Introduction
Sentinel lymph node biopsy (SLNB) has replaced
complete axillary lymph node dissection (ALND) as the current
standard of care for axillary node staging in patients with
clinically node-negative breast cancer (1). When the sentinel nodes are found to be
free from metastatic disease, no further axillary treatment is
recommended (1). The role of ALND,
for patients with positive sentinel lymph nodes, is currently being
redefined. Research has indicated that patients with sentinel lymph
node (SLN) micrometastases treated with SLNB alone have similar
disease-free and overall survival to those receiving ALND (2). This has also been observed for stage
T1-2 disease with ≤2 macro-metastatic SLNs treated with breast
conservation surgery, whole-breast radiotherapy and adjuvant
systemic therapy (3). Therefore,
ALND may be over-treatment for patients with early breast cancer
and a low burden of axillary node involvement.
A reliable intraoperative technique that predicts
non-sentinel lymph node (NSLN) involvement would offer selective
and more conservative treatment of the axilla in a single surgical
procedure. This would avoid unnecessary surgery and its associated
morbidity while providing benefits to the patient, conserving
resources and complying with emerging clinical practice guidelines
(4).
SLN assessment with one-step nucleic acid
amplification (OSNA) provides an intraoperative molecular-based
objective whole-node assessment of SLN disease burden that is
independent of the size or number of lymph nodes tested (5). For these reasons, OSNA has greater
potential to predict NSLN involvement than routine histopathology
assessment, which cannot offer timely intraoperative SLN
evaluation, remains subjective, categorical, and is exposed to
sampling errors with sub-total node assessment (6,7). OSNA
amplifies cytokeratin-19 (CK19) mRNA in SLN samples, typically
providing a quantitative measurement of metastatic disease burden
in 35 min. The total CK19 mRNA copy number of a SLN biopsy (total
tumour load, TTL) may predict NSLN involvement and axillary node
disease burden, facilitating the decision to proceed with, or
avoid, complete ALND.
The present study compared SLN OSNA TTL with NSLN
involvement following ALND, and assessed the sensitivity and
specificity of TTL, and patient and tumour characteristics to
predict NSLN metastatic burden. The present findings have generated
a selective treatment protocol for the conservative management of
the axilla in patients with early breast cancer that may be used in
the pre- and intraoperative setting.
Patients and methods
Patients
The present study was a retrospective, single-centre
cohort study of patients diagnosed with breast cancer from
symptomatic and screening services. A total of 700 consecutive
patients (681 females and 3 males; mean age, 62 years; range, 23–93
years) treated between December 2012 and August 2015 at the Royal
Hallamshire Hospital (Sheffield, UK) who underwent a successful SLN
biopsy with OSNA assessment were studied. The patients had primary
invasive cT1-3 breast carcinoma, a clinically negative axilla,
normal pre-operative axillary ultrasound, or benign
ultrasound-guided axillary node biopsy, and were medically fit for
general anaesthetic and axillary treatment. Patients with prior
neo-adjuvant chemotherapy, ipsilateral axillary surgery, recurrent
disease or extensive ductal carcinoma in situ (DCIS) without
invasion were excluded. Subset analysis was performed for patients
who had metastatic axillary disease identified by OSNA and
subsequent ALND. The following parameters were recorded: Age,
tumour size and grade, multifocality, histological subtype, type of
surgery, oestrogen receptor status, human epidermal growth factor
receptor 2 (HER2) status, Ki67, the presence of lymphovascular
invasion, total number of SLNs and NSLNs, the number of positive
and negative SLNs and NSLNs, and ratio of the number of positive
SLNs to the total number of removed SLNs and NSLNs following ALND.
CK19 mRNA copy number (copies/µl) in each SLN were recorded. CK19
expression was not routinely tested on pre-operative biopsy
specimens. For statistical analysis, when ≥2 SLNs were involved,
the combined value of CK19 mRNA copies was calculated. The TTL was
defined as the total CK19 mRNA copy number in the positive SLNs
(with units of copies/µl). The TTL of the macrometastatic SLN
sample was compared with the total lymph node status and NSLN
status of ALND, following routine histological assessment with
haematoxylin and eosin staining (8).
SLN identification
SLNs were identified using a standard protocol of
combination radiopharmaceutical and blue dye, as described by
Mansel et al (9).
99mTc-labelled albumin nanocolloid
(Nanocoll®; GE Healthcare Life Sciences, Little
Chalfont, UK) was injected intradermally (0.1–0.5 ml) at a single
periareolar site corresponding to the tumour quadrant; 40 MBq the
day before surgery or 20 MBq on the day of surgery. Patent Blue V
dye (Laboratoire Guebet, Aulnay-sous-Bois, France), 2 ml undiluted,
was injected subdermally at a single periareolar site corresponding
to the tumour quadrant immediately prior to surgery. Under general
anaesthetic, SLNs were identified and removed prior to breast
tumour excision, and sent on ice to the Pathology Department. No
more than two nodes were sent for assessment by OSNA. Any
additional SLNs were sent for routine fixation, hematoxylin and
eosin staining (8) and delayed
reporting. Therapeutic local excision, therapeutic mammoplasty or
mastectomy was performed as part of the planned breast cancer
treatment. Each SLN, trimmed of fat, was weighed and recorded. SLNs
weighing <50 mg were too small to be processed by OSNA, and were
therefore diverted to routine histological assessment. SLNs
weighing >600 mg were divided into two or more pieces and
processed separately, and the results combined.
OSNA
The OSNA assay (Sysmex Europe GmbH, Norderstedt,
Germany) was performed according to the manufacturer's protocols,
as described by Tsujimoto et al (5). Each SLN was homogenized in 4 ml
homogenizing buffer on ice. The lysate was centrifuged to remove
fat, cellular debris and other contaminants and the mRNA containing
supernatant was extracted and diluted. A 2-µl aliquot of the
buffered lymph node lysate was used for automated real-time
amplification of CK19 mRNA via reverse transcription loop-mediated
isothermal amplification with a ready-to-use reagent kit on the
RD-100i (Sysmex Europe GmbH). The rate of amplification was
measured spectrophotometrically and the CK19 copy number was
calculated by comparison to a standard curve. Based on the number
of CK19 mRNA copies/µl, the result was assessed in accordance with
the cut-off levels determined in a study by Tsujimoto et al
(5), with macrometastasis (OSNA ++)
defined as >5,000 copies/µl of CK19 mRNA, micrometastasis (OSNA
+) as 250–5,000 copies/µl and non-metastasis (OSNA -) as <250
copies/µl. The OSNA results were immediately communicated by
telephone to the surgeon within 45 min of sample receipt. Patients
with at least one macrometastasis on intraoperative OSNA analysis
underwent levels I, II and III ALND. Between December 2012 and June
2013, a positive OSNA result for one or two nodes with
micro-metastases also triggered an immediate ALND. In June 2013,
the departmental protocol was amended to recommend the removal of
two further nodes for routine histological processing, with a
delayed ALND if these returned macrometastatic involvement.
The remaining lymph nodes not involved in the OSNA
test were processed according to the UK Breast Cancer pathology
protocol (8). Lymph nodes <5 mm
were bisected whereas larger nodes were sliced at 3-mm intervals
and single sections assessed using haematoxylin and eosin staining.
Immunohistochemical staining was not used for evaluation of
NSLNs.
Preoperative assessment of axilla
Patients underwent axillary ultrasonography (US) at
the time of breast assessment, or soon after the diagnosis of
breast carcinoma. Those with abnormal lymph node morphology
according to local protocol (cortical thickening 2–3 mm, focal
bulge, rounded shape, partial or complete loss of fatty hilum,
non-hilar blood flow, or partial/complete replacement of node with
mass) underwent US-guided lymph node biopsy. Patients with
confirmed invasive disease on lymph node biopsy proceeded to
neo-adjuvant therapy or ALND without SLNB.
