Introduction
Glioblastoma (GBM) is the most frequently occurring
primary tumor of the central nervous system and represents one of
the most lethal malignancies. Surgical resection and postoperative
radiotherapy with concomitant and adjuvant temozolomide (TMZ) are
employed as a first-line treatment for GBM (1,2), and
more recently bevasizumab and/or carmustine wafers have also been
used in Japan (3,4). We report here an unusual case of
temporal lobe GBM fed from the middle meningeal artery that
underwent double implantation of carmustine wafers and triple
surgery.
The present study was approved by the Ethics
Committee of Nihon University Itabashi Hospital (Tokyo, Japan) and
written informed consent was obtained from the patient and his
family.
Case report
A 66-year-old male was admitted to the Department of
Neurosurgery at Nihon University Itabashi Hosipital (Tokyo, Japan)
with epilepsy. He had complained of headache a week before the
epilepsy. Laboratory evaluations including tumor markers
demonstrated no abnormalities. His consciousness level was clear
and neurological examinations revealed no abnormalities except for
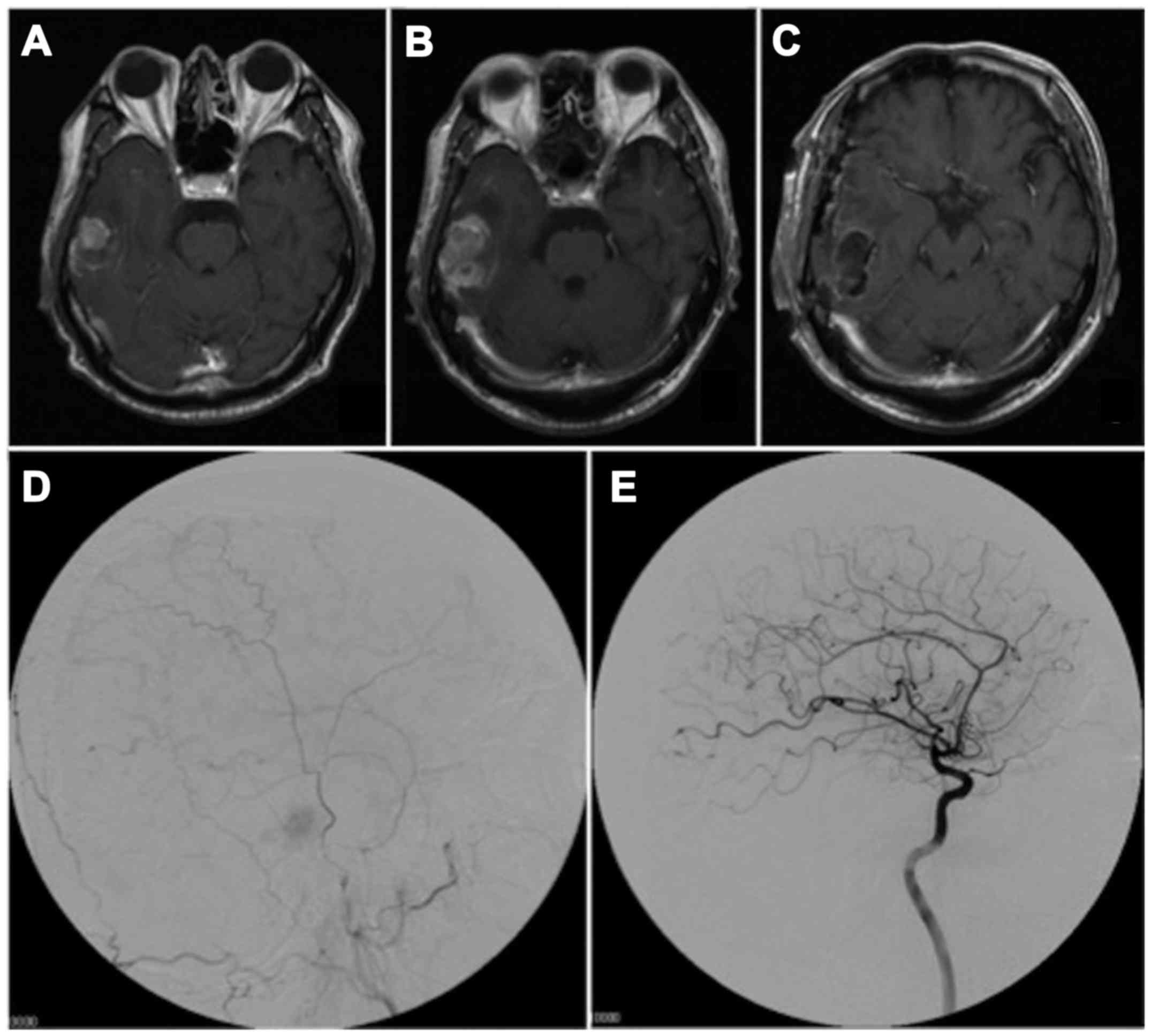
headache and deja vu. Magnetic resonance imaging (MRI) disclosed a
1.8 cm-diameter right middle fossa mass lesion which was attached
to the dura mater, and displayed low-intensity on T1-weighted MRI
and high-intensity on T2-weighted MRI; enhancement was evident
