit S: Manganese superoxide dismutase and breast cancer
recurrence: A Danish clinical registry-based case-control study and
a meta-analysis. PLoS One. 9:e874502014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tengström M, Mannermaa A, Kosma VM, Soini
Y, Hirvonen A and Kataja V: MnSOD rs4880 and XPD rs13181
polymorphisms predict the survival of breast cancer patients
treated with adjuvant tamoxifen. Acta Oncol. 53:769–775. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sosa V, Moline T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: A comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures,
evolution, and expression. Free Radic Biol Med. 33:337–349. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lebovitz RM, Zhang H, Vogel H, Cartwright
J Jr, Dionne L, Lu N, Huang S and Matzuk MM: Neurodegeneration,
myocardial injury, and perinatal death in mitochondrial superoxide
dismutase-deficient mice. Proc Natl Acad Sci USA. 93:9782–9787.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ho YS and Crapo JD: Isolation and
characterization of complementary DNAs encoding human
manganese-containing superoxide dismutase. FEBS Lett. 229:256–260.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Janicka A, Szymanska-Pasternak J and Bober
J: Polymorphisms in the oxidative stress-related genes and cancer
risk. Ann Acad Med Stetin. 59:18–28. 2013.(In Polish). PubMed/NCBI
|
|
71
|
Oberley LW and Buettner GR: Role of
superoxide dismutase in cancer: A review. Cancer Res. 39:1141–1149.
1979.PubMed/NCBI
|
|
72
|
Stewart LA and Parmar MK: Meta-analysis of
the literature or of individual patient data: Is there a
difference? Lancet. 341:418–422. 1993. View Article : Google Scholar : PubMed/NCBI
|