Patient data were anonymised, and collected
retrospectively, without influence on patient therapy. The ethical
considerations of the present study were approved by the Clinical
Effectiveness Unit of the Sheffield Teaching Hospitals NHS Trust
(Sheffield, UK).
Statistical analysis
Distributions of continuous variables were
determined by visual inspection of frequency-distribution plots;
variables were summarised as the mean and confidence interval
(after transformation if required), or median and interquartile
range, as appropriate. Tests for association between categorical
variables were determined using the Chi-square and Fisher's exact
tests. P<0.05 was considered to indicate a statistically
significant difference. Receiver operating characteristic (ROC)
analyses were performed to compute the area under the curve (AUC)
to estimate concordance. The statistical analysis was conducted
using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC,
USA) for Windows 7.
Results
Clinical cohort
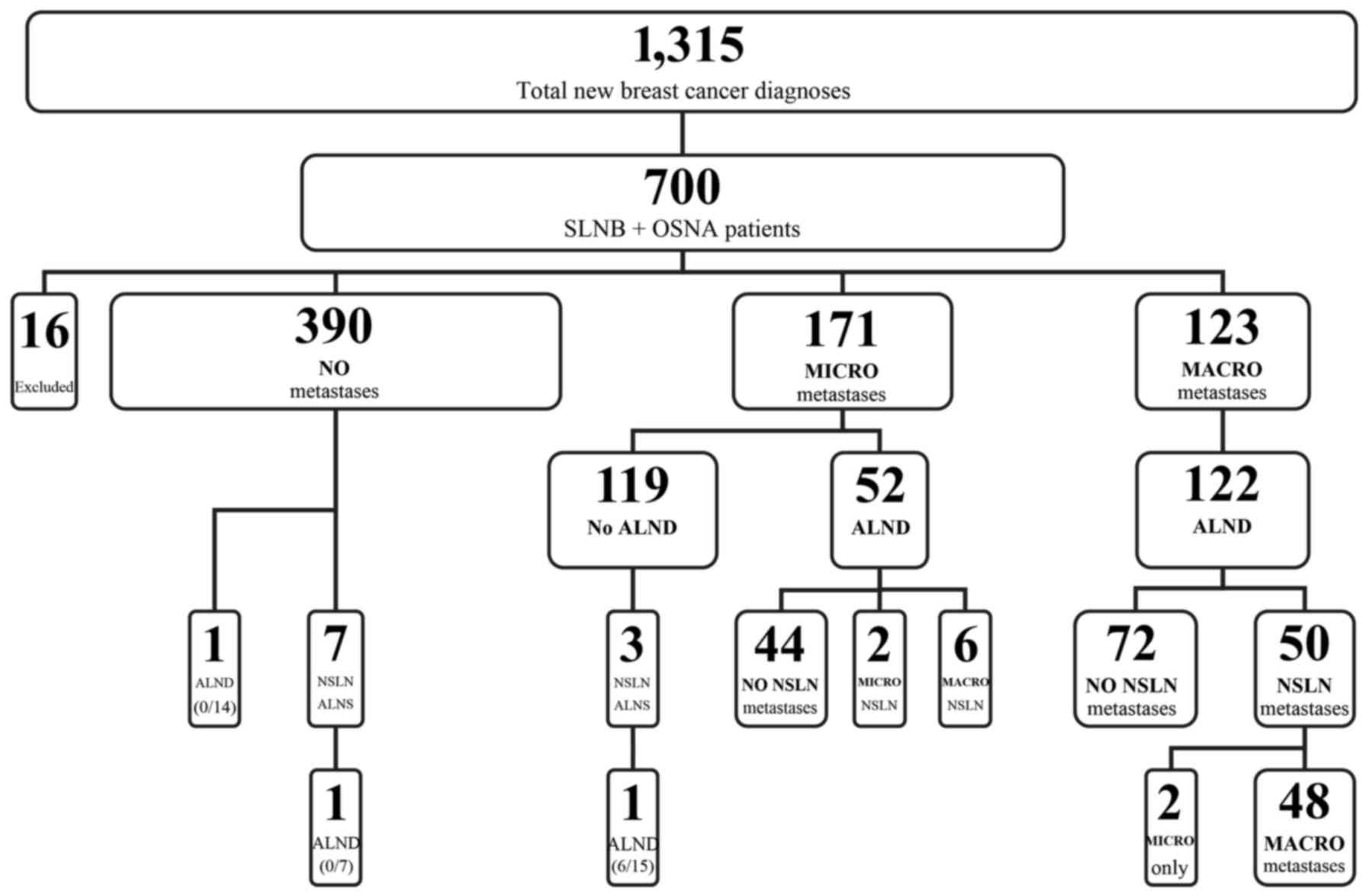
A total of 1,315 patients were diagnosed with breast
carcinoma between December 2012 and August 2015 at the institution
(Fig. 1). Of these, 700 consecutive
patients received OSNA during surgery for a preoperatively
identified cT1-T3 cN0 breast carcinoma. Sixteen cases were excluded
from the present study; 15 patients had extensive DCIS only and 1
patient had received incomplete neo-adjuvant therapy. There were
681 female patients and 3 male cases (mean age, 62 years; range,
23–93 years) in the remaining study cohort. Of the cases, 9 were
bilateral.
In contrast to the American College of Surgeons
Oncology Group (ACOSOG) Z0011 study (3), all the patients in our unit underwent
axillary US prior to breast surgery. Those with abnormal lymph node
morphology underwent immediate US-guided biopsy, and those with
confirmed metastatic disease were recommended to undergo ALND
without SLNB/OSNA, which was the protocol at the time of the
present study. These assessments removed 14.5% (191/1315) of our
total new diagnosis patient cohort from analysis. These patients
did not receive further assessment to explore the role of
conservative management of the axilla as part of their
treatment.