following contrast medium administration (Fig. 1A). The lesion was diagnosed
preoperatively as a meningioma. However, preoperative MRI one month
after the first MRI, disclosed rapid mass growth (Fig. 1B). The patient underwent surgical
resection and the tumor was completely removed (Fig. 1C). An angiogram showed a stain fed
from the right middle meningeal artery without branches of the
internal carotid artery (Fig. 1D and
E). The intraoperative findings indicated that the tumor was
attached to the dura mater and the tumor border was relatively
clear. Eight pieces of carmustine wafers were implanted in the
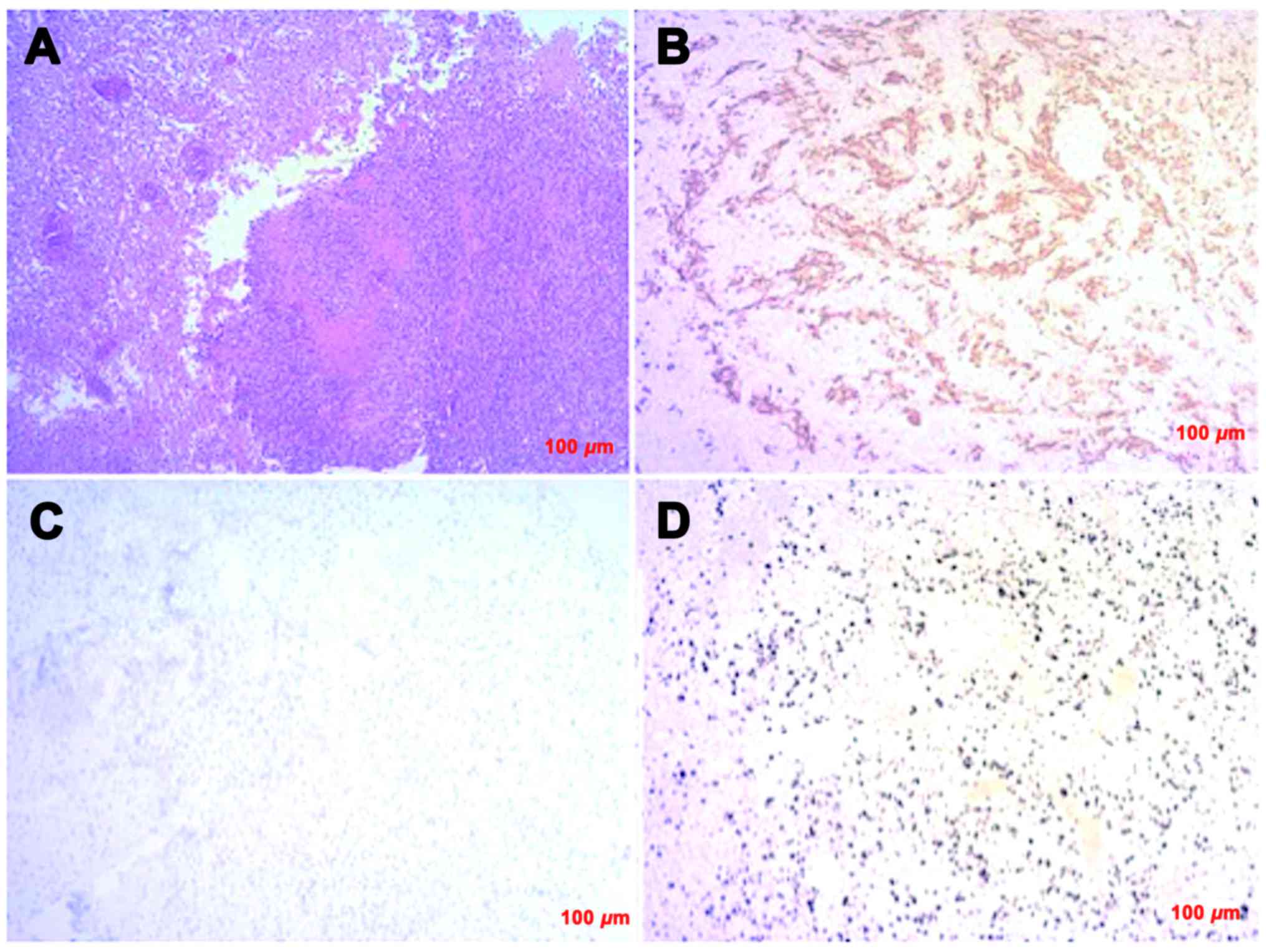
tumor resection cavity. Pathological examinations of the tumor
specimen demonstrated a high cellularity, mitosis,
pseudopalisading, necrosis, and microvascular proliferation
(Fig. 2A). Immunohistochemically,
the tumor cells exhibited positive expression for glial fibril acid
protein (Fig. 2B), whereas they were
negative for cytokeratin and epithelial membrane antigen. These
pathological findings were consistent with GBM. The tumor cells
were negative for IDH1-R132H and EGFR (Fig. 2C). The MIB-1 labeling index was 55%
(Fig. 2D). The patient underwent TMZ
chemotherapy with 60 gray radiation therapy and was discharged from
hospital, with a Karnofsky Performance Status score of 100, at 70
days after the first surgery.