Clinicopathological
characteristics
The mean number of nodes harvested for OSNA per
patient was 1.94, with a total of 1,356 SLNs assessed. A total of
123/684 patients (17.9%) were found to have OSNA CK19 mRNA copy
numbers indicative of macro-metastasis and all but 1 patient
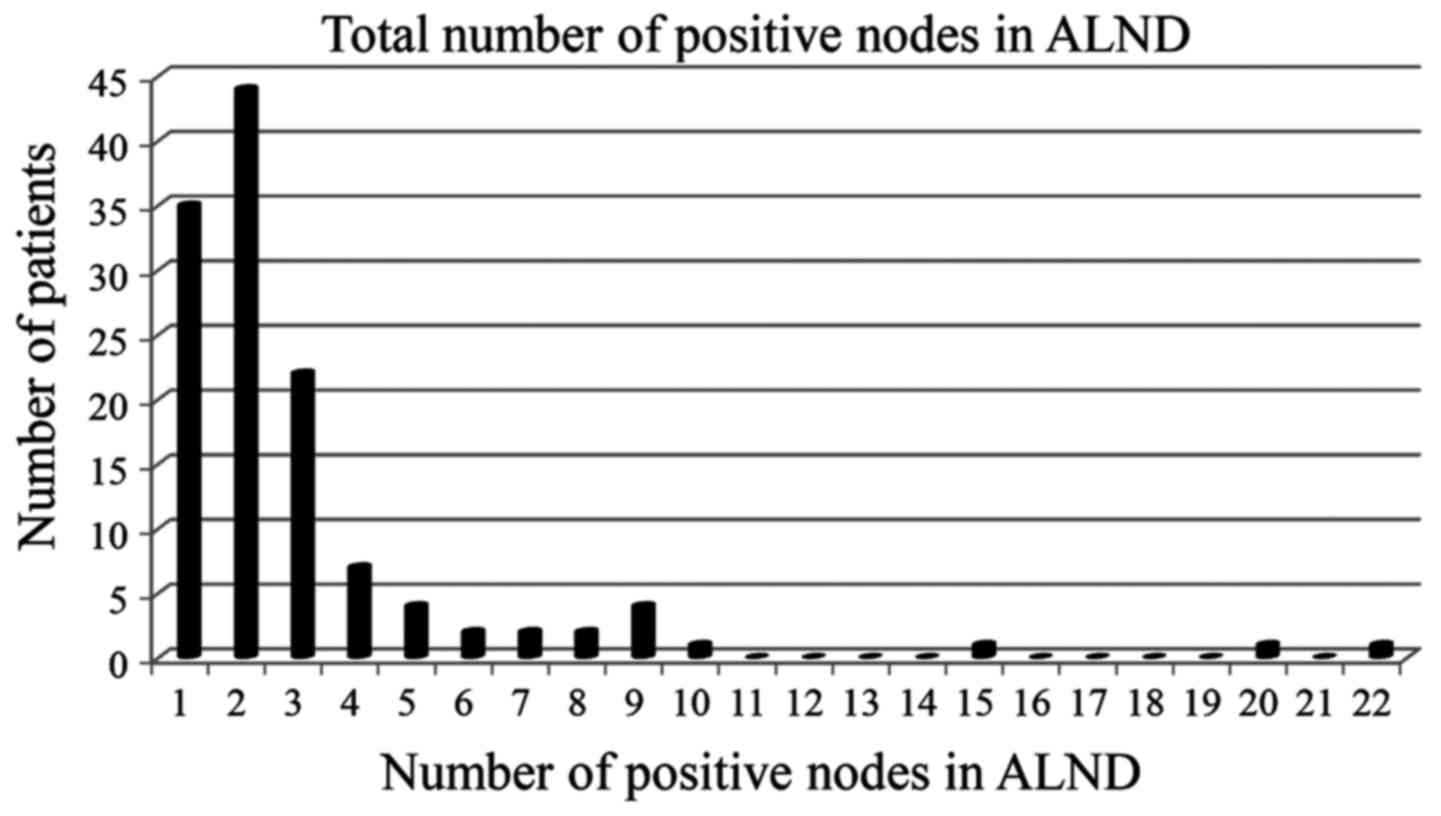
underwent ALND. In total, 45/122 (37%) patients had NSLN metastases
on ALND with a total positive lymph node burden exceeding the
ACOSOG Z0011 threshold of two macro-metastatic nodes (Fig. 2).
The distribution of tumour sizes within the cohort
displayed the expected log-normal distribution for total and
sub-group distribution of size by node involvement. There were 143
tumours with a diameter ≤10 mm, and none were associated with >2
macrometastases in ALND. TTL was the only clinicopathological
variable significantly associated with risk of NSLN involvement and
three or more positive nodes (P<0.0001). Tumour size (P=0.14),
tumour grade (P=0.84), oestrogen receptor status (P=0.09), HER2
(P=1.00) and lymphovascular invasion (P=0.30) were not
significantly associated with the risk of NSLN involvement and
three or more positive nodes (data not shown).
TTL, axillary lymph node burden and
prediction
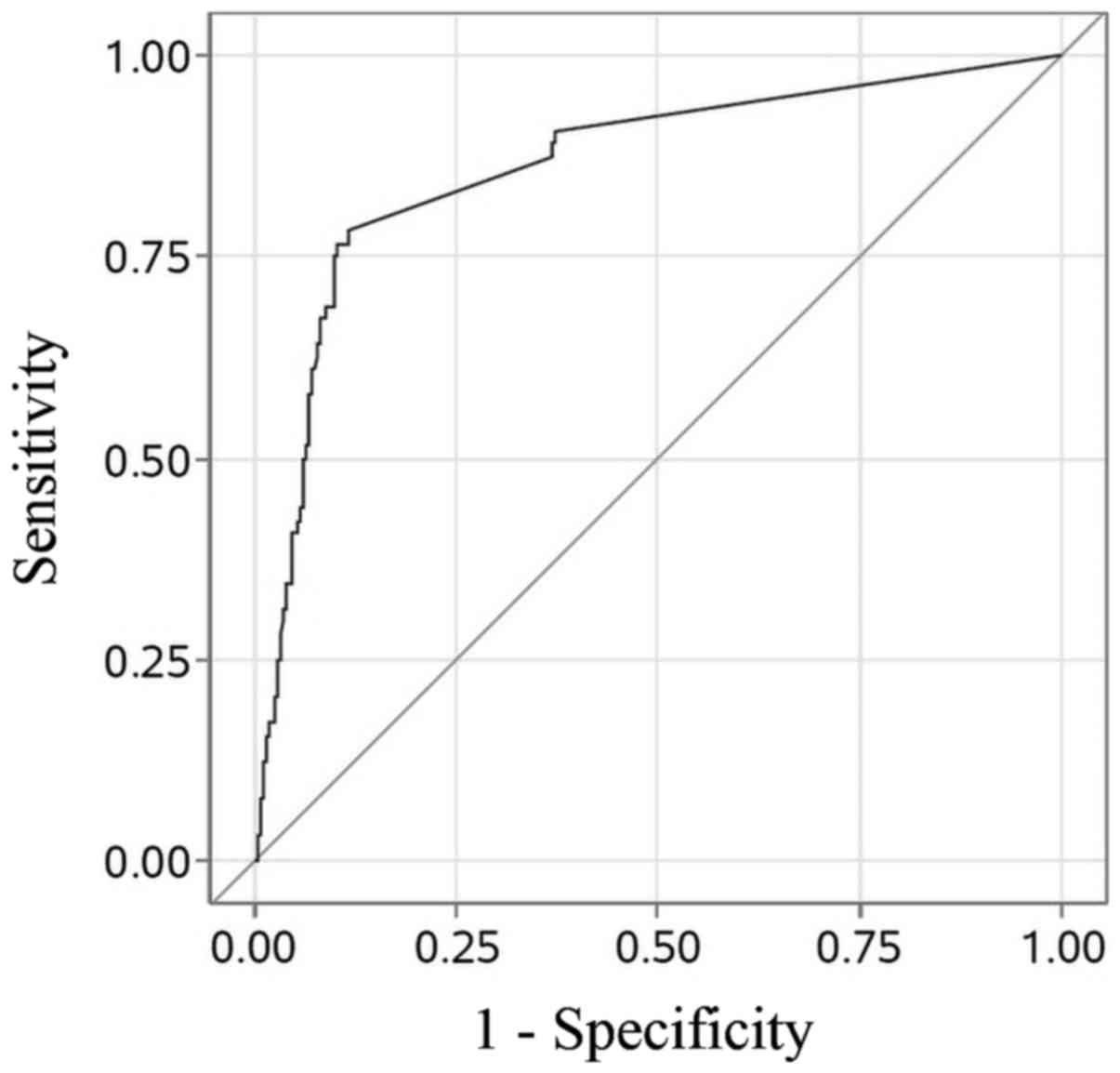
Sensitivity and specificity of OSNA TTL vs. NSLN
status using the protocol thresholds of TTL ≥250, ≥5000 and ≥15,000
copies/µl are detailed in Table I.
Diagnostic accuracy of TTL, as demonstrated by ROC AUC, was 0.86
(Fig. 3).
 | Table I.Sensitivities and specificities of
OSNA TTL of cytokeratin-19 mRNA copy numbers. |
Table I.
Sensitivities and specificities of
OSNA TTL of cytokeratin-19 mRNA copy numbers.
| A, OSNA by NSLN
using TTL cut-off of 250a copies/µl for all the
patients |
|
|
| NSLN |
|
|
|
|
|
|
|
|
|
|
|
| OSNA | + | − | Total |
Sensitivityb |
Specificityb | PPVb | NPVb |
|
| + | 58 | 231 | 289 | 0.906
(0.835–0.978) | 0.627
(0.589–0.665) | 0.201
(0.155–0.247) | 0.985
(0.973–0.997) |
| − | 6 | 388 | 394 |
|
|
|
|
| Total | 64 | 619 | 683 |
|
|
|
|
|
| B, OSNA by NSLN
using TTL cut-off of 5,000c copies/µl for all patients |
|
|
| NSLN |
|
|
|
|
|
|
|
|
|
|
|
| OSNA | + | − | Total |
Sensitivityb |
Specificityb | PPVb | NPVb |
|
| + | 50 | 72 | 122 | 0.781
(0.680–0.883) | 0.884
(0.858–0.909) | 0.410
(0.323–0.497) | 0.975
(0.962–0.988) |
| − | 14 | 547 | 561 |
|
|
|
|
| Total | 64 | 619 | 683 |
|
|
|
|
|
| C, OSNA by NSLN
using TTL cut-off of 15,000c copies/µl for all patients |
|
|
| NSLN |
|
|
|
|
|
|
|
|
|
|
|
| OSNA | + | − | Total |
Sensitivityb |
Specificityb | PPVb | NPVb |
|
| + | 44 | 58 | 102 | 0.815
(0.711–0.918) | 0.908
(0.885–0.930) | 0.431
(0.335–0.528) | 0.983
(0.972–0.992) |
| − | 10 | 571 | 581 |
|
|
|
|
| Total | 54 | 629 | 683 |
|
|
|
|
|
| D, OSNA by NSLN
using TTL cut-off of 15,000c copies/µl for patients undergoing
breast conserving surgery |
|
|
| NSLN |
|
|
|
|
|
|
|
|
|
|
|
| OSNA | + | − | Total |
Sensitivityb |
Specificityb | PPVb | NPVb |
|
| + | 20 | 35 | 55 | 0.870
(0.732–1.000) | 0.927
(0.903–0.950) | 0.364
(0.237–0.491) | 0.993
(0.986–1.000) |
| − | 3 | 441 | 444 |
|
|
|
|
| Total | 23 | 476 | 499 |
|
|
|
|
In the present cohort, 11.4% (13/114) of the grade 1
tumours had evidence of SLN or NSLN metastases, compared with 19.9%
(70/351) of the grade 2 and 19.4% (42/216) of the grade 3 tumours.
The maximum total axillary burden for any grade 1 tumour was four
nodes for an 18-mm tumour.
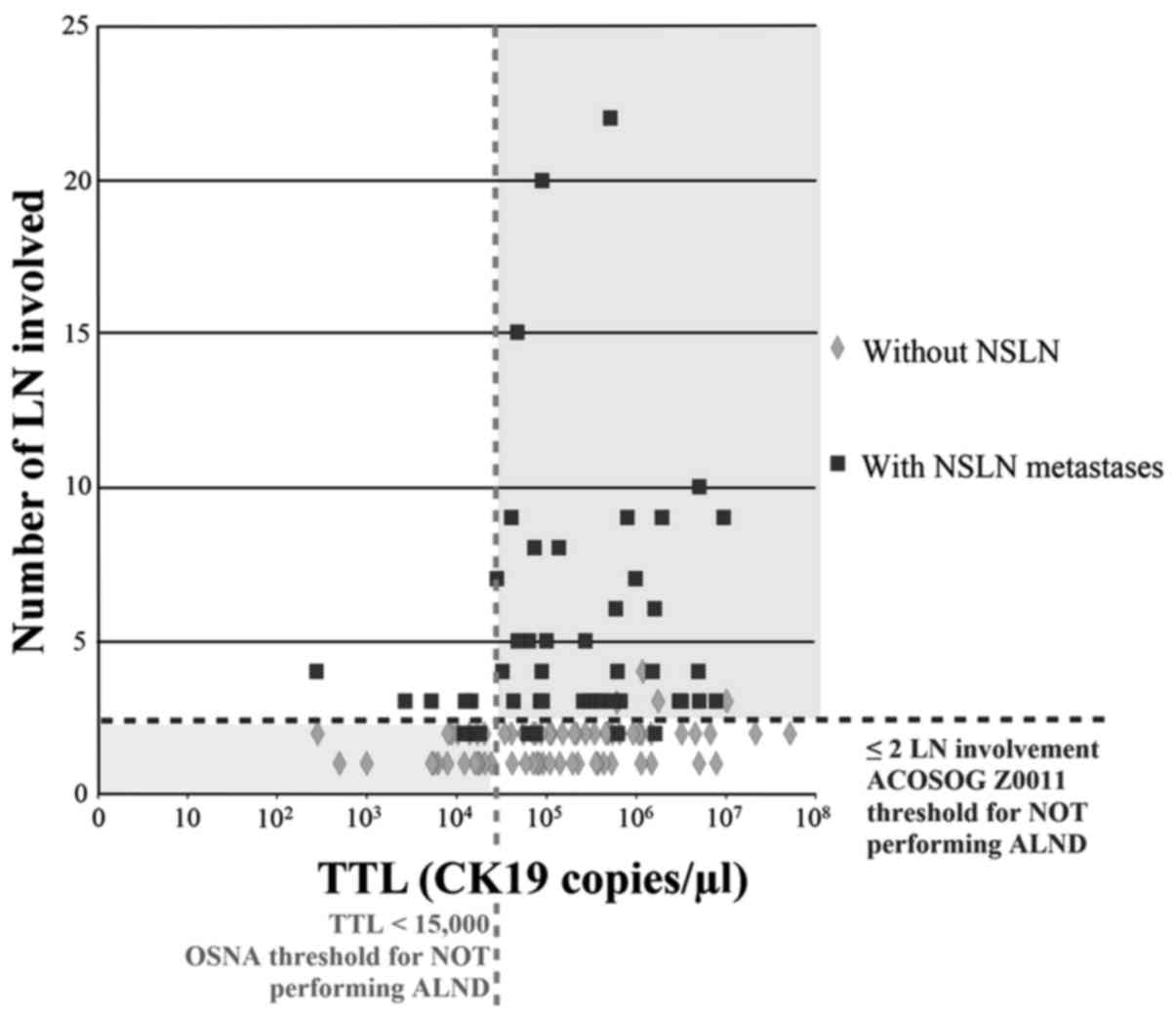
SLNB TTL vs. total lymph node involvement for the
entire cohort, and for the subset of patients undergoing breast
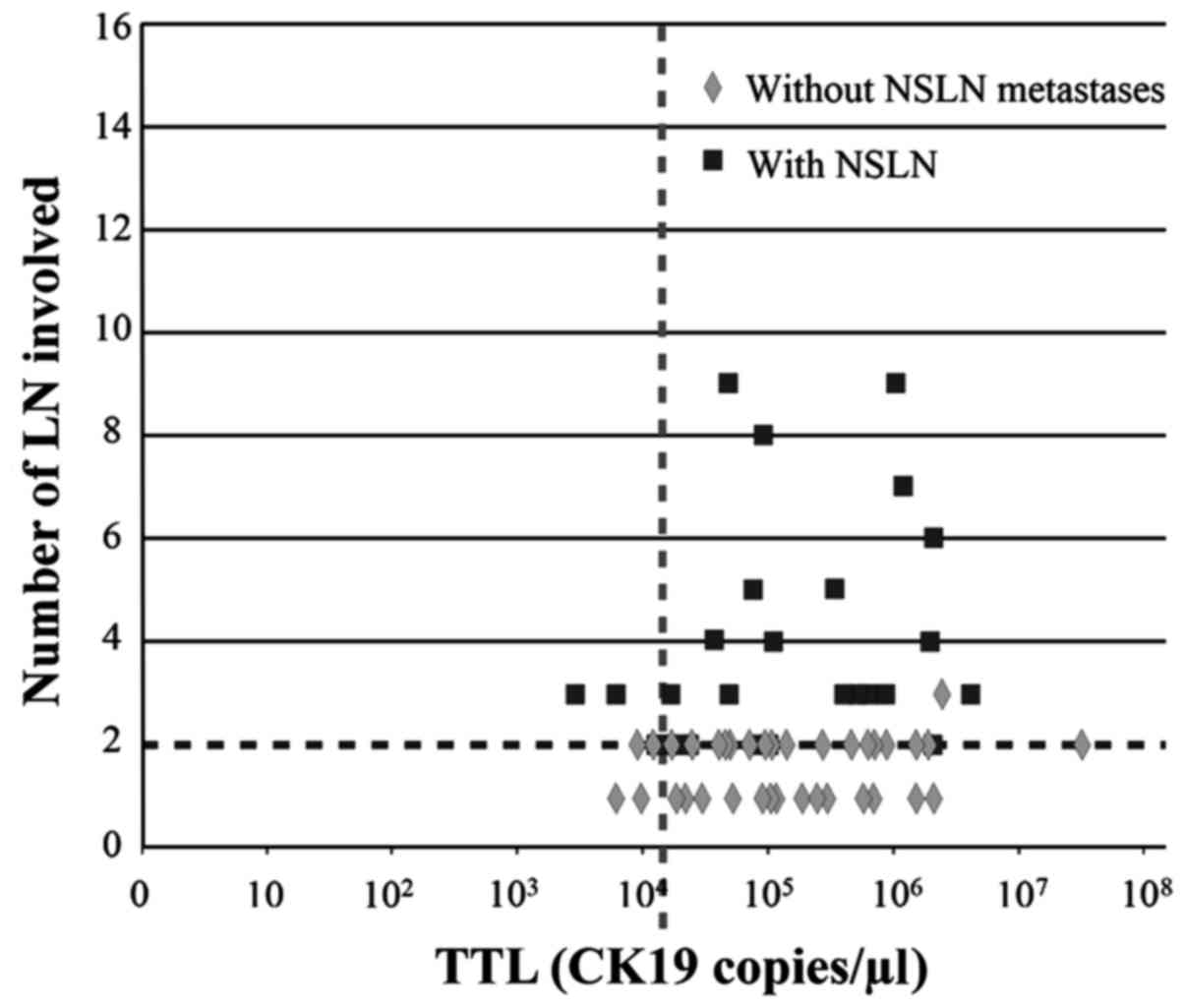
conserving surgery (BCS), was plotted (Figs. 4 and 5, respectively). The distribution of NSLN
metastases was partitioned vertically by the point separation of
data found at 15,000 copies/µl and horizontally by the two lymph
node involvement threshold by which the ACOSOG Z0011 trial would
not recommend ALND (3). BCS was
performed in 499/700 (71.2%) patients and they represented a
surrogate sub-group for comparison with the ACOSOG Z0011 (3) trial. However, the present group had
broader inclusion criteria and was selection-modified by
preoperative axillary US filtering. In total, 66/499 patients
(13.2%) had ALND, 27 (41%) with NSLN involvement and 39 (59%)
without NSLN involvement. In total, 20/23 (87%) patients with TTL
>15,000 copies/µl of CK19 mRNA had more than two involved NSLNs
on ALND. Sensitivity, specificity, positive predictive value and
negative predictive value of SLNB TTL vs. NSLN status using the
threshold of TTL ≥15,000 copies/µl for the subset of patients
undergoing BCS are detailed in Table
I. For patients with a SLNB TTL <15,000 copies/µl and who
had ≤2 nodes involved, the NPV was 0.983. According to Z0011
criteria, 35/499 (7.0%) patients could be regarded as being
over-treated with a 3/499 (0.6%) false-negative rate (potential
under-treatment).
Discussion
SLNB remains the standard of care for staging breast
carcinoma in the clinically uninvolved axilla (1). Research has suggested that ALND may be