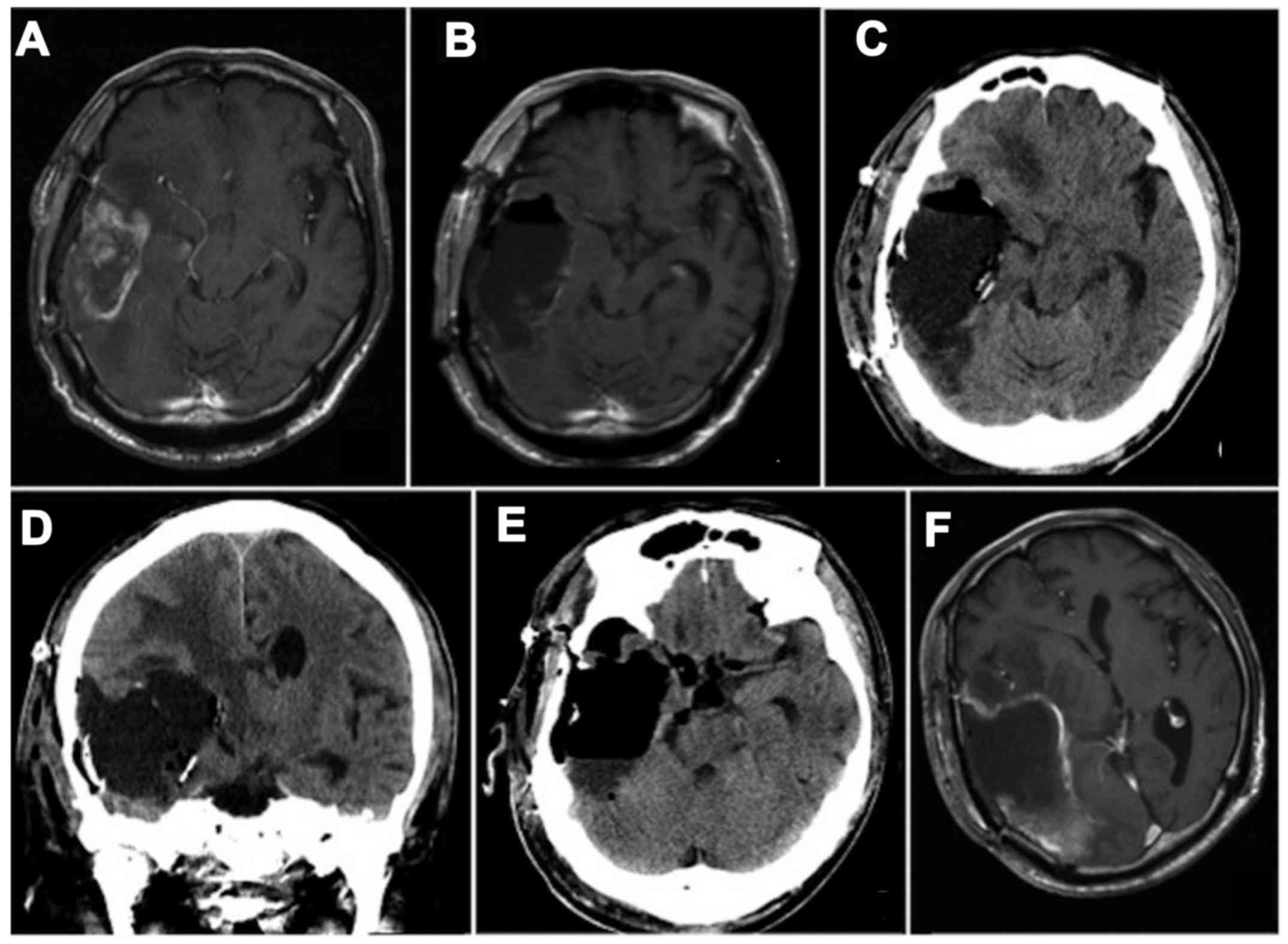
Six months later, after five courses of TMZ
maintenance therapy, evidence of tumor recurrence was found around
the resection cavity (Fig. 3A). The
patient underwent a second operation via the same approach as for
the first operation, and the tumor was again completely removed
(Fig. 3B). Eight pieces of
carmustine wafers were implanted in the cavity. Pathological
examinations of the tumor specimen demonstrated the same pattern as
for the first tumor specimen. Five days after the second surgery, a
third operation was performed because a cyst formed in the surgical
cavity (Fig. 3C and D). The cyst was
opened to the basal cisterns and the carmustine wafers were removed
at the third operation (Fig. 3E).