safely omitted in patients with a low burden of axillary disease
(3,10,11),
particularly with micrometastatic disease only (12), but also where only one or two nodes
with macrometastases are identified (3). Identifying patients who should proceed
with ALND has been attempted with nomograms (13) and prediction models (7) with varying degrees of accuracy. Several
studies have reported methods to predict ≥4 lymph node metastases
(14–17). However, the majority of these
prediction models were constructed using predictors derived from
breast resection specimen pathology, such as lymphovascular
invasion, and have not been widely adopted as they fail to
confidently facilitate one-stage intraoperative decision making
about the role of ALND. A small number of studies have reported on
the prediction of NSLN metastases in SLN-positive patients,
including the use of SLN OSNA TTL (7,18,19).
OSNA was formally approved by the UK National
Institute of Clinical Excellence and included in routine surgical
practice in 2013 (20). OSNA is at
least equally cost effective as routine histology; however, it has
substantial patient benefits (21,22). It
remains the only intraoperative diagnostic test recommended for
whole-node analysis for detecting SLN metastases during breast
surgery in individuals with early invasive breast cancer.
As a categorical diagnostic tool, OSNA alone is
insufficient to determine which patients with macro-metastatic
nodal disease may be spared ALND. The present study revealed that
60.7% of the cohort with macrometastatic lymph nodes on SLN OSNA
had no further nodal involvement on ALND. Indeed, only 7.9%
(54/683) of the entire cohort had more than two nodes with
macrometastases. However, the continuous exponential quantification
of TTL extends its utility considerably as a predictive tool
(7,23–25).
Stratification of CK19 mRNA copy numbers facilitates intraoperative
decision-making for management of the axilla. In the present cohort
of patients, the ROC AUC of 0.86 for two node TTL indicated that
SLN TTL represents a good association with the presence of NSLN
metastases. However, developing a model that predicts more than two
node involvement and NSLN involvement is challenged by the small
numbers of patients who satisfy this subset. In total, 21% of
patients in the ALND arm of the Z0011 trial had additional positive
nodes (3) whereas this occurred in
37% of the present cohort, despite our routine use of preoperative
axillary US assessment. This observation may reflect a cohort of
patients with more favourable disease in the ACOSOG Z0011 study,
and/or higher sensitivity of lower disease burden detected with
OSNA. Furthermore, the present cohort included 21/683 (3%) patients
with pT3 tumours and the overall mean tumour size was 20 mm
compared to 16 mm in Z0011 (3). The
larger proportion of patients with additional NSLN node involvement
in the present cohort may have contributed to the generation of an
ROC AUC >0.8. Identifying any predictive markers with
statistical significance was not possible using traditional
clinicopathological factors in the present study. When
lymphovascular invasion was reported in the resection specimen, it
was twice as likely that NSLN metastases would be present than
otherwise. If lymphovascular invasion was reported as being absent,
the likelihood of NSLN metastases was 30% less. These findings,
however, did not translate into statistical significance for
predicting the risk of additional NSLN disease.
A study by Kubota et al (18) reported a single-centre standardised
method for SLN detection that used indocyanine green; whereas, the
types of injection and the use of radioisotope, with or without
dye, varied according to institutional practice in a multi-centre
study by Piñero-Madrona et al (19). The present single-centre dual
technique of SLN detection is uniform and standardised (9). Differences in the methods of SLN
detection, in particular OSNA TTL quantification restricted to two
nodes in the present study, may account for some of the differences
observed in ROC AUC values and in the association of NSLN
positivity with other putative predictive variables. The mean
number of SLNs removed in the studies by Kubota et al
(18) and Piñero-Madrona et
al (19) are not reported for
comparison. An average SLN harvest >2.0 may suggest some
persistence in retrieval, diluting the residual pool of NSLN with
the removal of some nodes that would otherwise be accounted for in
a subsequent ALND analysis. This would impact on how the
relationship between SLN TTL burden and associated NSLN positivity
is interpreted and compared in practice.
The present study identified an extension of the TTL
threshold for conservative management of the axilla beyond 5,000 to
15,000 copies/µl and confirmed the findings of Peg et al
(7). In the present study, 70.0%
(14/20) of patients with a SLN TTL between 5,000 and 15,000
copies/µl had no further metastatic disease identified on ALND.
Those patients with a SLN TTL ≥15,000 copies/µl continue to attract
the recommendation to proceed with ALND. In the present cohort,
43.1% (44/102) of these patients (44/683, 6.4% of total cohort) had
more than two involved nodes in total. The remaining 56.9% (58/683,
8.5% of total cohort) had only one or two positive nodes, and could
be regarded as having their axilla over-treated. In those patients
who underwent BCS, 20/23 (87%) patients who had more than two
involved NSLNs on ALND had a SLNB TTL >15,000 copies/µl. The
3/23 (13%) patients who had more than two nodes involved on ALND
following TTL <15,000 copies/µl on OSNA was markedly less than
the 23% of patients estimated to have had residual axillary disease
in the non-ALND arm of Z0011, which reported a 0.9 and 1.5%
regional recurrence rate at 5 and 10 years, respectively, for SLN
positive disease without ALND (3,26). Our
new recommended threshold for no ALND of TTL <15,000 copies/µl
of CK19 mRNA is also markedly less than the recently adjusted
threshold demonstrated by Peg et al (27) that correlates with disease-free,
local recurrence-free and overall survival. They defined a TTL
threshold of 25,000 CK19 mRNA copies/µl for a low-risk group below,
and a high-risk group above, this threshold (27).
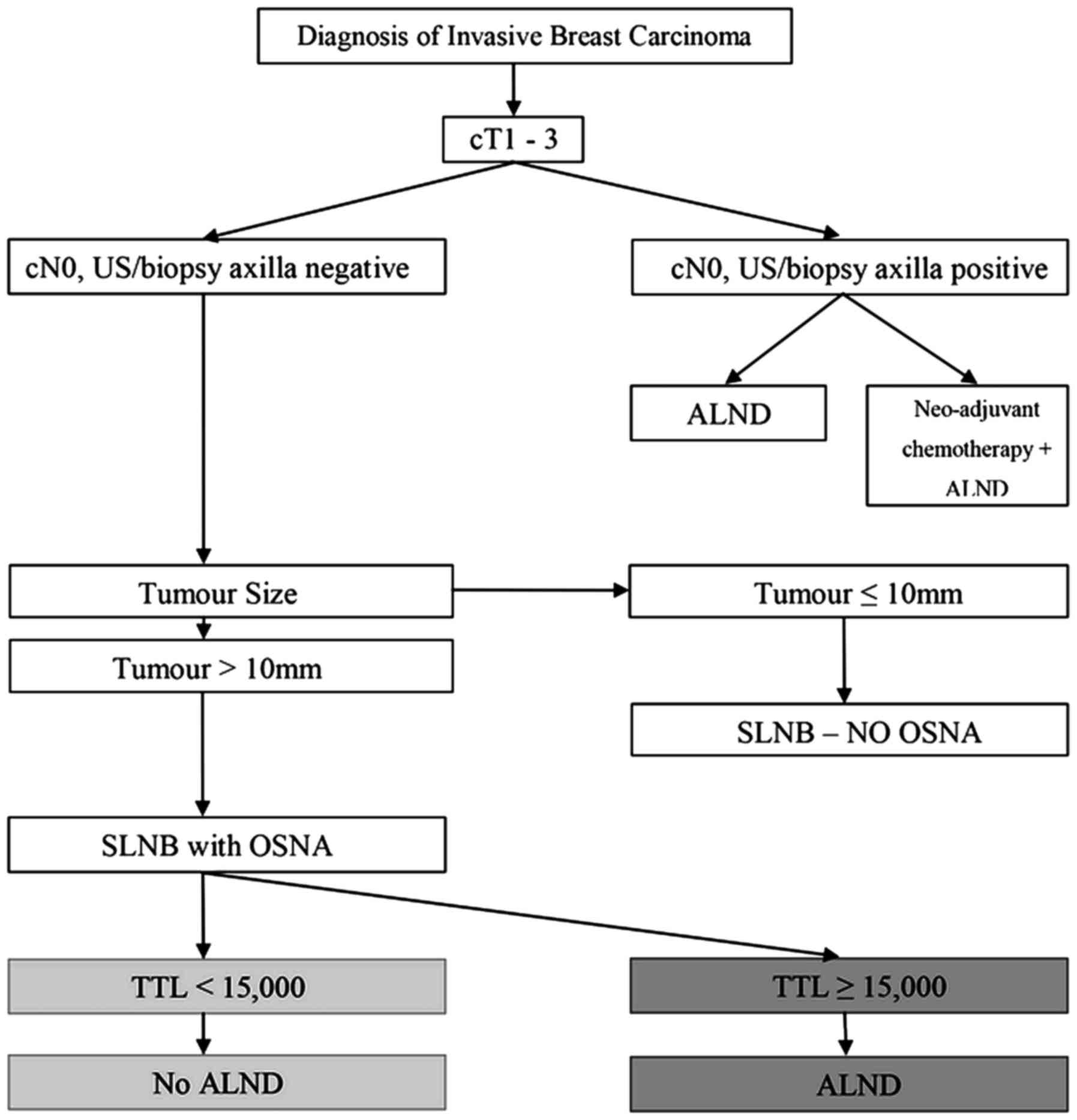
ALND is not recommended for patients when the SLNB
is deemed negative (OSNA TTL <250 copies/µl) or interpreted as
micrometastatic disease only (OSNA TTL between 250 and 5,000
copies/µl) [25.0% (171/683) in the present cohort] (2). Only 2.3% (4/171) of these patients had
more than two involved nodes. We would recommend that patients with
a tumour of <10 mm diameter receive standard SLNB without OSNA.
Only 5.6% (8/143) of these patients had evidence of metastatic
axillary disease, none had more than two involved nodes and the
rate of involvement was less than the reported 9% false-negative
rate for a two-node assessment (28).
False-negative SLN assessment by OSNA is very low
(1.4%) and also well within the two SLNB false-negative rate of
9.0% (29). We suggest that pre-SLNB
testing of the tumour biopsy specimen with CK19 immunostaining is
unnecessary or restricted to defining an inclusion role for OSNA in
uncommon circumstances where absent or minimal CK19 mRNA expression
may be suspected, such as metaplastic or high Ki67 scoring tumours,
inversely reflecting the aggressive nature of the tumour (29,30).
Much higher rates of CK19 negative tumours, up to 20%, have been
reported; however, these are related to assessments of CK19
immunostaining of protein rather than CK19 mRNA levels (30–32). A
study by Pegolo et al (33),
reported no correlation between CK19 protein expression and CK19
mRNA levels within primary breast cancer or the associated
metastatic lymph node. In their study, CK19 mRNA was detected in
all cases by OSNA. Similarly, a study by Fujisue et al
(29), reported that the incidence
of CK19 negative tumours was 12.3% with immunostaining; however,
CK19 mRNA expression was absent in only 1.4% of the cases (29). These observations have allowed us to
generate a protocol for the management of the axilla in our
practice (Fig. 6).