The cyst contents were found to comprise cerebrospinal fluid with
slight xanthochromia. The opened cyst reformed within a few days
after the third operation, and tumor recurrence was found at 25
days after the third operation (Fig.
3F). Despite another four courses of TMZ maintenance therapy
with additional administration of bevasizumab, the tumor became
uncontrollable and the patient died at 11 months after the first
operation.
Discussion
The extent of resection by surgery affects survival
time in GBM patients (5,6). A better prognosis might therefor be
predicted for right temporal lobe localized GBM, since total
removal can be carried out.
The present case was initially diagnosed as
meningioma, because the tumor stain was clearly seen from the
middle meningeal artery but not from the internal carotid artery.
Only a few case reports of such GBMs have been described (7,8). The
timing of surgical treatment may easily be delayed if the lesion is
misdiagnosed as a meningioma, and may be of concern for the
prognosis of GBM patients.
Cyst formation is known to occur as an adverse event
of carmustine wafer implantation for malignant gliomas (9), and there have been several reports of
space-occupying cysts in the cavity, which required additional
surgical treatment (10–13). Yoshida et al reported that
adverse events associated with implantation of carmustine wafers
tend to occur in the repeated surgery for the malignant gliomas
(11). Although there have been few
reports on multiple implantation of carmustine wafers, the risk of
adverse events, including cyst formation, might be high when double
implantation is performed as in the present case. Basic research
has not been done about the mechanism of cyst formation induced by
carmustine wafers implantation, so that further studies including
basic researches are clearly needed to clarify the mechanism.
In conclusion, we have described an atypical and
suggestive case of GBM. We need to be aware GBM should not be
excluded in preoperative diagnoses made from imaging studies, even
if the tumor is fed only by the middle meningeal artery. Moreover,
careful attention should be exercised when carmustine wafers have
to be implanted in repeated surgery.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aoki T, Nishikawa R, Sugiyama K, Nonoguchi
N, Kawabata N, Mishima K, Adachi J, Kurisu K, Yamasaki F, Tominaga
T, et al: A multicenter phase I/II study of the BCNU implant
(Gliadel(®) Wafer) for Japanese patients with malignant
gliomas. Neurol Med Chir (Tokyo). 54:290–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanai N, Polley MY, McDermott MW, Parsa AT
and Berger MS: An extent of resection threshold for newly diagnosed
glioblastomas. J Neurosurg. 115:3–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okuyama T, Saito K, Hirano A, Takahashi A,
Inagaki T, Inamura S and Nakazato Y: Glioblastoma fed by meningeal
branches of the external carotid artery: A case report. No Shinkei
Geka. 27:447–452. 1999.(In Japanese). PubMed/NCBI
|
|
8
|
Patel M, Son Nguyen H, Doan N, Gelsomino
M, Shabani S and Mueller W: Glioblastoma mimicking meningioma:
Report of 2 cases. World Neurosurg. 95:624.e9–624.e13. 2016.
View Article : Google Scholar
|
|
9
|
Chowdhary SA, Ryken T and Newton HB:
Survival outcomes and safety of carmustine wafers in the treatment
of high-grade gliomas: A meta-analysis. J Neurooncol. 122:367–382.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dörner L, Ulmer S, Rohr A, Mehdorn HM and
Nabavi A: Space-occupying cyst development in the resection cavity
of malignant gliomas following Gliadel®
implantation-Incidence, therapeutic strategies, and outcome. J Clin
Neurosci. 18:347–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida M, Yamaguchi S, Ishi Y, Endo S,
Motegi H, Kobayashi H, Asaoka K, Kamoshima Y, Terasaka Y and Houkin
K: Risk factors for adverse events after implantation of BCNU
wafers in high-grade gliomas. No Shinkei Geka. 43:603–610. 2015.(In
Japanese). PubMed/NCBI
|