The ACOSOG Z0011 trial demonstrated non-inferiority
of no ALND for patients with T1 or T2 primary breast lesions and
one or two positive axillary lymph nodes having BCS, whole-breast
radiotherapy and adjuvant systemic therapy (3). The findings and recommendations of the
Z0011 trial have attracted notable controversy and review. These
issues have been extensively discussed (34), recently updated (26) and remain defensible in clinical
practice. Regardless of controversy, it is clear that not all
patients with a positive SLN require an ALND if there is minimal
risk of axillary recurrence or compromise of their overall
survival. In the present study, 92.1% of patients had two or less
positive nodes on ALND. Axillary recurrence risk is low, even with
a reported false-negative SLNB rate varying between 6.7 and 14.8%
(1,9,35,36). The
reported rates of isolated axillary recurrence in SLN negative
disease are ~0.6% at 3 years and 1.1% at 5 years (37), similar to 0.9 and 1.5% reported at 5
and 10 years, respectively, for SLN-positive disease without ALND
in the Z0011 trial (3,26). It appears illogical to remain
preoccupied with the requirement to perform ALND on patients with
cT1-T2 disease and a clinically negative axilla when local
recurrence is 2.5–11 fold more likely to occur in the fully
irradiated breast than the partially irradiated ipsilateral axilla
(26). These rates of axillary
recurrence are markedly less than the rates of ALND morbidity
affecting up to 36% of patients with lymphoedema (13-19.1%),
paraesthesia (31-37.7%), pain (21.1%) and decreased mobility
(11.3%) (38,39).
In the present study, the results of testing two
nodes have provided a robust threshold for clinical
decision-making. Four-node testing would potentially alter the
management of just 8.5% (58/683) of the present cohort. Used in
combination with TTL, categorical four-node status may facilitate
the intraoperative decision to perform ALND along traditional
lines, bridging and translating the utility of SLNB TTL in existing
models of prognosis and in guiding adjuvant treatment. Enhancement
in prediction and treatment guidance is expected with extended
genomic biomarker analyses of the OSNA sample homogenate (40).
The observation that 92.1% of the present total
cohort had two or less nodes involved with macrometastatic disease
and 63% of the present patients with positive SLNs did not have
additional node metastases supports the move toward selective
conservative axillary management. The present study did not
demonstrate any benefit in performing ALND on patients with an OSNA
TTL ≥15,000 copies/µl. However, while there are no studies that
confirm otherwise, it remains practical to identify the ACOSOG
two-node macrometastases threshold for guiding clinical practice,
informing systemic treatment and axillary radiotherapy field
planning, and to putatively improve local disease control in the
axilla.
The present study confirmed the thresholds and
utility of including OSNA TTL in intraoperative ALND decision
modelling that predict and discriminate between ≤2 involved nodes
and >2 involved nodes. The present study identified, and
confirmed, an extended threshold of OSNA TTL that may independently
predict when ALND may be avoided, facilitating adoption of the
emerging acceptance and recommendations for selective conservative
management of the node positive axilla.
References
|
1
|
Krag DN, Anderson SJ, Julian TB, Brown AM,
Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP,
Jalovec LM, et al: Sentinel-lymph-node resection compared with
conventional axillary-lymph-node dissection in clinically
node-negative patients with breast cancer: Overall survival
findings from the NSABP B-32 randomised phase 3 trial. Lancet
Oncol. 11:927–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galimberti V, Cole BF, Zurrida S, Viale G,
Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, et
al: Axillary dissection versus no axillary dissection in patients
with sentinel-node micrometastases (IBCSG 23–01): A phase 3
randomised controlled trial. Lancet Oncol. 14:297–305. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giuliano AE, Hunt KK, Ballman KV, Beitsch
PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM and
Morrow M: Axillary dissection vs no axillary dissection in women
with invasive breast cancer and sentinel node metastasis: A
randomized clinical trial. JAMA. 305:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lyman GH, Temin S, Edge SB, Newman LA,
Turner RR, Weaver DL, Benson AB III, Bosserman LD, Burstein HJ,
Cody H III, et al: Sentinel lymph node biopsy for patients with
early-stage breast cancer: American Society of Clinical Oncology
clinical practice guideline update. J Clin Oncol. 32:1365–1383.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsujimoto M, Nakabayashi K, Yoshidome K,
Kaneko T, Iwase T, Akiyama F, Kato Y, Tsuda H, Ueda S, Sato K, et
al: One-step nucleic acid amplification for intraoperative
detection of lymph node metastasis in breast cancer patients. Clin
Cancer Res. 13:4807–4816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Filippo F, Giannarelli D, Bouteille C,
Bernet L, Cano R, Cunnick G and Sapino A: Elaboration of a nomogram
to predict non sentinel node status in breast cancer patients with
positive sentinel node, intraoperatively assessed with one step
nucleic acid amplification method. J Exp Clin Cancer Res.
34:1362015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peg V, Espinosa-Bravo M, Vieites B,
Vilardell F, Antúnez JR, de Salas MS, Delgado-Sánchez JJ, Pinto W,
Gozalbo F, Petit A, et al: Intraoperative molecular analysis of
total tumor load in sentinel lymph node: A new predictor of
axillary status in early breast cancer patients. Breast Cancer Res
Treat. 139:87–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
NHS Cancer Screening Programmes/Royal
College of Pathologists: Pathology Reporting of Breast Disease.
NHSBSP publication no. 58. 2005 http://www.cancerscreening.nhs.uk/breastscreen/publications/nhsbsp58.htmlAccessed.
May 21–2016.
|
|
9
|
Mansel RE, MacNeill F, Horgan K, Goyal A,
Britten A, Townson J, Clarke D, Newcombe RG, Keshtgar M Guildford
Breast Surgeons, et al: Results of a national training programme in
sentinel lymph node biopsy for breast cancer. Br J Surg.
100:654–661. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koca B, Kuru B, Ozen N, Yoruker S and Bek
Y: A breast cancer nomogram for prediction of non-sentinel node
metastasis-validation of fourteen existing models. Asian Pac J
Cancer Prev. 15:1481–1488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glechner A, Wöckel A, Gartlehner G, Thaler
K, Strobelberger M, Griebler U and Kreienberg R: Sentinel lymph
node dissection only versus complete axillary lymph node dissection
in early invasive breast cancer: A systematic review and
meta-analysis. Eur J Cancer. 49:812–825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Straver ME, Meijnen P, van Tienhoven G,
van de Velde CJ, Mansel RE, Bogaerts J, Demonty G, Duez N,
Cataliotti L, Klinkenbijl J, et al: Role of axillary clearance
after a tumor-positive sentinel node in the administration of
adjuvant therapy in early breast cancer. J Clin Oncol. 28:731–737.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nadeem RM, Gudur LD and Saidan ZA: An
independent assessment of the 7 nomograms for predicting the
probability of additional axillary nodal metastases after positive
sentinel lymph node biopsy in a cohort of British patients with
breast cancer. Clin Breast Cancer. 14:272–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meretoja TJ, Audisio RA, Heikkilä PS, Bori
R, Sejben I, Regitnig P, Luschin-Ebengreuth G, Zgajnar J, Perhavec
A, Gazic B, et al: International multicenter tool to predict the
risk of four or more tumour-positive axillary lymph nodes in breast
cancer patients with sentinel node macrometastases. Breast Cancer
Res Treat. 138:817–827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katz A, Smith BL, Golshan M, Niemierko A,
Kobayashi W, Raad RA, Kelada A, Rizk L, Wong JS, Bellon JR, et al:
Nomogram for the prediction of having four or more involved nodes
for sentinel lymph node-positive breast cancer. J Clin Oncol.
26:2093–2098. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chagpar AB, Scoggins CR, Martin RC II,
Cook EF, McCurry T, Mizuguchi N, Paris KJ, Carlson DJ, Laidley AL,
El-Eid SE, et al: Predicting patients at low probability of
requiring post-mastectomy radiation therapy. Ann Surg Oncol.
14:670–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rivers AK, Griffith KA, Hunt KK, Degnim
AC, Sabel MS, Diehl KM, Cimmino VM, Chang AE, Lucas PC and Newman
LA: Clinicopathologic features associated with having four or more
metastatic axillary nodes in breast cancer patients with a positive
sentinel lymph node. Ann Surg Oncol. 13:36–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kubota M, Komoike Y, Hamada M, Shinzaki W,
Azumi T, Hashimoto Y, Imoto S, Takeyama Y and Okuno K: One-step
nucleic acid amplification assay for intraoperative prediction of
advanced axillary lymph node metastases in breast cancer patients
with sentinel node metastasis. Mol Clin Oncol. 4:173–178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piñero-Madrona A, Ruiz-Merino G, Bernet L,
Miguel-Martínez B, Vicente-García F, Viguri-Díaz MA and
Giménez-Climent J: Tumoral load quantification of positive sentinel
lymph nodes in breast cancer to predict more than two involved
nodes. Breast. 23:859–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Intraoperative tests (RD-100i OSNA system
and Metasin test) for detecting sentinel lymph node metastases in
breast cancer. NICE diagnostics guidance 8. August;2013 http://www.nice.org.uk/dg8Accessed. May
21–2016.
|
|
21
|
Raia-Barjat T, Trombert B, Khaddage A,
Douchet C, Seffert P, Peoc'h M, Falk AT, Magné N and Chauleur C:
OSNA (one-step nucleic acid amplification) sentinel lymph node
intraoperative molecular analysis in breast cancer: A cost-benefit
analysis. Med Oncol. 31:3222014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brambilla T, Fiamengo B, Tinterri C,
Testori A, Grassi MM, Sciarra A, Abbate T, Gatzemeier W, Roncalli M
and Di Tommaso L: One-step nucleic acid amplification in breast
cancer sentinel lymph node: A single institutional experience and a
short review. Front Med (Lausanne). 2:372015.PubMed/NCBI
|
|
23
|
Espinosa-Bravo M, Sansano I, Pérez-Hoyos
S, Ramos M, Sancho M, Xercavins J, Rubio IT and Peg V: Prediction
of non-sentinel lymph node metastasis in early breast cancer by
assessing total tumoral load in the sentinel lymph node by
molecular assay. Eur J Surg Oncol. 39:766–773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohi Y, Umekita Y, Sagara Y, Rai Y,
Yotsumoto D, Matsukata A, Baba S, Tamada S, Matsuyama Y, Ando M, et
al: Whole sentinel lymph node analysis by a molecular assay
predicts axillary node status in breast cancer. Br J Cancer.
107:1239–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Osako T, Iwase T, Kimura K, Horii R and
Akiyama F: Sentinel node tumour burden quantified based on
cytokeratin 19 mRNA copy number predicts non-sentinel node
metastases in breast cancer: Molecular whole-node analysis of all
removed nodes. Eur J Cancer. 49:1187–1195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giuliano A, Ballman K, McCall L, Beitsch
P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M and
Hunt KK: Locoregional recurrence after sentinel lymph node
dissection with or without axillary dissection in patients with
sentinel lymph node metastases: Long-term Follow-up From the
American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011
Randomized Trial. Ann Surg. 264:413–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peg V, Sansano I, Vieites B, Bernet L,
Cano R, Córdoba A, Sancho M, Martín MD, Vilardell F, Cazorla A, et
al: Role of total tumour load of sentinel lymph node on survival in
early breast cancer patients. Breast. 33:8–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goyal A, Newcombe RG and Mansel RE:
Axillary Lymphatic Mapping Against Nodal Axillary Clearance
(ALMANAC) Trialists Group: Clinical relevance of multiple sentinel
nodes in patients with breast cancer. Br J Surg. 92:438–442. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujisue M, Nishimura R, Okumura Y, Tashima
R, Nishiyama Y, Osako T, Toyozumi Y and Arima N: Clinical
significance of CK19 negative breast cancer. Cancers (Basel).
5:1–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vilardell F, Novell A, Martin J, Santacana
M, Velasco A, Díez-Castro MJ, Cuevas D, Panadés MJ, González S,
Llombart A, et al: Importance of assessing CK19 immunostaining in
core biopsies in patients subjected to sentinel node study by OSNA.
Virchows Arch. 460:569–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tamaki Y: One-step nucleic acid
amplification assay (OSNA) for sentinel lymph node biopsy. Breast
Cancer. 22:230–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alvarenga CA, Paravidino PI, Alvarenga M,
Dufloth R, Gomes M, Zeferino LC and Schmitt F: Expression of CK19
in invasive breast carcinomas of special histological types:
Implications for the use of one-step nucleic acid amplification. J
Clin Pathol. 64:493–497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pegolo E, Puppin C, Gerometta A, Damante
G, Puglisi F and Di Loreto C: One-step nucleic acid amplification
(OSNA) for intraoperative evaluation of sentinel lymph node status
in breast cancer: A comparative study between CK19 protein
expression and CK19 mRNA level in primary tumors and lymph node
metastasis. Virchows Arch. 463:7–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shah-Khan M and Boughey JC: Evolution of
axillary node staging in breast cancer: Clinical implications of
the ACOSOG Z0011 trial. Cancer Control. 19:267–276. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kohrt HE, Olshen RA, Bermas HR, Goodson
WH, Wood DJ, Henry S, Rouse RV, Bailey L, Philben VJ, Dirbas FM, et
al: New models and online calculator for predicting non-sentinel
lymph node status in sentinel node positive breast cancer patients.
BMC Cancer. 8:662008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goyal A, Newcombe RG, Chhabra A and Mansel
RE: ALMANAC Trialists Group: Factors affecting failed localisation
and false-negative rates of sentinel node biopsy in breast
cancer--results of the ALMANAC validation phase. Breast Cancer Res
Treat. 99:203–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bergkvist L, de Boniface J, Jönsson PE,
Ingvar C, Liljegren G and Frisell J: Swedish Society of Breast
Surgeons: Axillary recurrence rate after negative sentinel node
biopsy in breast cancer: Three year follow-up of the Swedish
multicenter cohort study. Ann Surg. 247:150–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mansel RE, Fallowfield L, Kissin M, Goyal
A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny
I, et al: Randomized multicenter trial of sentinel node biopsy
versus standard axillary treatment in operable breast cancer: The
ALMANAC trial. J Natl Cancer Inst. 98:599–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Langer I, Guller U, Berclaz G, Koechli OR,
Schaer G, Fehr MK, Hess T, Oertli D, Bronz L, Schnarwyler B, et al:
Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and
completion axillary lymph node dissection after breast cancer
surgery: A prospective Swiss multicenter study on 659 patients. Ann
Surg. 245:452–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martín-Sánchez E, Pernaut-Leza E, Mendaza
S, Cordoba A, Vicente-Garcia F, Monreal-Santesteban I, Vizcaino JP,
De Cerio MJ, Perez-Janices N, Blanco-Luquin I, et al: Gene promoter
hypermethylation is found in sentinel lymph nodes of breast cancer
patients, in samples identified as positive by one-step nucleic
acid amplification of cytokeratin 19 mRNA. Virchows Arch.
469:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|